Introduction
Rotavirus is a double-stranded RNA virus which
remains a primary etiologic agent of viral gastroenteritis and
diarrhea in young children and animals (1). In humans, rotavirus is responsible for
~450,000 deaths worldwide per year in children under 5 years old
(2). Rotavirus infection mainly
occurs in the small intestine, which plays a role in food digestion
and absorption (3). Current
strategies to prevent rotavirus transmission are limited as the
virus is particularly resistant to common disinfectants (4). At present, two commercially available
vaccines, Rotarix (GlaxoSmithKline plc) and RotaTeq (Merck KGaA),
have been approved in many countries (5). These vaccines seem to be effective at
reducing rotavirus-induced diarrhea. However, the vaccines only
exert positive effects against the target strain of the virus, and
the immunization schedule of vaccines must be strictly controlled
(6). Therefore, it is important to
develop other natural antiviral agents to treat rotavirus
infection.
Macleaya cordata extract is a member of the
Macleaya genus in the family Papaveraceae, which contains
various active alkaloids (7). M.
cordata has been reported to have a wide spectrum of biological
activities, including antiviral, anti-inflammatory, antioxidative,
detoxifying and antimicrobial effects (8). In China, M. cordata has also
been used as a traditional medicinal herb to treat cancer,
including thyroid cancer and cervical cancer (9). M. cordata is also a traditional
medicinal plant in Europe and North America, used for treating
ringworm infection and insect bites (8).
The present study hypothesized that M.
cordata may play key roles in mediating antiviral responses and
consequently reduce the incidence of rotavirus-induced diarrhea.
The aim of the present study was to assess the biological influence
of M. cordata on rotavirus-induced diarrhea by detecting
rotavirus antigens and inflammatory cytokines, evaluating the
histopathological changes in the small intestine and exploring the
underlying mechanisms by which M. cordata mediates its
antiviral and anti-inflammatory effects.
Materials and methods
Cells and virus
Simian rotavirus SA11 and rhesus monkey kidney
MA-104 cells (American Type Culture Collection) were used for
rotavirus infection and cultivation (10). MA-104 cells were grown in DMEM
(Beyotime Institute of Biotechnology) supplemented with 10% FBS
(Beyotime Institute of Biotechnology) at 37˚C under 5%
CO2. SA11 was activated with 15 µg/ml trypsin for 30 min
and cultured in MA-104 cells for 48 h. The virus titer used in the
present study was 4x107 focus-forming units/ml (11).
Animals and experimental design
A total of 72 newborn BALB/c mice (female, 16-18 g)
were obtained from Nanjing Medical University Animal Laboratory and
maintained as previously described (12). Animal procedures were in line with
the National Institutes of Health Guidelines for the Care and Use
of Laboratory Animals and were approved by the Animal Committee of
the Jiangsu Agri-animal Husbandry Vocational College. All mice were
negative for rotavirus antibodies before rotavirus exposure. Mice
were randomly assigned to 6 groups (n=12 in each group) and
ear-coded as follows: Control; rotavirus (virus-inoculated and
administered saline); virus-inoculated and administered 1 mg/kg/day
M. cordata (Phytobiotics Futterzusatzstoffe GmbH);
virus-inoculated and administered 2 mg/kg/day M. cordata;
virus-inoculated and administered with 4 mg/kg/day M.
cordata; and virus-inoculated and administered with 4 mg/kg/d
ribavirin (Sigma-Aldrich; Merck KGaA). At 3 days of age, mice were
inoculated with 30 µl rotavirus-infected MA-104 cells via oral
gavage. At 2 days post-inoculation (DPI 2), the mice showed signs
of diarrhea. Inoculated mice were evaluated for diarrhea (0 to 4)
determined as the following: No fecal discharge recorded, score 0;
brown molded stool, score 1; brown soft stool, score 2; soft yellow
stool, score 3; yellow watery stool, score 4; and perianal stool
contamination, also score 4. 1 was considered no diarrhea; 2 was
considered common diarrhea; 3 was considered severe diarrhea; and 4
was considered very severe diarrhea. Mice with a score >2 were
identified as having diarrhea. The mice were treated with M.
cordata (1, 2, 4 mg/kg/day) or ribavirin (4 mg/kg/day). The
antiviral effects of M. cordata were evaluated on fecal
material, serum and small intestine histology. At the predetermined
times, mice were anesthetized prior to euthanasia by decapitation.
The experiments were based on humane endpoint criteria, that mice
with a score >2 were identified. All the animal procedures were
in accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals. The small intestines were
harvested at DPI 3 for pathological analysis (n=6). Samples were
quickly obtained and frozen in liquid nitrogen and stored at -80˚C
for further analysis. The remaining six mice in each group were
sacrificed at DPI 7 for diarrhea observation and the detection of
inflammatory cytokines.
Diarrhea score
To evaluate the antiviral effects of M.
cordata, fecal consistency scores (1, normal; 2, soft feces; 3,
mild diarrhea; 4, severe diarrhea) were determined for each rat
(13). Scores ≥2 were considered a
symptom of diarrhea. The severity of diarrhea was calculated by
dividing the total number of all scores by the number of total
experimental mice.
ELISA
Fecal samples were collected at DPI 3, dissolved in
PBS and centrifuged at 1,000 x g for 15 min at 4˚C. The supernatant
was collected for rotavirus antigen detection using an ELISA kit
(cat. no. CSB-EQ027718MO; Cusabio Technology, LLC) according to the
manufacturer's protocol. The absorbance was read at a wavelength of
450 nm using an EL808 microplate reader (BioTek Instruments, Inc.).
An optical density at 450 nm of the sample minus that of the
control group ≥0.1 was considered to be positive.
Macrophage migration inhibitory factor (MIF) (cat.
no. abx258931; Biolead Co., Ltd.), interleukin (IL)-8 (cat. no.
KHC0081; Invitrogen; Thermo Fisher Scientific, Inc.), IL-10 (cat.
no. 29-8101-65; eBioscience; Thermo Fisher Scientific, Inc.), IL-β
(cat. no. BMS224-2; Invitrogen; Thermo Fisher Scientific, Inc.),
interferon (IFN)-γ (cat. no. E-EL-R0009c; Elabscience), tumor
necrosis factor (TNF)-α (cat. no. BMS223HS; Invitrogen; Thermo
Fisher Scientific, Inc.) and IL-6 (cat. no. BMS213HS; Invitrogen;
Thermo Fisher Scientific, Inc.) in serum were also determined using
commercial ELISA kits. A total of 500 µl of blood was collected
each day and centrifuged at 1,000 x g for 10 min at 4˚C to obtain
serum samples.
Reverse transcription-quantitative PCR
(RT-qPCR)
Rotavirus VP6 RNA in fecal samples was extracted as
previously described (14). Total
RNA was extracted using a QIAamp Viral RNA Mini kit (cat. no.
52904; Qiagen China Co., Ltd.) and stored at -80˚C for further
analysis. RT was performed under the following thermocycling
conditions: 61˚C for 3 min, followed by 95˚C for 5 min and chilling
at 4˚C. The gene expression levels were measured using the SYBR
Premix Ex TaqTM (Takara Bio, Inc.). The temperature protocol was as
follow: 95˚C for 5 sec, 60˚C for 45 sec and 72˚C for 1 min for 40
cycles using a Real Time PCR system (Illumina, Inc.). A standard
curve established using Light-Cycler 480 gene scanning software
V1.5 (Roche) was used to measure RNA concentration by performing
linear analysis of the data. The following primer pair was used for
the qPCR: The amount of rotavirus RNA in fecal samples was
quantified based on the standard curve derived from 10-fold serial
dilutions of plasmid DNA, which was used as an external standard
for all RT-qPCR experiments (11).
The data were calculated using the
2-ΔΔCT method (12).
Hematoxylin and eosin (H&E)
staining
Mouse small intestines were excised at DPI 3,
perfused with 10% formalin for 24 h at 4˚C and embedded in
paraffin. Sections with a thickness of 5 µm were stained with 0.5%
hematoxylin for 5 min at room temperature, followed by staining
with 0.5% eosin solution for 1 min at room temperature. The images
were examined under a light microscope (Olympus Corporation) at
x200 magnification.
TUNEL assay
To evaluate the effects of M. cordata on
intestinal epithelial cell apoptosis, mice were sacrificed at DPI
3. Small intestine samples were fixed in 10% formalin for 24 h at
4˚C and embedded in paraffin. Sections with 5-µm thickness were
used for TUNEL staining (according to the instructions of In
Situ Cell Death Detection kit (cat. no. 11684817910; Roche
Diagnostics GmbH). In brief, the sections were washed and incubated
with proteinase K for 30 min at 37˚C, followed by incubation with a
terminal deoxynucleotidyl transferase. Then the sections were
treated with 3% hydrogen peroxide for 5 min and subsequently
incubated with the peroxidase-conjugated antibodies from the
aforementioned kit, for 10 min at room temperature. Then the DAB
solution, with 3% hydrogen peroxide and the methyl green was added
for 2 min at room temperature. After treating with Mayer's
hematoxylin for 1 min at room temperature, the TUNEL-positive cells
were observed under a light microscope (Olympus Corporation) at
x200 magnification. Five fields of view were selected randomly from
one section.
Western blotting
Total protein was extracted from small intestines
using RIPA buffer (Beyotime Institute of Biotechnology) and the
concentration was detected using the BCA kit (Beyotime Institute of
Biotechnology). Proteins (30 µg per lane) were separated by 10%
SDS-PAGE and transferred to a PVDF membrane (EMD Millipore).
Subsequently, the membrane was blocked with 5% non-fat milk for 2 h
at room temperature. The membrane was incubated overnight at 4˚C
with the following primary antibodies: Janus kinase 2 (JAK2;
1:1,000; cat. no. 74987), phosphorylated (p)-JAK2 (1:750; cat. no.
66245), STAT3 (1:1,000; cat. no. 9139), p-STAT3 (1:750; cat. no.
4113) and GAPDH (1:2,000; cat. no. 97116). The membranes were
subjected to three 5-min washes with 0.5% TBS with Tween-20, then
probed with HRP-conjugated secondary mouse antibodies (1:2,000;
cat. no. 7076) for 1 h at room temperature. Following washing,
protein levels were examined with an ECL kit (Beyotime Institute of
Biotechnology). All antibodies were purchased from CST Biological
Reagents Co., Ltd. The gray values of the bands were calculated
using ImageJ software (version 4.3; National Institutes of Health)
with GAPDH as the loading control.
Immunohistochemical analysis
At DPI 3, the aforementioned paraffin-embedded
sections were used to assess the expression of p-JAK2 and p-STAT3
based immunohistochemical staining. Sections with 5-µm thickness
were incubated with primary antibodies for JAK2 (1:1,000; cat. no.
74987), p-JAK2 (1:750; cat. no. 66245), STAT3 (1:1,000; cat. no.
9139) and p-STAT3 (1:750; cat. no. 4113) at 37˚C overnight,
followed by incubation with biotinylated secondary antibody (cat.
no. 31926, 30-40 µl) at room temperature for 30 min. All antibodies
were purchased from CST Biological Reagents Co., Ltd. All images
were captured under a light microscope (Olympus Corporation) at
x200 magnification.
Statistical analysis
Data were analyzed using GraphPad Prism 5.0
(GraphPad Software, Inc.) and are expressed as the mean ± SD. All
experiments were repeated at least three times. One-way ANOVA
followed by Tukey's post hoc test was used to compare the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
M. cordata decreases the severity of
diarrhea in rotavirus-infected newborn mice
To investigate the effects of M. cordata on
rotavirus infection in vivo, newborn mice were inoculated
with SA11 rotavirus cultured in MA104 cells by oral gavage. As
expected, mice without rotavirus treatment did not exhibit diarrhea
during the entire experimental period, whereas mice inoculated with
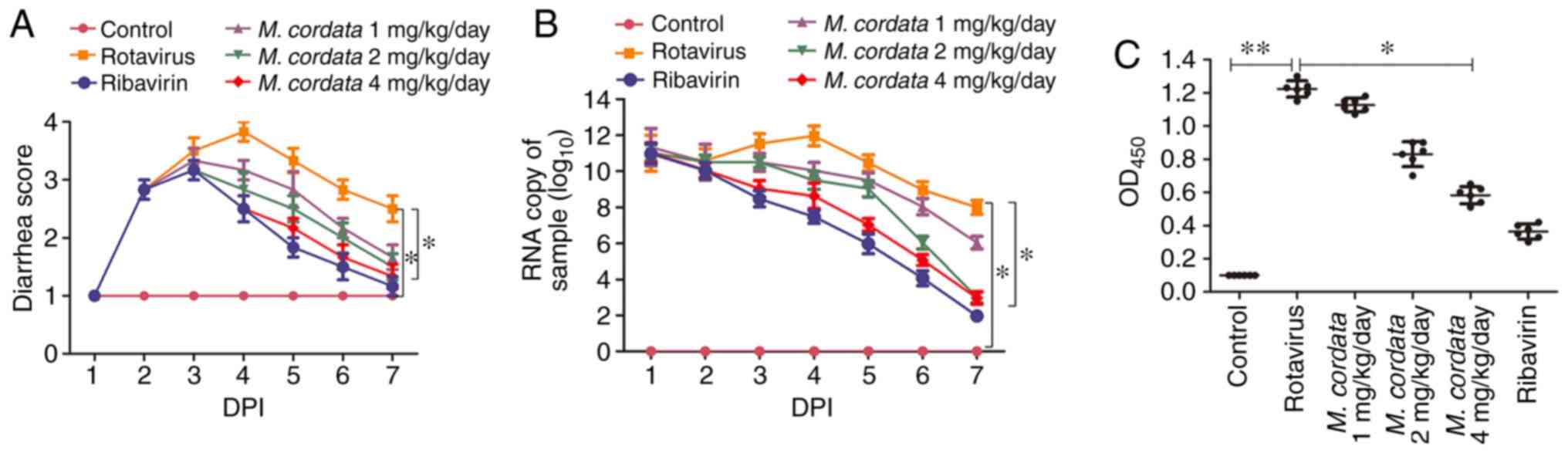
rotavirus presented with severe diarrhea at DPI 2 (Fig. 1A). Diarrhea was inhibited after
ribavirin treatment ranging from DPI 4-7, when compared to
Rotavirus-treated group, as evidenced by the decreased diarrhea
score in the ribavirin group. M. cordata treatment resulted
in decreased diarrhea scores at DPI 4 compared with
rotavirus-inoculated mice, and diarrhea scores appeared reduced at
DPI 6. No significant difference was observed in the diarrhea
scores between M. cordata- and ribavirin-treated mice. The
results demonstrated that M. cordata could effectively
inhibit rotavirus-induced diarrhea.
M. cordata inhibits rotavirus
replication in rotavirus-infected newborn mice
In order to determine whether M. cordata
suppressed rotavirus infectivity, rotavirus VP6 RNA and antigen
expression were evaluated using RT-qPCR and ELISA, respectively. No
expression of rotavirus antigens was detected in mouse feces prior
to the experiments. As depicted in Fig.
1B, no increase was measured in the rotavirus copy number of
control mice, whereas M. cordata remarkedly reduced
rotavirus copy numbers at the three tested doses. Similarly, the
M. cordata- and ribavirin-treated groups presented with
reduced rotavirus antigen levels compared with virus-inoculated
mice (Fig. 1C). No significant
difference in antigen levels between the M. cordata (4
mg/kg/day) and ribavirin-treated groups was measured.
M. cordata restores the levels of
inflammatory cytokines in rotavirus-infected newborn mice
ELISA was performed to quantify inflammatory
cytokine levels in rotavirus-infected newborn mice. As shown in
Fig. 2, the levels of the
pro-inflammatory cytokines MIF, IL-6, IL-8, TNF-α, IFN-β and IFN-γ
increased, but the levels of anti-inflammatory IL-10 declined,
after rotavirus infection. However, M. cordata or ribavirin
treatment significantly reversed the upregulation of
pro-inflammatory mediator levels and the downregulation of
anti-inflammatory molecule levels (P<0.05; Fig. 2). The results indicated that M.
cordata may exert immunomodulatory and anti-inflammatory roles
in rotavirus-induced diarrhea.
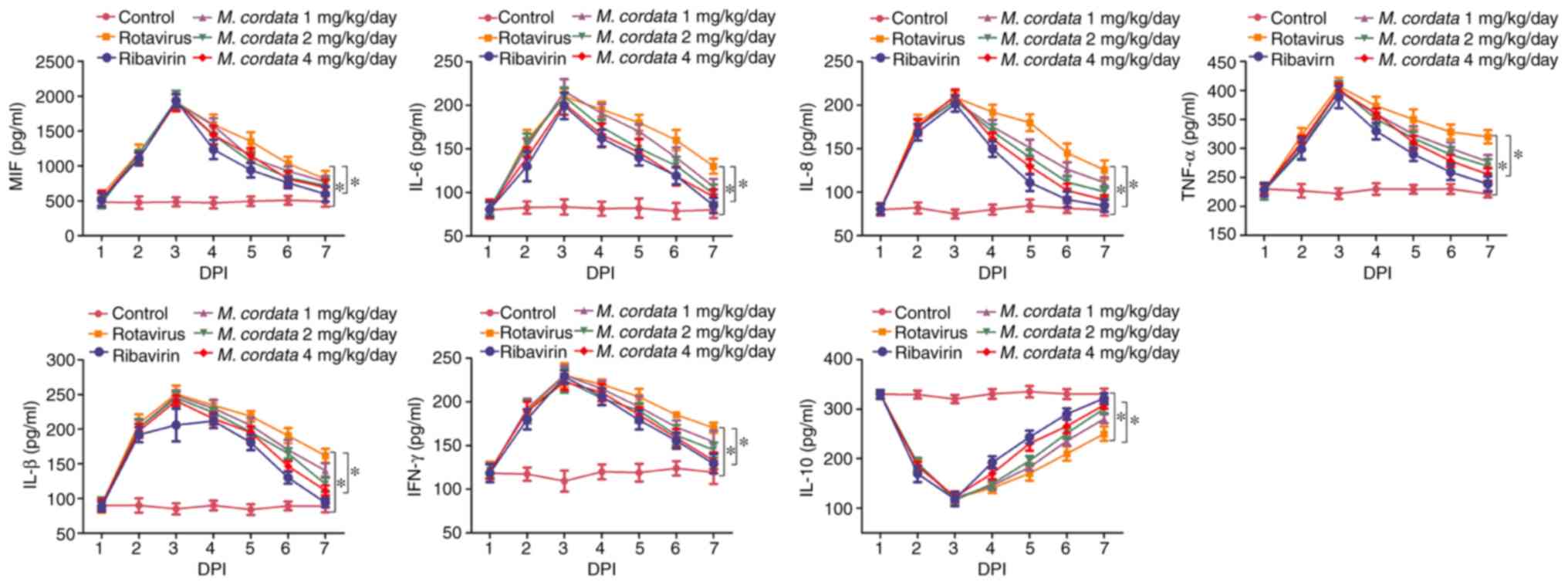 | Figure 2Influence of M. cordata on
rotavirus-induced levels of IL-8, IFN-β, IFN-γ, TNF-α, IL-6 and
IL-10 in mice. *P<0.05. MIF, migration inhibitory
factor; IL, interleukin; IFN, interferon; TNF, tumor necrosis
factor; DPI, days post-inoculation; M. cordata, Macleaya
cordata. |
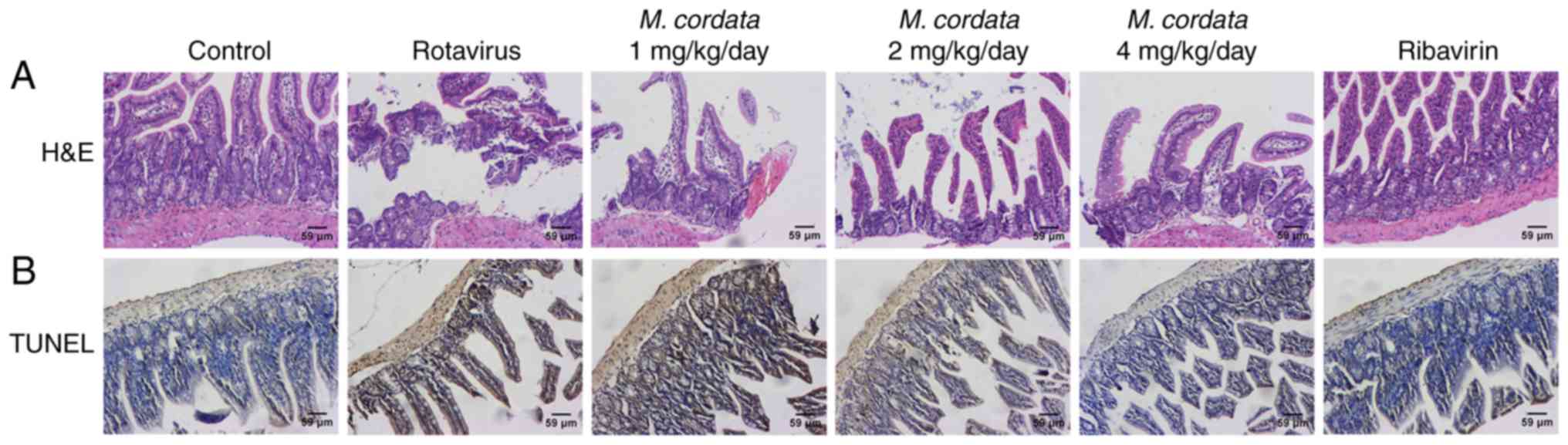
M. cordata induces histopathological
changes in the small intestine
H&E staining was used to examine the effects of
M. cordata on rotavirus-induced changes in the pathology of
the small intestine. As shown in Fig.
3A, enterocytes were clearly polarized and the nuclei were
localized at the base of the enterocytes in control mice.
Virus-inoculated mice showed notable lesions in the small
intestinal villi, including epithelium defluxion, edema and
vacuolar degeneration. However, M. cordata and ribavirin
treatment led to an attenuation of the lesions in the small
intestinal villi. These data indicated that M. cordata
treatment could improve the pathological damage in the small
intestine caused by rotavirus.
M. cordata suppresses enterocyte
apoptosis in rotavirus-infected newborn mice
A TUNEL assay was performed to further investigate
the potential effect of M. cordata on enterocyte apoptosis
in rotavirus-induced mice. Virus-inoculated mice showed notable
enterocyte apoptosis compared with control mice (Fig. 3B). However, M. cordata
treatment resulted in reduced apoptotic cell death as confirmed by
the decreased number of TUNEL-positive cells, indicating that
enterocyte apoptosis was repressed in rotavirus-infected mice.
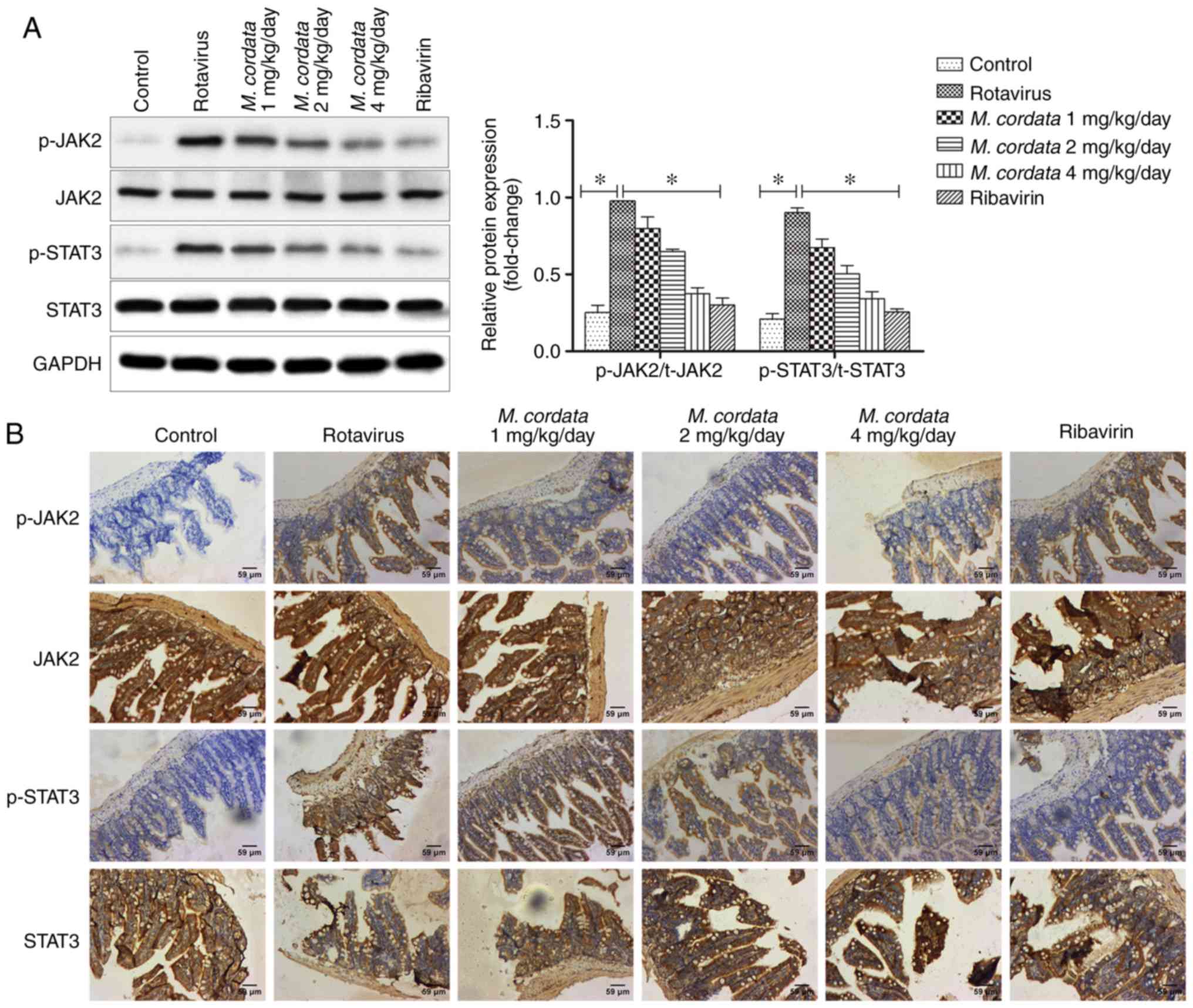
M. cordata exerts antiviral effects
via the JAK2/STAT3 pathway in vivo
To verify whether M. cordata regulated the
activity of the JAK2/STAT3 signaling pathway, the protein
expression levels of JAK2 and STAT3 were detected by western
blotting and immunohistochemical assays. As shown in Fig. 4A, rotavirus increased the levels of
p-JAK2 and p-STAT3 compared with the control, whereas the levels of
p-JAK2 and p-STAT3 markedly decreased following M. cordata
treatment compared with rotavirus-induced samples (P<0.05).
M. cordata was found to markedly decrease the
Rotavirus-induced phosphorylation of JAK2 and STAT3. On the other
hand, no significant differences existed between the M.
cordata and ribavirin-treated groups, indicating a comparable
therapeutic effect of M. cordata to ribavirin. Consistent
with the western blotting results, immunohistochemical analysis
also revealed that M. cordata markedly reversed the
increased expression levels of p-JAK2 and p-STAT3 induced by
rotavirus (Fig. 4B). Overall, the
results suggested that M. cordata may regulate antiviral and
inflammatory responses by inactivating the JAK2/STAT3 signaling
pathway.
Discussion
Rotavirus infection can result in severe diarrhea
with high morbidity and mortality (15). However, no specific drug against
rotavirus infection is currently available. Therefore, herbal
products can be potentially used as therapeutic drugs against
rotavirus-induced diarrhea. In the present study, M.
cordata, a traditional medicinal herb, showed an antiviral role
in mice with rotavirus-induced diarrhea.
The employment of rotavirus-infected animal models
has been used to better understand the pathogenesis of
rotavirus-associated diseases (16,17). The
physiopathology of rotavirus-induced diarrhea has been investigated
in different animal species including cows, pigs, sheep, rabbits
and mice (18). Murine models, which
are cost-effective, easy to maintain and fast-breeding, have been
frequently used to study rotavirus infection (19). In the present study, a
rotavirus-infected mouse model was established by inoculating SA11
virus passaged in MA104 cells into newborn mice. A previous study
showed that targets of 26 isoquinoline alkaloids were identified
from M. cordata therefore the exact active ingredient(s) in
this present study remain unclear (20). The targets in the interaction
network of M. cordata were implicated in various biological
progresses including cancer development, neurodegeneration,
inflammation and autoimmunity, parasitosis, injury and pain
(20,21). Among these targets,
dihydrochelerythrine (C6) was found to hit 23 targets, while MIF
was found to hit 15 alkaloids (C1-2, C11-16, C19-25), and appeared
to be the most promising target related to cancer (20). These findings further demonstrated
that M. cordata had pleiotropic antiviral, anticancer and
immunomodulatory effects. In the present study, M. cordata
suppressed the diarrhea caused by rotavirus infection. In addition,
M. cordata inhibited virus replication, since it reduced the
fecal shedding of rotavirus RNA and antigens in mice. The present
findings corroborate the results of a previous study, in which
M. cordata was found to alleviate rotavirus-induced diarrhea
(14).
Rotavirus infection is known to affect the secretion
of inflammatory cytokines (22).
M. cordata was demonstrated to have immunomodulatory
properties and anti-inflammatory effects (23). M. cordata was shown to
stimulate the anti-inflammatory enzyme heme oxygenase, which is
associated with the pathogenesis of autoimmune diseases (24,25). The
inflammatory response is characterized by the release of
inflammatory cytokines (26,27). MIF, a widely expressed pleiotropic
proinflammatory cytokine, is a part of the innate immune response
to inflammatory diseases (28,29). MIF
promotes the release of inflammatory indicators such as IL-6, IL-1
and TNF-α (30). In the present
study, rotavirus infection elevated MIF levels compared with the
control group, whereas M. cordata treatment significantly
reduced MIF levels compared with rotavirus-induced samples.
Subsequently, the levels of inflammatory cytokines including IL-8,
IFN-β, IFN-γ, TNF-α and IL-10 were determined. Similar to MIF,
rotavirus infection led to increased levels of the pro-inflammatory
cytokines IL-8, IFN-β, IFN-γ and TNF-α, as well as decreased levels
of the anti-inflammatory cytokine IL-10, whereas M. cordata
reversed the levels of inflammatory cytokines in the serum of mice
with rotavirus-induced diarrhea, which is in accordance with
previous reports (23,2).
Considering that rotavirus was documented to affect
histopathological changes of the small intestine (31,32), the
pathology of the small intestine was determined by histological
analysis. Rotavirus-induced lesions in the small intestine were
markedly restored following M. cordata or ribavirin
treatment. The TUNEL assay results further confirmed that rotavirus
promoted epithelial cell apoptosis, and M. cordata or
ribavirin treatment reversed the apoptosis induced by rotavirus
infection.
Traditional Chinese medicine may improve
inflammatory processes by regulating various protein kinases, such
as mitogen-activated protein kinase, PI3K and AKT (33). M. cordata exerts an important
role in the development of immunoinflammatory diseases and cancer
by inhibiting the PI3K/AKT/mTOR pathway (34,35). The
PI3K/AKT/mTOR axis is involved in the pathogenesis of rotavirus
infection (36). The JAK2/STAT3
pathway is an important intracellular signal transduction pathway;
phosphorylation of JAK2 results in STAT3 phosphorylation, which
consequently regulates the transcription of target genes encoding
inflammatory cytokines (37).
Previous studies confirmed that the JAK2/STAT3 signaling pathway is
not only associated with various important functions in normal and
malignant cells including differentiation, proliferation,
angiogenesis and apoptosis (38),
but is also confirmed as a pivotal inflammatory and immune mediator
during disease progression, including hyperglycemia-associated
inflammation, atherosclerosis and rheumatoid arthritis (39-41).
Aberrant activation of the JAK2/STAT3 pathway generally occurs
during the process of neuroinflammatory disease (42). In the present study, mice exhibited a
significant activation in JAK2/STAT3 phosphorylation following
rotavirus infection compared with the control group, while M.
cordata treatment reduced the levels of p-JAK2 and p-STAT3. The
immunohistochemistry results also demonstrated reduced expression
of p-JAK2 and p-STAT3 following M. cordata treatment. These
data suggested that the JAK2/STAT3 signaling pathway was inhibited
following M. cordata or ribavirin treatment. These findings
are in accordance with previous research, which reported that the
JAK2/STAT3 pathway was involved in the inflammatory response by
inducing chemokine release (43-45).
In conclusion, M. cordata was demonstrated to
exert antiviral and anti-inflammatory effects, which protected
against rotavirus-induced diarrhea. Furthermore, the present
findings showed that M. cordata repressed rotavirus-induced
diarrhea by suppressing the activation of the JAK2/STAT3 signaling
pathway. These results suggested that M. cordata could be
used as a therapeutic agent for rotavirus-induced diarrhea.
Acknowledgements
Not applicable.
Funding
This work was supported by the Jiangsu Province
Agricultural Three New Projects [grant no. SXGC (2017)249], Taizhou
Agricultural Technology Supporting Project (grant no. TN201711) and
Jiangsu Agri-animal Husbandry Vocational College project (grant no.
NSF201901).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CJ and CW conceived and planned the experiments of
this study. CJ performed the experiments. HY and XC performed and
analyzed the ELISA and reverse transcription-quantitative PCR
experiments. SQ, BZ and LJ performed the hematoxylin and eosin,
TUNEL, immunohistochemistry and western blot analyses. CW
supervised the project. CJ and HY wrote the manuscript. All authors
analyzed and interpreted the data. All authors contributed to and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Animal procedures were in line with the National
Institutes of Health Guidelines for the Care and Use of Laboratory
Animals and were approved by the Animal Committee of the Jiangsu
Agri-animal Husbandry Vocational College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boshuizen JA, Reimerink JH, Korteland-van
Male AM, van Ham VJ, Koopmans MP, Büller HA, Dekker J and Einerhand
AW: Changes in small intestinal homeostasis, morphology, and gene
expression during rotavirus infection of infant mice. J Virol.
77:13005–13016. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tate JE, Burton AH, Boschi-Pinto C, Steele
AD, Duque J and Parashar UD: WHO-coordinated Global Rotavirus
Surveillance Network: 2008 estimate of worldwide
rotavirus-associated mortality in children younger than 5 years
before the introduction of universal rotavirus vaccination
programs: A systematic review and meta-analysis. Lancet Infect Dis.
12:136–141. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pesavento JB, Crawford SE, Estes MK and
Prasad BV: Rotavirus proteins: Structure and assembly. Curr Top
Microbiol Immunol. 309:189–219. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kawahara T, Tomono T, Hamauzu Y, Tanaka K
and Yasui H: Inhibitory effect of a hot-water extract of leaves of
Japanese big-leaf magnolia (magnolia obovata) on rotavirus-induced
diarrhea in mouse pups. Evid Based Complement Alternat Med.
2014(365831)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Anderson EJ: Rotavirus vaccines: Viral
shedding and risk of transmission. Lancet Infect Dis. 8:642–649.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lepage P and Vergison A: Prevention of
childhood rotavirus disease through the use of Rotarix and RotaTeq
vaccines. Expert Opin Biol Ther. 7:1881–1892. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu M, Lin YL, Chen XR, Liao CC and Poo
WK: In vitro assessment of Macleaya cordata crude extract
bioactivity and anticancer properties in normal and cancerous human
lung cells. Exp Toxicol Pathol. 65:775–787. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin L, Liu YC, Huang JL, Liu XB, Qing ZX,
Zeng JG and Liu ZY: Medicinal plants of the genus Macleaya
(Macleaya cordata, Macleaya microcarpa): A review of their
phytochemistry, pharmacology, and toxicology. Phytother Res.
32:19–48. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Ke W, Lin X, Yu Z, Sun Q and Zhang Q:
Molluscicidal activity and physiological toxicity of Macleaya
cordata alkaloids components on snail oncomelania hupensis. Pestic
Biochem Physiol. 143:111–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arnold M, Patton JT and McDonald SM:
Culturing, storage, and quantification of rotaviruses. Curr Protoc
Microbiol. 15(Unit 15C 3)2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tian Z, Shen Z, He H, Zhang J, Li J and Wu
Y: Antiviral effects of cyclosporin a in neonatal mice with
rotavirus-induced diarrhea. J Pediatr Gastroenterol Nutr. 60:11–17.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen Z, He H, Wu Y and Li J: Cyclosporin a
inhibits rotavirus replication and restores interferon-beta
signaling pathway in vitro and in vivo. PLoS One.
8(e71815)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu G, Guan G, Fang J, Martínez Y, Chen S,
Bin P, Duraipandiyan V, Gong T, Tossou MC, Al-Dhabi NA and Yin Y:
Macleaya cordata extract decreased diarrhea score and enhanced
intestinal barrier function in growing piglets. Biomed Res Int.
2016(1069585)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dung TT, Phat VV, Nga TV, My PV, Duy PT,
Campbell JI, Thuy CT, Hoang NV, Van Minh P, Le Phuc H, et al: The
validation and utility of a quantitative one-step multiplex RT
real-time PCR targeting rotavirus A and norovirus. J Virol Methods.
187:138–143. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alfajaro MM, Kim HJ, Park JG, Ryu EH, Kim
JY, Jeong YJ, Kim DS, Hosmillo M, Son KY, Lee JH, et al:
Anti-rotaviral effects of Glycyrrhiza uralensis extract in piglets
with rotavirus diarrhea. Virol J. 9(310)2012.
|
|
17
|
Ciarlet M, Conner ME, Finegold MJ and
Estes MK: Group A rotavirus infection and age-dependent diarrheal
disease in rats: A new animal model to study the pathophysiology of
rotavirus infection. J Virol. 76:41–57. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guerin-Danan C, Meslin JC, Lambre F,
Charpilienne A, Serezat M, Bouley C, Cohen J and Andrieux C:
Development of a heterologous model in germfree suckling rats for
studies of rotavirus diarrhea. J Virol. 72:9298–9302.
1998.PubMed/NCBI
|
|
19
|
Ward RL: Possible mechanisms of protection
elicited by candidate rotavirus vaccines as determined with the
adult mouse model. Viral Immunol. 16:17–24. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lei Q, Liu H, Peng Y and Xiao P: In silico
target fishing and pharmacological profiling for the isoquinoline
alkaloids of Macleaya cordata (Bo Luo Hui). Chin Med.
10(37)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Freitas K, Carroll FI and Damaj MI: The
antinociceptive effects of nicotinic receptors α7-positive
allosteric modulators in murine acute and tonic pain models. J
Pharmacol Exp Ther. 344:264–275. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jiang B, Snipes-Magaldi L, Dennehy P,
Keyserling H, Holman RC, Bresee J, Gentsch J and Glass RI:
Cytokines as mediators for or effectors against rotavirus disease
in children. Clin Diagn Lab Immunol. 10:995–1001. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khadem A, Soler L, Everaert N and Niewold
TA: Growth promotion in broilers by both oxytetracycline and
Macleaya cordata extract is based on their anti-inflammatory
properties. Br J Nutr. 112:1110–1118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vrba J, Orolinova E and Ulrichova J:
Induction of heme oxygenase-1 by Macleaya cordata extract and its
constituent sanguinarine in RAW264. .7 cells. Fitoterapia.
83:329–335. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fagone P, Patti F, Mangano K, Mammana S,
Coco M, Touil-Boukoffa C, Chikovani T, Di Marco R and Nicoletti F:
Heme oxygenase-1 expression in peripheral blood mononuclear cells
correlates with disease activity in multiple sclerosis. J
Neuroimmunol. 261:82–86. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ronco C, Ronco F and Mccullough PA: A call
to action to develop integrated curricula in cardiorenal medicine.
Rev Cardiovasc Med. 18:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jin J, Liu Y, Huang L and Tan H: Advances
in epigenetic regulation of vascular aging. Rev Cardiovasc Med.
20:19–25. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yilmaz E, Coskun EI, Gul M, Sahin N,
Tuncay G and Simsek Y: Nuclear factor-kappa β pathway and
endometrial cancer: A pilot study. Eur J Gynaecol Oncol.
38:536–540. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tian JS, Zhai QJ, Zhao Y, Chen R and Zhao
LD: 2-(2-benzofuranyl)-2-imidazoline (2-BFI) improved the
impairments in AD rat models by inhibiting oxidative stress,
inflammation and apoptosis. J Integr Neurosci. 16:385–400.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fagone P, Mazzon E, Cavalli E, Bramanti A,
Petralia MC, Mangano K, Al-Abed Y, Bramati P and Nicoletti F:
Contribution of the macrophage migration inhibitory factor
superfamily of cytokines in the pathogenesis of preclinical and
human multiple sclerosis: In silico and in vivo evidences. J
Neuroimmunol. 322:46–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Presti M, Mazzon E, Basile MS, Petralia
MC, Bramanti A, Colletti G, Bramanti P, Nicoletti F and Fagone P:
Overexpression of macrophage migration inhibitory factor and
functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in
glioblastoma. Oncol Lett. 16:2881–2886. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lang T, Lee JPW, Elgass K, Pinar AA, Tate
MD, Aitken EH, Fan H, Creed SJ, Deen NS, Traore DAK, et al:
Macrophage migration inhibitory factor is required for NLRP3
inflammasome activation. Nat Commun. 9(2223)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gandhi GR, Santos VS, Denadai M, da Silva
Calisto VK, de Souza Siqueira Quintans J, de Oliveira E Silva AM,
de Souza Araújo AA, Narain N, Cuevas LE, Júnior LJQ and Gurgel RQ:
Cytokines in the management of rotavirus infection: A systematic
review of in vivo studies. Cytokine. 96:152–160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Feng N, Kim B, Fenaux M, Nguyen H, Vo P,
Omary MB and Greenberg HB: Role of interferon in homologous and
heterologous rotavirus infection in the intestines and
extraintestinal organs of suckling mice. J Virol. 82:7578–7590.
2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu M, Zhao G, Cao S, Zhang Y, Li X and
Lin X: Development of certain protein kinase inhibitors with the
components from traditional chinese medicine. Front Pharmacol.
7(523)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mammana S, Bramanti P, Mazzon E, Cavalli
E, Basile MS, Fagone P, Petralia MC, McCubrey JA, Nicoletti F and
Mangano K: Preclinical evaluation of the PI3K/Akt/mTOR pathway in
animal models of multiple sclerosis. Oncotarget. 9:8263–8277.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Horie R, Nakamura O, Yamagami Y, Mori M,
Nishimura H, Fukuoka N and Yamamoto T: Apoptosis and antitumor
effects induced by the combination of an mTOR inhibitor and an
autophagy inhibitor in human osteosarcoma MG63 cells. Int J Oncol.
48:37–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yin Y, Dang W, Zhou X, Xu L, Wang W, Cao
W, Chen S, Su J, Cai X, Xiao S, et al: PI3K-Akt-mTOR axis sustains
rotavirus infection via the 4E-BP1 mediated autophagy pathway and
represents an antiviral target. Virulence. 9:83–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Raible DJ, Frey LC and Brooks-Kayal AR:
Effects of JAK2-STAT3 signaling after cerebral insults. JAKSTAT.
3(e29510)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sansone P and Bromberg J: Targeting the
interleukin6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cui J, Zhang F, Cao W, Wang Y, Liu J, Liu
X, Chen T, Li L, Tian J and Yu B: Erythropoietin alleviates
hyperglycaemia-associated inflammation by regulating macrophage
polarization via the JAK2/STAT3 signalling pathway. Mol Immunol.
101:221–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang X, Jia J, Yu Z, Duanmu Z, He H, Chen
S and Qu C: Inhibition of JAK2/STAT3/SOCS3 signaling attenuates
atherosclerosis in rabbit. BMC Cardiovasc Disord.
20(133)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li CH, Xu LL, Zhao JX, Sun L, Yao ZQ, Deng
XL, Liu R, Yang L, Xing R and Liu XY: CXCL16 upregulates RANKL
expression in rheumatoid arthritis synovial fibroblasts through the
JAK2/STAT3 and p38/MAPK signaling pathway. Inflamm Res. 65:193–202.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Y, Holdbrooks AT, De Sarno P, Rowse
AL, Yanagisawa LL, McFarland BC, Harrington LE, Raman C, Sabbaj S,
Benveniste EN and Qin H: Therapeutic efficacy of suppressing the
Jak/STAT pathway in multiple models of experimental autoimmune
encephalomyelitis. J Immunol. 192:59–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Villarino AV, Kanno Y, Ferdinand JR and
O'Shea JJ: Mechanisms of Jak/STAT signaling in immunity and
disease. J Immunol. 194:21–27. 2015.PubMed/NCBI View Article : Google Scholar
|