Introduction
The prevalence of obesity has increased; according
to the World Health Organization, >1.9 billion adults were
overweight in 2014, and of these, >600 million were obese
(1). Increased energy intake or
decreased physical activity are the two most obvious contributing
factors. The type of diet has an important role in excessive
caloric intake and excessive body fat accumulation. Recently, the
gut microbiota has been implicated in the etiology of obesity
(1). The diet and intestinal
microbiota have crucial effects on health, since they are involved
in nutritional supply, immunology and tumorigenesis (2).
Intestinal metabolites are produced by diet
catabolism and bacterial metabolism (3,4). Food is
processed through physical and chemical breakdown in the digestive
tract, producing nutrients to be absorbed. Most nutrients cannot be
absorbed without the presence of the intestinal microbiota. The
intestinal microbiota provides an indispensable array of enzymes
that degrade complex dietary substrates. Different metabolites have
varying roles in disease development. According to current studies,
microbial metabolism of dietary carbohydrates results mainly in the
formation of short-chain fatty acids (SCFAs) and gases, while the
production of acetate, propionate and butyrate take part in
regulating the intestinal barrier, bone mass and central nervous
system autoimmunity (5-7).
The consumption of different diets not only provides
substrates for the body's metabolism, but also affects the
structure and metabolism of the microbial community (2,3). The
post-weaning role of the dietary composition in shaping the adult
microbiome composition is of particular importance. The amount,
type and balance of the major dietary macronutrients
(carbohydrates, proteins and fats) have a considerable effect on
the large intestinal microbiota (3,8). Diet is
a major factor that shapes the composition and activity of enteric
dysbacteriosis (9,10). Effective manipulation of the gut
microbiota by altering dietary patterns has been proved to have the
potential to prevent metabolic disorders by numerous studies
(8). Unfortunately, little is known
about how diet and intestinal bacteria affect cellular health.
Owing to the complexity of the microbiota,
interactions of the intestinal bacteria with the host are difficult
to study. In recent years, high-throughput sequencing technology
has been used to study the intestinal flora, and the components of
the fecal flora have been almost entirely elucidated (11,12).
However, this technology has limitations in testing for bacterial
genera and species, and the same species of bacteria have numerous
different effects at the subspecies levels. Since intestinal
bacteria cannot penetrate the gastrointestinal tract, the present
study hypothesizes that the possible molecular mechanisms
associated with obesity rely on intestinal metabolites.
In the present study, the levels of intestinal
metabolites and expression of appetite control factors were
assessed in Sprague Dawley (SD) rats fed different diets. The
present study aimed to explore the role of intestinal metabolites
in obesity development and prevention from a novel perspective.
Materials and methods
Animals and diets. The present study was
approved and monitored by the Ethics Committee of the Second
Xiangya Hospital of Central South University (Changsha, China),
where the animal experiment was performed, and the protocol was in
accordance with the guiding principles covered in the Guide for the
Use and Care of Experimental Animals. All surgical procedures were
performed under chloral hydrate anesthesia and all efforts were
made to minimize the suffering of the experimental animals. SD rats
of 5-week-old (Hunan SJA Laboratory Animal Co., Ltd.) weighing
62.1-102.3 g with the same genetic background were used for all the
experiments. After a 1-week acclimation, all 35 male specific
pathogen-free SD rats were weaned and immediately randomly assigned
to the FAT, SUG, FIB, PRO and control diet (CON) groups (seven rats
in each). The CON group was provided ad libitum access to a
normal chow diet, while the FAT, SUG, FIB and PRO groups were fed a
high-fat, high-sugar, high-fibre and high-protein diet,
respectively, for 4 weeks. Protein, fat, sugar and fibre are
responsible for 60% of the calories in the PRO, FAT, SUG and FIB
diet group, respectively. All of the diets are listed in Table I. Water and food were provided ad
libitum and the body weights were recorded weekly. At the end
of the feeding protocol, body composition analysis and oral glucose
tolerance test (OGTT) were performed as described below. After 4
weeks, all the rats were sacrificed, blood samples were collected
and the contents of the cecum and stool samples, as well as fat,
gastric, intestinal, liver and brain tissues were harvested.
 | Table IComposition (g/kg) and energy density
of the five diets. |
Table I
Composition (g/kg) and energy density
of the five diets.
| Component | Normal control | High-protein | High-fibre | High-sugar | High-fat |
|---|
| Casein | 200 | 619.9 | 181.8 | 200 | 271.9 |
| Corn starch | 547.0 | 122.6 | 377.2 | 75.5 | 133.2 |
| Dextrin | 0 | 0 | 120.0 | 0 | 0 |
| Sucrose | 100 | 99.3 | 90.9 | 571 | 135.9 |
| Soy oil | 70 | 69.5 | 63.6 | 70 | 345.2 |
| Fibre | 50 | 49.6 | 90.9 | 50 | 68.0 |
| Pectin | 0 | 0 | 45.5 | 0 | 0 |
| Minerals | 27.3 | 27.1 | 24.8 | 27.3 | 37.1 |
| Vitamins | 0.26 | 0.26 | 0.2 | 0.26 | 0.35 |
| L-cystine | 3 | 9.3 | 2.7 | 3 | 4.1 |
| Choline
chloride | 2.5 | 2.5 | 2.3 | 2.5 | 4.2 |
| TBHQ | 0.014 | 0.014 | 0.013 | 0.014 | 0.07 |
| Energy density
(kcal/kg) | 3,810 | 3,810 | 3,528 | 3,810 | 5,179 |
Measurements of body length and
weight, and Lee's index
The animals were weighed prior to feeding weekly,
and the weight change trend of the five groups was monitored.
Furthermore, the body length and the final weight of the rats were
measured under anesthesia, prior to euthanasia. Lee's index was
calculated using the following formula: Lee's index =.
Measurements of body fat and fat
index
The fat tissue (omental-, perirenal- and
peritesticular adipose fat) was harvested and measured, and the fat
index was then calculated as follows: Fat index (%)=[fat content
(g)/body weight (g)] x100.
Fasting blood glucose (FBG) and
OGTT
After a 12-h fast, blood was collected from the tail
vein and FBG was measured using a glucose meter (LifeScan).
Subsequently, glucose (2 g/kg rat) was administered
intragastrically, and blood glucose was determined again at 30, 60,
90 and 120 min post-administration via tail.
Biochemical analysis
At the end of the experiment, all of the animals
were anesthetized by intraperitoneal injection of 10% chloral
hydrate (400 mg/kg) after a 12-h overnight fast. Blood samples
(~1.0 ml) were collected via cardiac puncture and centrifuged (4˚C;
3,000 r/min; 15 min) to obtain the serum. The concentrations of
serum triglyceride (TG), high-density lipoprotein cholesterol
(HDL-C) and low-density lipoprotein cholesterol (LDL-C) were
analyzed using an automatic biochemical analyzer provided by The
Second Xiangya Hospital of Central South University (Changsha,
China). The levels of serum leptin and ghrelin were assayed using
the rat leptin ELISA kit (cat. no. ELR-Leptin-001; RayBiotech) and
the rat ghrelin enzyme immunoassay kit (cat. no. EIA-GHR-1;
RayBiotech), respectively.
Reverse transcription-quantitative
(RT-q) PCR
Samples of fat, gastric, intestinal, liver and brain
tissues were collected and stored in dry ice (-80˚C) for further
evaluation. The RNA of the tissues were extracted using TRI reagent
(Takara Bio. Inc.). RT was performed using a PrimeScriptÔ RT
reagent kit with gDNAEraser (Takara Bio. Inc.) using the
appropriate primers. Levels of the genes of interest were
determined using qPCR with SYBR Green master mix (cat. no. RR820A;
Takara Bio. Inc) using Step One Plus equipment (Bio-Rad CFX96T M
real-time system, C1000 touch thermal cycle; Bio-Rad Laboratories).
The thermocycling conditions were as follows: 95˚C for 30 sec; PCR
(50 cycles), denaturation 5 sec at 95˚C, annealing for 30 sec at
60˚C; denaturation at 95˚C for 15 sec, annealing at 6˚C for 1 h,
heating at 95˚C for 10 sec. The gene expression was normalized to
that of endogenous expression of the genes of interest and
respective primers are listed in Table
II. The 2-∆∆Cq method was used for quantification of
data (13,14).
 | Table IISequences of primers used for
PCR. |
Table II
Sequences of primers used for
PCR.
| Primer | Forward
(5'-3') | Bp length | Reverse
(3'-5') | Bp length |
|---|
| Leptin |
ACCTGGAGAACCTGCGAGAC | 20 |
TAGAGGAGTAGGAGAAACGGACA | 23 |
| Leptin
receptor |
ACCCAGCACAATCCAATCACTA | 22 |
TTGAGCTCTGATGTAGGACGAATAG | 25 |
| Ghrelin |
GCCACTCTGGGTGTTCTTTTGT | 21 | GCAGATGA
GATGGGTCTTTATTG | 23 |
| Ghrelin
receptor |
GCTGGTCATCCTTGTCATCTG | 21 |
TTCACTGTCTGCTTGTGGTTCT | 22 |
| β-actin |
GGAGATTACTGCCCTGGCTCCTA | 23 |
GACTCATCGTACTCCTGCTTGCTG | 24 |
Pre-column derivatization
reverse-phase high-performance liquid chromatography (RP-HPLC)
The HPLC system used was an LC-20A HPLC system
(Shimadzu Corp.) equipped with a Shimadzu LC-20AD dual pump,
Rf-10ADvp fluorescence detector (Shimadzu Corp.), manual injection
port (20 µl) and LabSolution/LCSolution Lite chromatography
Chemstation (Shimadzu Corp.). The separation was performed using a
Thermo Hypersil C18 column (length, 150 mm; internal diameter, 4.6
mm; particle size, 4 µm; Shimadzu Corporation) by using a linear
gradient elution mode at a flow rate of 1 ml/min. All reagents used
in the present study were of analytical grade unless otherwise
specified.
Determination of SCFAs
In the present study, a pre-column derivatization
method was used to determine the SCFAs using RP-HPLC. The samples
were first acid-extracted by adding 1 mg of the feces or ileocecal
content samples to 1 ml 12% sulfuric acid or 0.5 ml of blood sample
to 0.5 ml 12% sulfuric acid and vortexing (2 min), followed by
ultrasonic processing (35 kHz; 10 min). Subsequently, the mixture
was added to 2 ml methyl tertiary butyl ether (Sinopharm Chemical
Reagent Co., Ltd), and after extraction and centrifugation (8,000 x
g; 4˚C; 5 min), the upper organic phase was collected, mixed with
20 µl 50 mmol/l potassium hydroxide-methanol solution, and then
dried under a nitrogen stream. Standard solutions of the SCFAs
(chromatographic grade; Supelco) formic, acetic, propionic,
butyric, isobutyric, malic, valeric, lactic and succinic acid were
accurately diluted to obtain a series of mixed reference solutions
of various concentrations using acetonitrile (chromatographic
grade; TEDIA Co.). The derivative reagent was
2,4'-dibromoacetophenone (chromatographic grade; Sigma-Aldrich;
Merck KGaA) and 18-crown-6 (chromatographic grade; Sigma-Aldrich;
Merck KGaA) was used as a catalyst for the derivatization reaction.
A Thermo Hypersil C18 column (length, 150 mm; internal diameter,
4.6 mm; particle size, 4 µm) was used at 35˚C, and the mobile phase
consisted of a methanol-water (75:25, v/v) with a flow rate of 1.0
ml/min. The detection wavelength was 255 nm.
Determination of amino acids
In the present study, a pre-column derivatization
method was used to determine the amino acids using RP-HPLC. The
feces or ileocecal samples were first acid-extracted by adding 1 mg
to 1 ml 0.1 mol/l hydrochloric acid or 100 µl of the blood samples
to 100 µl anhydrous ethanol, followed by centrifugation for 5 min
(4˚C; 8,000 x g). Standard solutions of the following amino acids
(thin layer chromatography grade; Sigma-Aldrich; Merck KGaA),
aspartic acid, glutamic acid, histidine, glycine, serine,
threonine, arginine, alanine, tyrosine, methionine, valine,
phenylalanine, isoleucine, leucine and lysine were confected into a
1.00 mmol/l solution by adding 0.1 mol/l hydrochloric acid
accurately, and the stock was then diluted to obtain a series of
mixed reference substance solutions of varying concentrations using
double-distilled water. The derivative reagent was a mixture of 10
mg o-phthalaldehyde (chromatographic grade; Sigma-Aldrich; Merck
KGaA), 0.6 ml methyl alcohol (chromatographic grade, Bcl
International Trading Co., Ltd.), 2.4 ml 2% boric acid and 30 µl
mercaptoethanol (Sigma-Aldrich; Merck KGaA). A Thermo Hypersil C18
column (150x4.6 mm, 4 µm) was used at 35˚C with a mobile phase
consisting of solution A (a mixture of 160 ml methyl alcohol, 900
ml sodium acetate and 10 ml tetrahydrofuran) and solution B (a
mixture of 800 ml methyl alcohol, 200 ml sodium acetate and 10 ml
tetrahydrofuran) run at a flow rate of 1.0 ml/min. The emission and
excitation wavelengths for detection were 430 and 338 nm,
respectively.
Statistical analysis
All values are expressed as the mean ± standard
error and the statistical analyses were performed using SPSS
version 22.0 (IBM Corp.). Values were compared among the five
groups using analysis of variance (ANOVA), and a post-hoc
least-significant differences test was performed when ANOVA
indicated significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Test diets have a significant role in
initiating metabolic disorders
In the present study, the SD rats were provided with
different diets ad libitum for 4 weeks. The abdominal
anatomy of representative animals from the five groups is presented
in Fig. 1A-E. The FAT group had more
abdominal fat accumulation and fatty livers (the liver color turned
yellow and had a dull appearance). The animals from the PRO group
had a lean body. The body weight, Lee's index, fatty content,
lipids and glucose index were also analyzed. As presented in
Fig. 1F, the body weight of each
group exhibited a linear increase over time. At the beginning of
the experiment, no significant difference in body weight was
present between the groups. At the end of the experiment, the FAT
group exhibited a significantly greater weight increase and fat
accumulation, and developed obesity when compared to the CON group.
However, the PRO group had a significantly lower weight, whereas
the high-fibre and high-glucose diets did not lead to any
significant changes compared with the control diet. There was a
positive association among the trends regarding Lee's index,
visceral fat index and body weight. As presented in Fig. 1G and H, respectively, Lee's index and the
visceral fat index of the PRO group was significantly lower than
that of the CON group. However, the values in the FAT group were
significantly higher than those in the CON group. Fig. 1I presents the changes in glucose
levels over time. The high-fat diet elevated the FBG levels, and
delayed the glucose level decrease in the OGTT, the glucose level
at 2 h post-glucose exposure was >11 mmol/l. Compared with that
in the CON group, the glucose level was also elevated in the FIB
and PRO groups. Furthermore, various lipid indexes (TG, HDL-C and
LDL-C) were compared among the five groups, indicating that the TG
levels were significantly decreased in the PRO group. However, the
FAT group exhibited a significant elevation compared with the other
groups. Furthermore, the HDL-C level was significantly decreased in
the FAT group and the LDL-C level was significantly elevated in the
SUG group (Table III). Therefore,
the high-fat diet led to impaired glucose tolerance and accumulated
fat, while the high-protein diet exhibited weight loss compared
with the CON group.
 | Figure 1Abdominal anatomy and nutritional
indicators in rats. (A-E) Features of abdominal anatomy of the five
groups are listed as follows: (A) CON, (B) PRO, (C) FIB, (D) SUG
and (E) FAT group. (F) Body weight, (G) Lee's index, (H) visceral
fat index and (I) oral glucose tolerance. *P<0.05 FAT
group vs. CON group. #P<0.05 PRO group vs. CON group.
Groups: CON, normal control diet; PRO, high-protein diet; FIB,
high-fibre diet; SUG, high-sugar diet; FAT, high-fat diet. |
 | Table IIIComparison of serum lipid levels in
the different experimental groups. |
Table III
Comparison of serum lipid levels in
the different experimental groups.
| Group | TG (mmol/l) | HDL-C (mmol/l) | LDL-C (mmol/l) |
|---|
| CON | 0.818±0.028 | 1.268±0.017 | 0.185±0.024 |
| PRO |
0.353±0.120a | 1.230±0.476 | 0.228±0.048 |
| FIB | 0.738±0.152 | 1.295±0.031 | 0.190±0.334 |
| SUG | 0.930±0.036 | 1.220±0.036 |
0.253±0.032b |
| FAT |
1.420±0.364c |
1.098±0.045d | 0.220±0.042 |
Potential mechanisms of obesity
assessed using metabolomics
SCFAs and amino acids are important nutrients taking
part in energy metabolism and regulation. In the present study, the
contents of SCFAs and amino acid in the feces, ileocecal content,
and blood samples were analyzed using HPLC analysis and
compared.
SCFAs
As presented in Fig.
2A, the total content of SCFAs in the feces, ileocecal content
and blood samples differed among the five groups. In the fecal
samples, the contents of total SCFAs were successively reduced in
the FAT, FIB, SUG, CON and PRO group, and the FAT group exhibited a
significant decrease compared with the other groups. In addition,
there was a significant difference between the FIB and PRO groups.
No difference between the groups was identified in the ileocecal
content. In the blood samples, the content in the FAT group samples
was significantly decreased compared with that in the all other
groups, the PRO and the SUG group exhibited a significant increase
when compared with the CON, FIB and FAT groups, while there was no
significant differences exhibited between the PRO and SUG group.
Acetic acid, propionic acid and butyric acid are popular SCFAs, and
to eliminate the influence of sample wetness, the percentage of the
three SCFAs among the total SCFAs in each sample was analyzed
(Fig. 2B). The feces samples of the
FAT group had significantly elevated levels than the other groups
except the FIB group. The blood samples of the FAT and FIB group
had significantly elevated levels than the PRO group and SUG group.
The propionic acid/butyric acid ratio of the feces and blood
samples did not differ among the five groups (Fig. 2C). The ileocecal content of the PRO
and FIB groups exhibited significantly decreased ratios of
propionic acid/butyric than the other groups, but there was no
differences exhibited between the PRO and FIB group. Comparing the
acetic acid/butyric acid ratio in the feces samples of the various
groups, no significant differences were identified. In the
ileocecal content, the acetic acid/butyric acid ratio of the SUG
group was significantly higher than that of PRO and FIB group, the
acetic acid/butyric acid ratio of the FAT group was significantly
higher compared with the FIB group. In addition, the values of
acetic acid/butyric acid ratio in the FAT group in blood sample
were significantly decreased compared with those in the PRO group
(Fig. 2D). As presented in Fig. 2E, the acetic acid/propionic acid
ratio in the FAT group was significantly higher in the fecal
samples than the FIB and SUG group, but was lower in the blood
samples compared with that of the PRO and SUG group. The ileocecal
acetic acid/propionic acid ratio exhibited no differences between
the groups.
 | Figure 2Comparison of the content of SCFAs
and AAs among different samples of the five groups. (A) Total SCFA
concentration, (B) content of acetic, propionic and butyric acid,
(C) acetic acid/propionic acid ratio, (D) acetic acid/butyric acid
ratio and (E) propionic acid/butyric acid ratio in different
samples. (F-H) Total content of AAs in (F) feces, (G) ileocecal
content and (H) blood samples, respectively; (I-K) Content of
branched chain amino acids in (I) feces, (J) ileocecal content and
(K) blood samples, respectively. *P<0.05 vs. two
assigned groups, #P<0.05 vs. one assigned group
compared with the other groups. Groups: CON, normal control diet;
PRO, high-protein diet; FIB, high-fibre diet; SUG, high-sugar diet;
FAT, high-fat diet. SCFA, short-chain fatty acids; AA, amino
acids. |
Amino acids
As presented in Fig.
2F, the total content of amino acids in the feces samples
differed among the five groups in the following order:
FIB<FAT<CON<SUG<PRO, and the levels in the FIB group
were significantly lower than those in the other groups. The PRO
group exhibited significantly higher levels than those in the other
groups in the feces and ileocecal contents (Fig. 2F and G). The blood samples of the PRO group had
significantly lower levels than those of the other groups (Fig. 2H). The total branched-chain amino
acid levels, including leucine, isoleucine and valine, in the feces
samples of the PRO group were significantly higher than those in
CON, FIB and FAT groups (Fig. 2I).
Furthermore, there was no significant difference among the five
groups in the amino acid levels in the ileocecal content (Fig. 2J). However, the levels of the PRO
group were significantly decreased than FIB, CON and FAT group in
the blood samples (Fig. 2K).
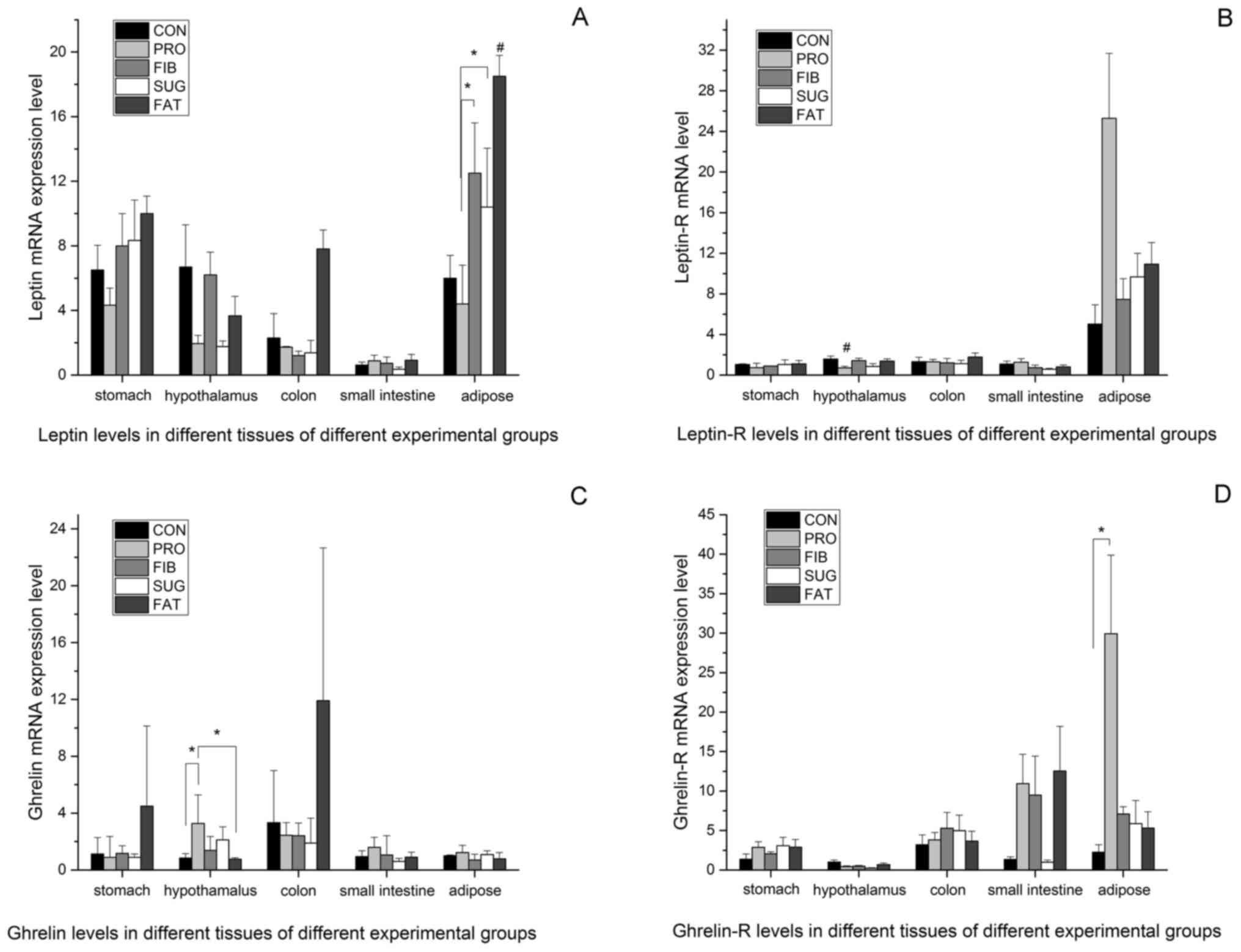
Leptin and ghrelin production in
different tissues
The above results demonstrate the differences in
intestinal metabolic products between the various diet groups, but
the mechanisms by which the diets affect food control and energy
metabolism have remained elusive. Therefore, the expression levels
of the appetite control factors leptin and ghrelin were examined in
different tissues from the rats. Fig.
3A presents the expression levels of leptin in the adipose,
stomach, small intestine, colon and hypothalamus tissues determined
using RT-qPCR. The mRNA expression levels of leptin in the adipose
tissue were higher than those in the other tissues, and were
consistent with the body weight and amount of fat mass in the
various groups (FAT>FIB>SUG>CON>PRO). The FAT group had
the highest level of leptin than the all other groups). The PRO
group had the lowest levels, which differed significantly from
those in the FIB and SUG group. In the stomach, small intestine,
colon and hypothalamus tissues, the leptin mRNA expression of the
FAT group was relatively high, but no significant differences were
observed between the groups. Fig. 3B
presents the leptin receptor mRNA expression levels in different
tissues of the five groups. In the adipose tissue, the expression
level was higher than that in the other tissues assessed, and the
PRO group had the highest level among the five groups; however, no
statistically significant difference was observed between the
groups. In the hypothalamus tissue, the PRO group had the lowest
level of leptin receptor, and there was a significant difference
compared with the all other groups. There were no differences in
leptin receptor expression among the groups in the stomach, small
intestine and colon tissues.
Fig. 3C and D present the mRNA expression levels of
ghrelin and ghrelin receptor in all of the tissues analyzed. The
level in the hypothalamus tissue was significantly higher in the
PRO group than it was in CON and FAT group; The ghrelin receptor
mRNA expression level of the PRO group was higher compared with the
CON group. The levels of ghrelin receptor in the other tissues did
not differ between the groups.
Discussion
The present study aimed to investigate the changes
in expression levels of metabolite and appetite control factors
caused by different diets. The present study reported that a 4-week
unrestricted diet of different compositions affected the
obesity-associated metabolic index, indicating that the dietary
composition is highly linked to the occurrence of obesity. All of
the rats in the present study were fed ad libitum and the
actual food intake per rat was not quantified. In order to avoid
the influence of diet, the experimental diets were designed based
on the percentage of energy of nutrients; for instance, the
percentage of energy of the casein content in all diets except for
the high-protein diet was 19.2%. The casein content was actually
equivalent between all diets. Furthermore, the micronutrient
content was almost equivalent between the diets. All diets of the
present study had an equivalent energy density of ~3,810 kcal/kg,
except for the high-fat diet (5,179 kcal/kg). This was due to the
equal weight of fat having higher calories. Consistent with
previous studies, the present study demonstrated that the SD rats
fed the high-fat diet successfully developed obesity with
disordered glucolipid metabolism. The high-fat diet has been widely
used to establish obesity models and has been recognized by
domestic and foreign studies (15-17).
In a previous study by our group rats on a high-fat diet developed
fatty liver after 12 weeks (18). At
present, there is limited evidence regarding the pathophysiological
effects of a high-protein diet. Numerous studies have proven that a
high-protein diet is beneficial for weight loss and improving
glucolipid metabolism (19). Wang
et al (20) reported that
male rats switched to a high-protein diet for 6 weeks after high
fat and sugar for 12 weeks had reduced body fat, as well as
restored glucose homeostasis and cholecystokinin sensitivity. One
study on Mexican adults with metabolic syndrome on hypocaloric
diets indicated that a diet with elevated protein (1.34 g/kg body
weight) is associated with significant weight loss compared with a
standard protein diet (0.8 g/kg), but there were no differences
between the groups in terms of glucose, insulin and triglycerides
(21). Furthermore, according to
certain clinicians, a high-protein diet may overload the body and
cause damage to the organs directly involved in protein metabolism
and excretion (22). The present
study indicated that the SD rats on a high-protein diet exhibited
significant weight loss, but also had a markedly disordered
glucolipid metabolism. The different effects of a high-protein diet
on glucolipid metabolism may depend on the protein type and content
in the diet (the percentage of energy derived from protein was 60%
in the present study). The nutritional importance of different
types of carbohydrate and their distinct physiological effects on
health have remained to be fully elucidated. The role of a
high-carbohydrate diet in obesity development has been debated. In
the present study, the SD rats on a high-sugar diet or a high-fibre
diet exhibited no significant weight increases or loss compared
with those in the CON group. The high-sugar and high-fibre diets
led to impaired glucose tolerance, while the high-sugar diet also
caused impaired fasting glucose compared with the normal control
diet. In the present study, certain expected results, including a
high-fibre diet reducing weight and improving glucose and lipid
metabolism (23-25),
were not obtained. When comparing the fibre group with the control
group, the high-fibre group exhibited a relatively high dietary
fibre content, but the soluble fibre content was not very high, and
this groups exhibited a higher glycemic index (GI). Studies
conducted with 48,631 European Prospective into Cancer and
Nutrition study participants, with a 5.5-year follow-up time, have
indicated a positive association between GI and obesity measures
(26). A systematic review and
meta-analysis of the effects of GI on rodent metabolism
demonstrated that a high-GI diet fed to male mice and rats
increases body weight, adiposity and fasting insulin levels
(27). Another study pointed out
that consuming a single high-fibre food source was not sufficient
to achieve a high-fibre diet in Polish adolescents, but that the
consumption of a wide variety of dietary fibre sources, including
relatively high-fibre and low-fibre food, may help Polish
adolescents achieve a relatively high-fibre diet (28). Overall, the present results suggest
that a high-fat diet was positively associated with the occurrence
of obesity and that a high-protein diet was associated with a
reduced body weight, but also led to glucose and lipid metabolism
disorders. In comparison with the control, there was no significant
increase in body weight in the high-sugar and high-fibre diet
groups after 4 weeks, and the effect of high-sugar and high-fibre
diet on glucose and lipid control may depend on the GI and fibre
content.
Previous studies have indicated that diet is
involved in the pathogenesis of obesity and glucolipid metabolism.
Diet is a major factor that modulates the composition and activity
of the gut microbiota (10). To
date, little is known regarding how diet and intestinal bacteria
affect cellular health. SCFAs and amino acids are important
metabolites produced by gut microbiota. Recent studies have
indicated that excessive SCFAs produced by a particular gut
microbiota represent an additional energy source and simultaneously
participate in glucose-stimulated insulin secretion from pancreatic
β-cells. These effects are mediated by the interaction of SCFAs
with the free fatty acid 2 (FFA2) and FFA3 receptors and the
release of peptide hormones, which control appetite (29). SCFAs are thought to inhibit the
accumulation of fat in adipose tissue, and therefore, decreased
levels may contribute to obesity (30). The present study demonstrated that SD
rats on a high-fat diet had lower SCFA levels and that a
high-protein diet is associated with higher total SCFA levels in
feces. This observation may reflect higher satiety and diet-induced
thermogenesis when the dietary protein content is increased. The
literature has reported that SCFA butyrate emerged is a significant
mediator of health, through its effects on inflammation and satiety
(31). Furthermore, the results of
the present study confirmed that the SD rats fed the high-fat diet
produced less SCFAs, but the percentage of the three SCFAs (acetic
acid, propionic acid and butyric acid vs. total SCFAs) was
significantly elevated in the feces and blood samples. In addition,
the FAT group of the present study had higher acetic acid/propionic
acid ratios in their fecal samples. Therefore, it may be inferred
that bacteria that produce a higher acetic/propionic ratio may
promote the development of obesity. The results of the present
study indicate that the high-protein diet resulted in higher amino
acid levels in the feces and ileocecal content. The PRO group
consumed a much higher proportion of protein than the other groups,
and the results indicated that the high protein-based calorie
intake was beneficial for reduction of weight gain. Fermentation of
dietary proteins may also contribute to SCFA production, but
typically provides branched-chain fatty acids, including
isobutyrate, 2-methylbutyrate and isovalerate, which originate
exclusively from branched-chain amino acids, including valine,
isoleucine and leucine. It has been reported that protein- or
fat-rich diets supplemented with dietary fibre promote the
restoration of beneficial microbes, which may increase SCFA
production and inhibit toxin production (32).
The homeostatic control of energy balance is highly
regulated by a complex neuronal network where the hypothalamus has
a key role in connecting the brainstem and various forebrain
regions. There are various peripheral anorexigenic hormones,
including leptin, which increase satiety, whereas ghrelin induces
hunger. The effect of the microbiota-gut-brain axis is becoming
increasingly recognized in psychological and psychiatric disorders
(33). In the present study, the
levels of the appetite control factors leptin and ghrelin were
compared between the groups. In order to avoid the influence of
diet itself, the experimental diets were designed based on the
percentage of energy of nutrients, for instance, the percentage of
energy from protein (casein) was 60% in the high-protein diet. The
percentage of energy from the casein content in all other diets was
19.2%. The micronutrient content was also almost equivalent between
diets. Certain studies have pointed out that the energy density
also influenced the food intake (34,35). All
diets in the present study had an equivalent energy density, except
for the high-fat diet. The results of the present study indicated
differences in intestinal metabolic products between the different
diet groups and differences in leptin and ghrelin expression in
different tissues. In addition to serving as an energy source,
acetate, propionate and butyrate have been recognized as ligands of
the G protein-coupled receptors FFAR2 and FFAR3(36). Activation of FFAR2 in adipocytes
triggers the release of leptin (37,38).
Consistent with the literature, the SD rats of the present study
fed the high-fat diet had markedly higher leptin levels and it was
noted that leptin mRNA was expressed mainly in adipose tissues.
Furthermore, leptin levels were positively increased in parallel
with the body weight and fat mass size, and in the digestive tract,
the leptin mRNA expression of the FAT group was relatively high. It
appears that bacteria, which produce higher acetic/propionic and
acetic/butyric ratios, may affect the expression of leptin and
leptin receptor, which promotes the development of obesity.
Furthermore, the results of the present study
indicated that the PRO group had higher ghrelin mRNA expression
levels in the hypothalamus tissues compared with that in other diet
groups The amino acid composition and digestibility of proteins,
which are affected by its source and amount of intake, have a
pivotal role in determining the microbiota. Elevated systemic
concentrations of certain amino acids, particularly the aromatic
and branched-chain amino acids, have been demonstrated to modulate
the development of insulin resistance and type 2 diabetes mellitus.
Changes in the microbiota may affect the gut barrier and the immune
system by regulating gene expression in relevant signaling pathways
and regulating the secretion of metabolites (39).
In conclusion, the present study demonstrated that
diet has an important role in the development of obesity. The
limited sample size of the present study may have been a limitation
by hampering the detection of other significant associations, and
the effect of metabolites on the regulation of dietary factors,
which may be elucidated in cell-based experiments. The lack of
other measurements, including the determination of factors from the
gut microbiota that induce obesity and metabolic disease, e.g.
endotoxin, is another limitation of the present study. Due to time
and cost constraints, no further intervention experiments were
included. It may be proposed that manipulation of intestinal
metabolites by dietary alterations may be a useful strategy to
prevent or treat obesity and associated complications in the
future. It is worth pointing out the physiological diet of rats, as
well as their metabolism, are not completely superimposable to
those of humans.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Zhejiang Province (grant no. LQ15H050002), Projects
of Traditional Chinese Medicine in Zhejiang Province (grant no.
2015ZQ004) and Projects of Medical and Health Technology
Development Program in Zhejiang Province (grant no. 2016KYA015;
2017KY012; 2018KY266).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
BL and WZh designed the experiments. YL, WZo and WZh
performed the experiments and calculations. YL and WZo wrote and
edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of the Second Xiangya Hospital of Central South
University (Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seganfredo FB, Blume CA, Moehlecke M,
Giongo A, Casagrande DS, Spolidoro JVN, Padoin AV, Schaan BD and
Mottin CC: Weight-loss interventions and gut microbiota changes in
overweight and obese patients: A systematic review. Obes Rev.
18:832–851. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ubeda C, Djukovic A and Isaac S: Roles of
the intestinal microbiota in pathogen protection. Clin Transl
Immunology. 6(e128)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsumoto M, Kibe R, Ooga T, Aiba Y,
Kurihara S, Sawaki E, Koga Y and Benno Y: Impact of intestinal
microbiota on intestinal luminal metabolome. Sci Rep.
2(233)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Z, Quan G, Jiang X, Yang Y, Ding X,
Zhang D, Wang X, Hardwidge PR, Ren W and Zhu G: Effects of
metabolites derived from gut microbiota and hosts on pathogens.
Front Cell Infect Microbiol. 8(314)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lucas S, Omata Y, Hofmann J, Böttcher M,
Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B,
Krönke G, et al: Short-chain fatty acids regulate systemic bone
mass and protect from pathological bone loss. Nat Commun.
9(55)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Haghikia A, Jörg S, Duscha A, Berg J,
Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, et al:
Dietary fatty acids directly impact central nervous system
autoimmunity via the small intestine. Immunity. 44:951–953.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
D'Souza WN, Douangpanya J, Mu S, Jaeckel
P, Zhang M, Maxwell JR, Rottman JB, Labitzke K, Willee A, Beckmann
H, et al: Differing roles for short chain fatty acids and GPR43
agonism in the regulation of intestinal barrier function and immune
responses. PLoS One. 12(e0180190)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ojeda P, Bobe A, Dolan K, Leone V and
Martinez K: Nutritional modulation of gut microbiota-the impact on
metabolic disease pathophysiology. J Nutr Biochem. 28:191–200.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
DeGruttola AK, Low D, Mizoguchi A and
Mizoguchi E: Current understanding of dysbiosis in disease in human
and animal models. Inflamm Bowel Dis. 22:1137–1150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kverka M and Tlaskalova-Hogenova H:
Intestinal microbiota: Facts and fiction. Dig Dis. 35:139–147.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Birch JM, Ullman K, Struve T, Agger JF,
Hammer AS, Leijon M and Jensen HE: Investigation of the viral and
bacterial microbiota in intestinal samples from mink (Neovison
vison) with pre-weaning diarrhea syndrome using next generation
sequencing. PLoS One. 13(e0205890)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ji W, Zhu Y, Kan P, Cai Y, Wang Z, Wu Z
and Yang P: Analysis of intestinal microbial communities of
cerebral infarction and ischemia patients based on high throughput
sequencing technology and glucose and lipid metabolism. Mol Med
Rep. 16:5413–5417. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Morsczeck C, Korenkov M, Nagelschmidt M,
Feher D and Schierholz JM: Total RNA-isolation of abdominal hernia
of rats for quantitative real-time reverse transcription (RT) PCR
assays. Prep Biochem Biotechnol. 38:87–93. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alcock J and Lin HC: Fatty acids from diet
and microbiota regulate energy metabolism. F1000Res.
4(738)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Candido FG, Valente FX, Grzeskowiak LM,
Moreira APB, Rocha DMUP and Alfenas RCG: Impact of dietary fat on
gut microbiota and low-grade systemic inflammation: Mechanisms and
clinical implications on obesity. Int J Food Sci Nutr. 69:125–143.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Pinho L, Andrade JM, Paraíso A, Filho
AB, Feltenberger JD, Guimarães AL, de Paula AM, Caldeira AP, de
Carvalho Botelho AC, Campagnole-Santos MJ and Sousa Santos SH: Diet
composition modulates expression of sirtuins and renin-angiotensin
system components in adipose tissue. Obesity (Silver Spring).
21:1830–1835. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu JP, Zou WL, Chen SJ, Wei HY, Yin YN,
Zou YY and Lu FG: Effects of different diets on intestinal
microbiota and nonalcoholic fatty liver disease development. World
J Gastroenterol. 22:7353–7364. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Araujo JR, Tomas J, Brenner C and
Sansonetti PJ: Impact of high-fat diet on the intestinal microbiota
and small intestinal physiology before and after the onset of
obesity. Biochimie. 141:97–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang L, Jacobs JP, Lagishetty V, Yuan PQ,
Wu SV, Million M, Reeve JR Jr, Pisegna JR and Taché Y: High-protein
diet improves sensitivity to cholecystokinin and shifts the cecal
microbiome without altering brain inflammation in diet-induced
obesity in rats. Am J Physiol Regul Integr Comp Physiol.
313:R473–R486. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Campos-Nonato I, Hernandez L and Barquera
S: Effect of a high-protein diet versus standard-protein diet on
weight loss and biomarkers of metabolic syndrome: A randomized
clinical trial. Obes Facts. 10:238–251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
da Rosa Lima T, Ávila ETP, Fraga GA, de
Souza Sena M, de Souza Dias AB, de Almeida PC, Dos Santos Trombeta
JC, Junior RCV, Damazo AS, Navalta JW, et al: Effect of
administration of high-protein diet in rats submitted to resistance
training. Eur J Nutr. 57:1083–1096. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi Y, Giovannucci E and Lee JE:
Glycaemic index and glycaemic load in relation to risk of
diabetes-related cancers: A meta-analysis. Br J Nutr.
108:1934–1947. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang J, Fang YJ, Xu M, Luo H, Zhang NQ,
Huang WQ, Pan ZZ, Chen YM and Zhang CX: Carbohydrate, dietary
glycaemic index and glycaemic load, and colorectal cancer risk: A
case-control study in China. Br J Nutr. 119:937–948.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dong JY, Zhang L, Zhang YH and Qin LQ:
Dietary glycaemic index and glycaemic load in relation to the risk
of type 2 diabetes: A meta-analysis of prospective cohort studies.
Br J Nutr. 106:1649–1654. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Romaguera D, Angquist L, Du H, Jakobsen
MU, Forouhi NG, Halkjaer J, Feskens EJ, van der A DL, Masala G,
Steffen A, et al: Dietary determinants of changes in waist
circumference adjusted for body mass index-a proxy measure of
visceral adiposity. PLoS One. 5(e11588)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Campbell GJ, Senior AM and Bell-Anderson
KS: Metabolic effects of high glycaemic index diets: A systematic
review and meta-analysis of feeding studies in mice and rats.
Nutrients. 9(E646)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Krusinska B, Kowalkowska J, Wadolowska L,
Wuenstel JW, Slowinska MA and Niedzwiedzka E: Fibre-related dietary
patterns: Socioeconomic barriers to adequate fibre intake in polish
adolescents. A short report. Nutrients. 9(E590)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Murugesan S, Nirmalkar K, Hoyo-Vadillo C,
Garcia-Espitia M, Ramirez-Sanchez D and Garcia-Mena J: Gut
microbiome production of short-chain fatty acids and obesity in
children. Eur J Clin Microbiol Infect Dis. 37:621–625.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tilg H and Moschen AR: Microbiota and
diabetes: An evolving relationship. Gut. 63:1513–1521.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lu Y, Fan C, Li P, Lu Y, Chang X and Qi K:
Short chain fatty acids prevent high-fat-diet-induced obesity in
mice by regulating G protein-coupled receptors and gut microbiota.
Sci Rep. 6(37589)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sanchez JI, Marzorati M, Grootaert C,
Baran M, Van Craeyveld V, Courtin CM, Broekaert WF, Delcour JA,
Verstraete W and Van de Wiele T: Arabinoxylan-oligosaccharides
(AXOS) affect the protein/carbohydrate fermentation balance and
microbial population dynamics of the simulator of human intestinal
microbial ecosystem. Microb Biotechnol. 2:101–113. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
van de Wouw M, Schellekens H, Dinan TG and
Cryan JF: Microbiota-gut-brain axis: Modulator of host metabolism
and appetite. J Nutr. 147:727–745. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Murakami K, Livingstone MB, Okubo H and
Sasaki S: Energy density of the diets of Japanese adults in
relation to food and nutrient intake and general and abdominal
obesity: A cross-sectional analysis from the 2012 national health
and nutrition survey, Japan. Br J Nutr. 117:161–169.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Raynor HA and Vadiveloo M: Understanding
the relationship between food variety, food intake, and energy
balance. Curr Obes Rep. 7:68–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brown AJ, Goldsworthy SM, Barnes AA,
Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn
I, Fraser NJ, et al: The Orphan G protein-coupled receptors GPR41
and GPR43 are activated by propionate and other short chain
carboxylic acids. J Biol Chem. 278:11312–11319. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Blaut M: Gut microbiota and energy
balance: Role in obesity. Proc Nutr Soc. 74:227–234.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiong Y, Miyamoto N, Shibata K, Valasek
MA, Motoike T, Kedzierski RM and Yanagisawa M: Short-chain fatty
acids stimulate leptin production in adipocytes through the G
protein-coupled receptor GPR41. Proc Natl Acad Sci USA.
101:1045–1050. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ma N, Tian Y, Wu Y and Ma X: Contributions
of the interaction between dietary protein and gut microbiota to
intestinal health. Curr Protein Pept Sci. 18:795–808.
2017.PubMed/NCBI View Article : Google Scholar
|