Introduction
Idiopathic epiretinal membrane (IERM) may be
classified into two types based on its composition of pathology,
complex (type 1) and simple (type 2) (1,2). The
symptoms experienced by patients with IERM are determined by the
extent of the impact that the ERM has on macular ultrastructure and
primarily include visual impairment and metamorphopsia. IERM may
easily be diagnosed through fundus examination and optical
coherence tomography (OCT).
Pars plana vitrectomy (PPV) remains the predominant
treatment for patients with IERM (3). In all, the surgical procedures involved
are not considered to be complex; however, the most common
post-operative complication of IERM treatment is cataracts, which
leads to further visual impairment in those patients (4). The incidence rate of post-operative
cataracts in patients with IERM reaches 80%. Cataracts generally
occur at 8-12 months post-surgery, potentially due to exposure of
the lens to oxidative stress damage, phototoxicity and
intra-operative perfusion following vitrectomy (5).
To avoid post-operative cataracts in IERM-PPV,
certain retinal surgeons attempted the application of non-pars
plana vitrectomy (N-PPV) with IERM peeling ~10 years ago. However,
during follow-up, it was revealed that, compared to conventional
PPV, the recurrence of IERM in N-PPV-treated cases was 7.5-38%, and
thus, this technique was not widely promoted (6).
Oshima et al (7) were the first to introduce 27G
vitrectomy in 2010. In recent years, 27G PPV, which involves a
smaller incision compared with 23G and 25G PPV, has been applied
for the treatment of macular diseases, vitreous haemorrhage and
retinal detachment. Previous studies have demonstrated that 27G PPV
is more efficient than 23G PPV and has a lower incidence of
post-operative incision-associated complications, including
subconjunctival air bubbles and hypotony (7). The aim of IERM surgery is to loosen and
remove the epiretinal proliferative membrane, which involves the
vitreous and macula, and no other procedures are required,
including laser and intraocular tamponade. Thus, application of 27G
core-PPV may be effective, combined with a double-staining
technique to perform partial resection of the epimacular posterior
vitreous cortex, followed by ERM and internal limiting membrane
(ILM) peeling. This may resolve the high post-operative recurrence
of IERM after N-PPV, while also retaining a large portion of the
vitreous body and decreasing damage to the lens.
The present study applied 27G core-PPV in patients
with IERM and evaluated the effects of this modified IERM surgery
for the lens, as well as the efficacy and safety of the
procedure.
Patients and methods
Patient information
The present study was approved by the Institutional
Ethical Review Board of Shanghai 10th People's Hospital affiliated
to Tongji University (Shanghai, China). Written informed consent
was obtained from all patients or their guardians prior to the
start of the study. A total of 38 patients who received 27G
core-PPV for IERM were recruited between October 2015 and December
2016. The inclusion criteria were as follows: Confirmation of ERM
using funduscopic examination under slit-lamp microscopy and OCT;
visual impairment; metamorphopsia, Amsler Grid Test-positive;
phakic eye and a complete 12-month follow-up period. The exclusion
criteria were as follows: History of other ocular diseases, surgery
and trauma; myopia ≤-6 diopters or hyperopia ≥+6 diopters; axial
length ≥26 mm or ≤22 mm; Grades of N2, C1 and above, according to
the Lens Opacities Classification System III (LOCS III) (8), and occurrence of diabetes, renal
dysfunction and other systemic diseases, which may have interfered
with the measurements.
Surgical methods
27G three-port PPV was performed (Alcon
Constellation Vision System). All surgeries were performed by an
experienced physician. The affected eye was retrobulbar
anesthetized using 2% lidocaine + 1% ropivacaine. Three cannula
trocar systems were transconjunctivally inserted in the eye. The
small sutureless incisions of 27G PPV were made by trocars, first
at 4 mm from the limbus in the infero-temporal quadrant (4:30 or
7:30) for the infusion line and then in the supero-temporal and
supero-nasal quadrants (10:30 and 2:30, respectively). The surgical
parameters were as follows: 6,000-7,000 cuts/min; vacuum of 500-600
mmHg and perfusion pressure of 25-30 mmHg. The OPMI LUMERA 700
surgical microscope (Carl Zeiss AG) was used and
RESIGHT® Fundus Viewing System (Carl Zeiss AG) was
applied. In brief, 0.05 ml from 10 mg/ml of triamcinolone acetonide
was injected above the optic disc using an ultra-wide-angle lens in
order to identify the posterior vitreous cortex before the disc and
macula, prior to performing core vitrectomy. After switching to a
60 D posterior-pole lens, the complete detachment of posterior
vitreous cortex and the posterior retinal pole was validated.
Subsequently, the intraocular perfusion pressure was decreased and
staining with 0.025% indocyanine green was performed for 30 sec.
The ILM and ERM within the macular area were subsequently peeled
using 27G ILM tweezers (27G™ Grieshaber Revolution® DSP;
Alcon). The peeling range was within the vascular arches, above and
below the posterior pole. The procedure was completed following
resection of the vitreous body within a 40˚ range centred on the
optic disc, which was roughly defined as the nearly circular region
with a radius of the distance (~5.5 mm) from the optic disc to the
temporal vascular arch, under an ultra-wide-angle lens. None of the
affected eyes received combined cataract surgery. The entire
surgical process was video-recorded and the operation time and
process were also recorded.
Pre-operative and post-operative
examinations
In addition to the patients' medical history, the
following information was recorded pre-operatively: Sex, age,
disease duration (the time of distorted vision complained by
patients), best-corrected visual acuity (BCVA), Amsler grid,
intraocular pressure and slit-lamp microscopy-assisted dilated
fundus examination with a 90 D funduscopic lens (Volk Digital Wide
Field Slit Lamp Indirect Ophthalmoscopy Lens; Volk optical, Inc.).
A precise fundus examination was required in order to prevent
retinal tears and detachment. In the case of the occurrence of
either, the affected patient was removed from the study group and
treated by retinal photocoagulation or vitrectomy.
Assessment of lens density was performed using 40˚
slit-lamp imaging of the lens after mydriasis, followed by cataract
grading using the LOCS III system. Bilateral fundus images were
captured using Model CR-2 (Canon, Inc.). OCT was performed using
the Zeiss Cirrus HD-OCT 400 Macular Cube 512 x 128 scanning mode
(Carl Zeiss AG) in order to scan the patients' macular area, and an
in-built software was used to automatically assess the CMT. OCT
angiography (OCTA) was performed using an angioscope with 3 mm x 3
mm measurement range (Optovue RTVue XR Avanti, Optovue, Inc.) to
scan the patients' macular are, whilst the flow density map
software AngioAnalytics in-built version 2016.1.0.26 was used to
quantity the FAZ, superficial retinal capillary density (SRCD) and
deep retinal capillary density (DRCD) automatically.
Panoramic ultrasound biomicroscopy (UBM; Model
UBMSW-3200L; Tianjin Suowei Electronic Technology) was performed to
monitor the PPV scleral incision. B-mode ultrasound (Aviso A/B;
Quantel Medical; Bozeman) was performed to observe the
post-operative vitreous body and vitreous base.
All patients returned for follow-up at 1 week and at
1, 3, 6 and 12 months post-operatively. During each follow-up, the
pre-operative examinations were repeated. UBM, B-mode ultrasound
and OCTA were also performed at 6 months post-operatively.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 21.0; IBM Corp.). Values are expressed as the
mean ± standard deviation. BCVA was converted to Logarithm of the
Minimum Angle of Resolution scoring. The repeated-measure analysis
of variance (ANOVA) followed by a paired t-test with Bonferroni's
corrections was used to compare differences in follow-up data
between the pre-operative and post-operative groups in Figs. 1 and 5, and in Tables
II, III and IV. Pearson correlation analysis was
performed between BCVA and FAZ. P<0.05 was considered to
indicate a statistically significant difference.
 | Figure 1Pre-operative and post-operative
changes in BCVA and time distribution of post-operative BCVA
recovery. (A) The mean post-operative BCVA at 1 week and 1, 3, 6
and 12 months are significantly improved compared with that in the
pre-operative BCVA. (B) During the 12-month follow-up, the
post-operative BCVA were >0.15 or ≥2 lines in 36 of 38 patients.
Among these, 33 showed significant improvement at 1 week
post-operatively, one at 1 month post-operatively and one at 12
months post-operatively. Post-operative BCVA of 18 patients
exhibited a score of >0.3, represented by the horizontal line in
the lower panel. P<0.05. BCVA, best-corrected visual acuity; w,
week; m, month; OP, operation; postop, post-operative. |
 | Table IIComparison of pre- and post-OP BCVA
in the 38 patients with idiopathic epiretinal membrane. |
Table II
Comparison of pre- and post-OP BCVA
in the 38 patients with idiopathic epiretinal membrane.
| Time-point | BCVA | F-value |
P-valuea |
|---|
| Pre-OP | 0.40±0.23 | - | - |
| 1 week post-OP | 0.52±0.20 | 5.18 |
2.68x10-2 |
| 1 month
post-OP | 0.65±0.20 | 12.43 |
1.26x10-3 |
| 3 months
post-OP | 0.65±0.18 | 18.92 |
1.05x10-4 |
| 6 months
post-OP | 0.68±0.16 | 22.80 |
3.54x10-5 |
| 12 months
post-OP | 0.75±0.16 | 21.85 |
3.84x10-5 |
 | Table IIIPre- and post-operative CMT of the 38
patients with idiopathic epiretinal membrane. |
Table III
Pre- and post-operative CMT of the 38
patients with idiopathic epiretinal membrane.
| Time-point | CMT (µm) | F-value |
P-valuea |
|---|
| Pre-OP | 435.1±86.36 | - | - |
| 1 week post-OP | 415.75±70.20 | 0.60 | 0.60 |
| 1 month
post-OP | 385.15±57.23 | 4.65 | 0.04 |
| 3 months
post-OP | 369.5±47.88 | 8.83 |
8x10-3 |
| 6 months
post-OP | 342.4±46.51 | 17.86 |
2x10-4 |
| 12 months
post-OP | 318.05±37.50 | 30.91 |
3.49x10-6 |
 | Table IVLOCS III score grades post-OP
time-points in the 38 patients with idiopathic epiretinal membrane
(38 eyes)a. |
Table IV
LOCS III score grades post-OP
time-points in the 38 patients with idiopathic epiretinal membrane
(38 eyes)a.
| Variable | Pre-OP | 1 month
post-OP | 3 months
post-OP | 6 months
post-OP | 12 months
post-OP |
|---|
| Nuclear opalescence
score | 2.53±0.94 | 2.86±0.87 | 2.97±0.89 | 3.33±0.78 | 3.54±0.63 |
| Cortical score | 0.89±0.42 | 1.18±0.35 | 1.32±0.34 | 1.54±0.46 | 1.66±0.49 |
| Posterior
subcapsular score | 0.60±0.29 | 0.76±0.27 | 0.92±0.27 | 1.08±0.30 | 1.18±0.33 |
| LOCS III score | 4.63±1.42 | 5.34±1.43 | 5.6±1.17 | 6.26±1.10 | 6.42±1.05 |
| F-value | - | 0.63 | 1.40 | 4.13 | 5.30 |
|
P-valueb | - | 0.60 | 0.38 | 0.96 | 0.08 |
Results
Pre- and post-operative BCVA
The baseline characteristics of the 38 patients (18
male, 20 female) are presented in Table
I. The mean age of these patients was 62.73±5.61 years. The
mean pre-operative BCVA and post-operative BCVA at 1 week and 1, 3,
6 and 12 months are listed in Table
II. The post-operative BCVA were all found to be significantly
improved compared with those in pre-operative BCVA (P<0.05;
Fig. 1A; Table II). Based on ≥2 Snellen lines
considered as the criterion for improvement (9), 36 of the 38 cases (94.74%) exhibited
improvements in post-operative BCVA. As Fig. 1B shows, 86.84% (33/38) of patients
exhibited improved visual acuity at 1 week post-operatively, which
means that the post-operative BCVA was improved >0.15 or by 2
lines compared with those of pre-operative BCVA. Furthermore, two
additional patients demonstrated improved visual acuity at 1 month
post-operatively and 1 additional patient exhibited improvement at
12 months post-operatively. During the entire follow-up, BCVA
improved >0.3 (lower panel, Fig.
1) in 50% of the patients (18/38).
 | Table IBaseline characteristics of 38 eyes
of 38 patients with IERM. |
Table I
Baseline characteristics of 38 eyes
of 38 patients with IERM.
| Item | Value |
|---|
| Age (years) | 45-76
(62.73±5.61) |
| Sex
(male/female) | 18/20 |
| Affected eye
right/left | 22/16 |
| Disease duration
(months) | 1-15
(7.55±4.21) |
| Axial length of eye
(mm) | 23.6±1.4 |
| IOP (mmHg) | 13.7±2.5 |
| Operation time
(min) | 11.52±2.21 |
| Rhegmatogenous
retinal detachment | 1/38 |
| IERM
recurrence | 2/38 |
Post-operative changes in CMT
Fig. 2 presents the
pre- and post-operative CMT of a patient with IERM. The patients'
pre-operative CMT ranged from 274 to 626 µm, with a mean value of
435.10±86.36 µm. The mean CMT values at 1, 3, 6 and 12 months
post-operatively are presented in Table III. The difference from the
pre-operative CMT at 1 week post-operatively was not statistically
significant (P=0.60). The subsequent decreases in CMT values were
all statistically significant (P<0.05) and the greatest drop of
CMT occurred between 1 week and 1 month post-operatively (Table III).
Changes in lens density
According to the LOCS III scoring system, nuclear
density of the lens was scored from 0.1 to 6.9 whilst the cortical
and posterior subcapsular densities were scored from 0.1 to
5.9(8). The total LOCS III score of
the normal lens is typically ≤5 (nuclear density, ≤2; cortical
density, ≤2 and posterior subcapsular density, ≤1) (8,10).
Patients with a total LOCS III score of ≥2 were regarded as
exhibiting development of post-operative lens opacity (10) compared with the pre-operative group.
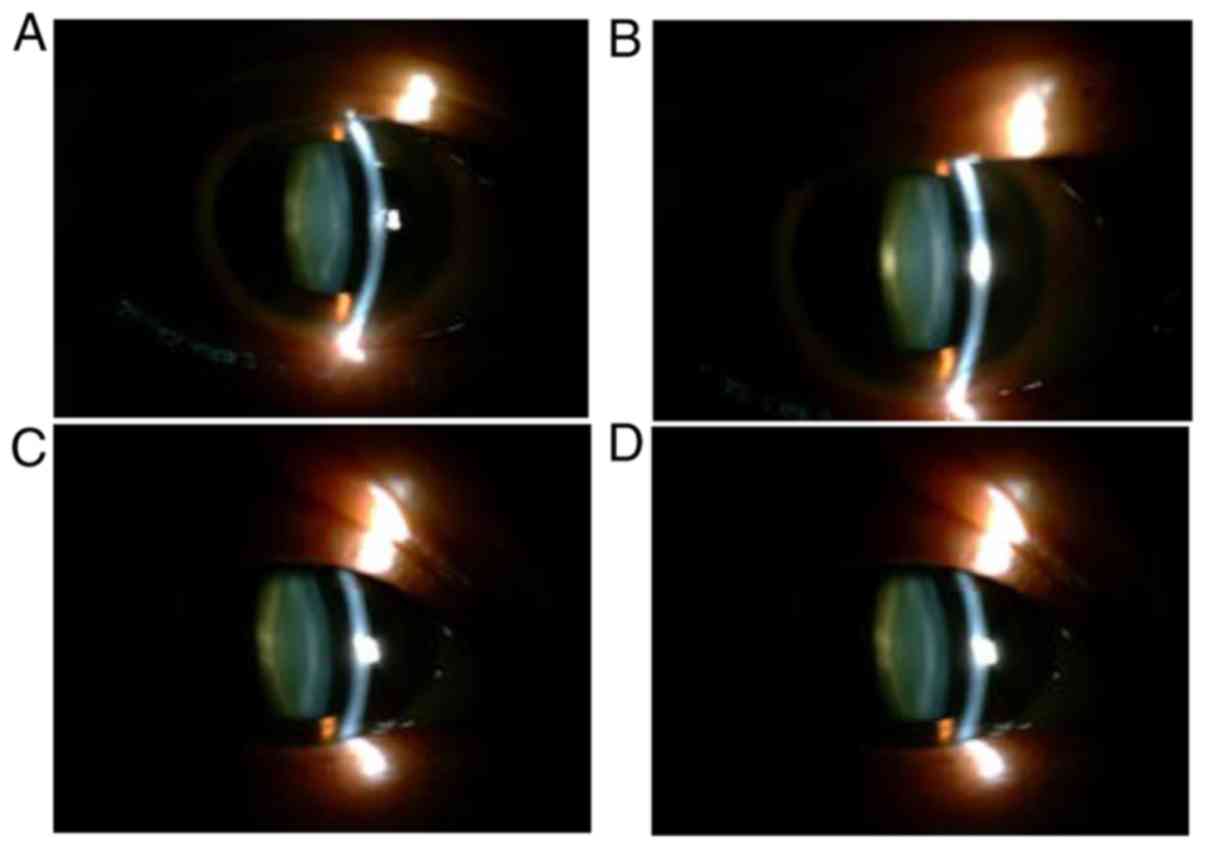
Fig. 3 presents the pre- and
post-operative lens images of a patient with IERM. The pre- and
post-operative mean LOCS III total scores of the 38 patients are
presented in Table IV. The density
changes at different parts of the lens from the pre-operative
levels were not statistically significant (P>0.05).
Association between post-operative
BCVA with FAZ and vascular density (VD)
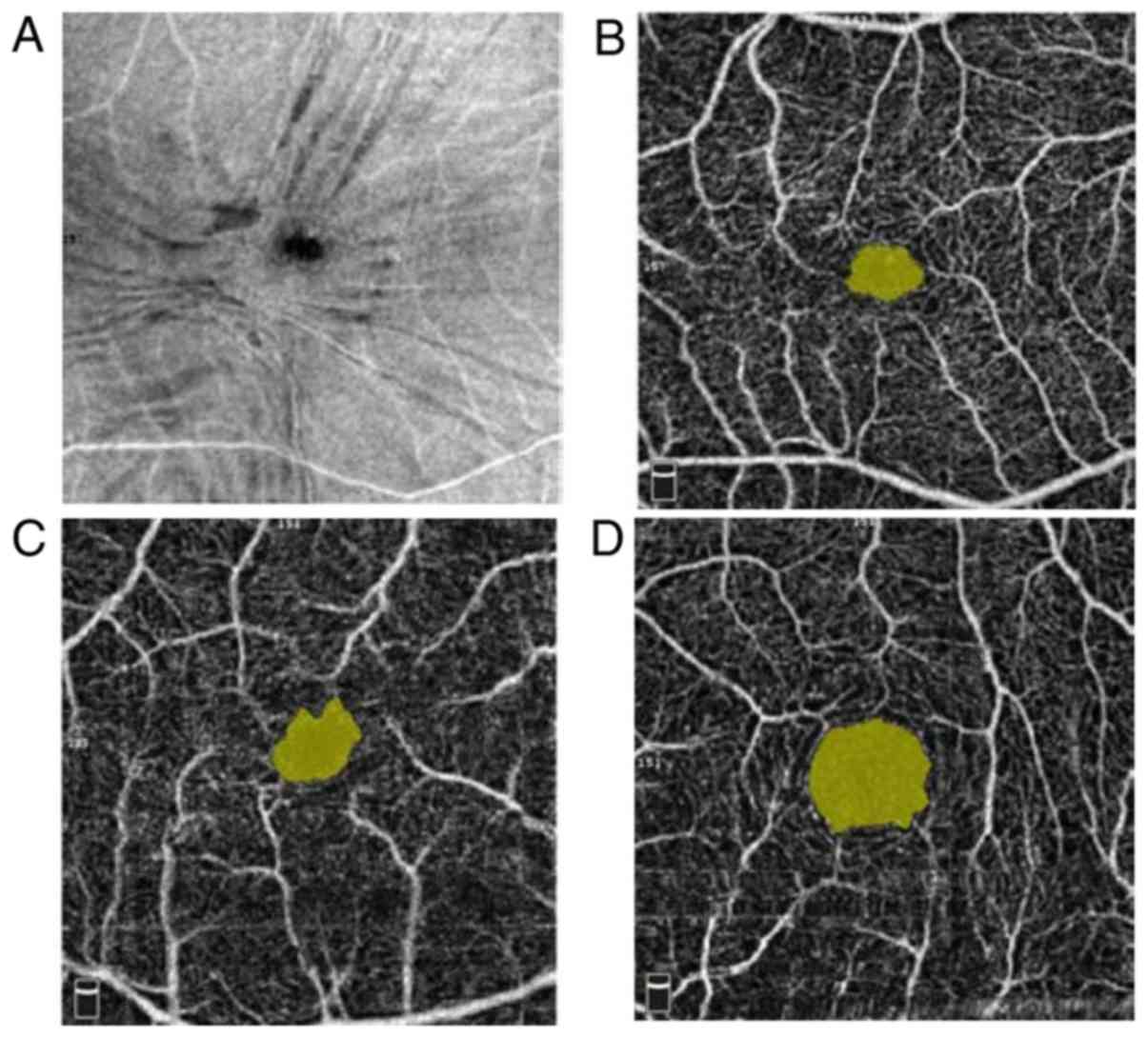
Fig. 4 presents the
images of pre- and post-operative FAZ in a patient with IERM. The
mean pre-operative FAZ was 0.19±0.08 mm² (range, 0.083-0.460 mm²),
which was low compared with that of the unaffected contralateral
eye (0.390±0.180 mm²). At 6 months post-operatively, the patients'
FAZ increased to 0.23±0.14 mm², which was higher than the
pre-operative value but still lower than the FAZ of the unaffected
contralateral eyes. The differences between the pre- and
post-operative FAZs in the affected eye, and that between the
affected and healthy eyes were statistically significant
(P<0.05; Fig. 5).
The post-operative SRCD and DRCD values (45.58±3.58
and 52.71±3.21%, respectively) were significantly lower than the
pre-operative values (50.58±3.55 and 54.58±3.46%, respectively) and
the values of the fellow eyes (51.76±5.83 and 56.40±5.21%,
respectively) at 6 months (P<0.05; Fig. 5).
Correlation analysis indicated that the patients'
post-operative BCVA was negatively associated with the FAZ
(r=-0.72; P≤0.05; Fig. 5).
B-mode ultrasound and UBM observation
of scleral incision
Imaging observation of the scleral incision was
performed for all patients at 6 months post-operatively using
B-mode ultrasound and UBM. The post-operative UBMs demonstrated no
abnormal echoes at the scleral incisions. Furthermore, the vitreous
body and vitreous base did not indicate any abnormalities compared
with the pre-operative observations (Fig. 6).
Discussion
PPV remains the predominant treatment for IERM.
Oshima et al (7) were the
first to introduce 27G vitrectomy in 2013. The 27G surgical
instruments have smaller diameters, a larger port and a shorter
distance between the port and tip compared with 25G vitrectomy.
Thus, 27G is able to be used closer to the retina, which increases
the space for delicate operations (11). In addition, the system operating
parameters, including cutting speed and vacuum level, have been
improved, whereby the 27G probe has an ultra-high cutting speed of
7,500 r/min and the valved sleeves of the 27G vitrectomy probe
strengthen its stiffness to a certain extent (12,13).
In contrast to treatments for other fundus diseases,
IERM surgery predominantly involves the macular area, while
intensive surgery on the peripheral vitreous and retina are not
required. Hence, it is the most preponderant indication for PPV
with minimal incision (14). Thus,
the present study applied the concept of core-PPV in IERM surgery.
Core vitrectomy was predominantly applied in conjunction with
anterior segment surgeries, including anterior vitrectomy of
malignant glaucoma, intraocular lens suspension and cornea
transplant (15,16). Naser et al (17) performed core-PPV prior to
intravitreal injection. Thus, core-PPV involves partial resection
of the vitreous body, in accordance with the treatment aims. This
technique is advantageous, as it protects the lens and does not
interfere with the anterior vitreous body, which in turn decreases
the incidence of post-operative cataracts (18).
Cataract is the most common complication following
IREM surgery, which has a high incidence rate of 42.5-81.0%
(19,20). The decline in oxygen absorption
following vitrectomy is considered to be the major cause of
subsequent oxidative stress damage to the lens (21). Saito et al (22) performed the N-PPV with IERM peeling
technique, which has been confirmed to protect the lens
post-operatively, and thus avoids cataracts in IERM-PPV. However,
the recurrence rate of IERM in N-PPV was reported to be between 10
and 38% (22-24),
which is higher than that of conventional PPV, at 1-16% (25,26). The
high recurrence rate is considered to be due to the inability to
remove the posterior vitreous cortex effectively and the presence
of residual ERM without the assistance of staining.
In the present study, the N-PPV with IERM peeling
was modified and core epimacular vitrectomy combined with
double-staining was performed (27).
As this modified surgery preserves the lens behind the vitreous
body, the lens density of the 38 patients was not significantly
increased compared with the pre-operative values after 12 months.
The combined use of staining techniques also ensured effective
removal of the ERM. The post-operative IERM recurrence among the 38
patients was only 5%, which is less than that reported for
conventional PPV [23G, 7.9% (28);
25G, 5.1 % (2)].
The results of the present study demonstrated a
higher and faster visual recovery rate in the 1st week
post-operatively. A total of 33 of the 38 patients (86.84%)
exhibited visual improvement of ≥2 Snellen lines, which is higher
than the post-operative visual acuity reported after conventional
PPV (44.5-80.0%) (9,29,30).
Similarly, Sandali et al (25) reported that using smaller incisions
to treat patients with ERM results in earlier post-operative
recovery of visual acuity In that previous study, visual
improvement was higher at 8 days postoperatively in the 25G group
compared with that observed in the 20G and 23G groups (P=0.035),
but not at 6 weeks postoperatively (P=0.186). The major factors
affecting earlier visual recovery include corneal astigmatism,
inflammatory reaction and post-operative cataract development
(26). In the present study,
post-operative cataract development of the 38 patients was not
statistically significant, which may be the major reason as to why
corneal astigmatism and inflammatory reaction were not analyzed.
Furthermore, the smaller incision and lower vitreous interference
of the 27G procedure may result in faster recovery of visual acuity
in patients who underwent the modified core-PPV.
Post-operative CMT of the 38 patients demonstrated a
significant decrease compared with the pre-operative value and the
maximum CMT decrease was observed at 1 month post-operatively,
which was consistent with a previous study by Jung et al
(31). However, no association was
observed between BCVA and CMT in the present study. CMT fails to
fully predict the patients' level of post-operative visual acuity
in clinical practice. According to certain scholars, the
preservation of the foveal photoreceptor inner/outer segment and
external limited membrane may be key factors in the prognosis of
BCVA (32). Laban et al
(33) reported that OCT
characteristics are not associated with post-operative BCVA and
according to them, pre-operative BCVA is the most influential
factor.
In the present study, OCTA was also performed to
assess the association between FAZ and VD with post-operative BCVA.
First of all, the refractive system of the eye is composed of the
cornea, aqueous humor, lens and vitreous body. The total refractive
force of the eyeball was +58.64 D, including +43.05 D for the
cornea and +16.0 - +20.0 D for the lens; thus, the vitreous has low
refractive power. A relevant study reported that most of the
progression of myopia after vitrectomy was linked to cataract
progression (34). However, in the
present study, no significant cataract progression was detected at
12 months post-operatively. Thus, it may be concluded that there
was almost no change in refraction after vitrectomy, which made the
FAZ and VD between the pre- and post-operative examination
comparable.
Kitagawa et al (35) reported that the mean post-operative
FAZs were significantly larger than the pre-operative value;
however, they still remained smaller than those of the fellow eyes,
which was consistent with the results of the present study. These
changes in the FAZ suggest that IERM may directly alter the
distribution of macular capillaries. Furthermore, the results of
the present study demonstrated that the FAZ was positively
associated with visual acuity. Thus, the FAZ of patients with IERM
may be a useful indicator of post-operative visual acuity.
Furthermore, the mean SRCD and DRCD at 6 months post-operatively
were significantly lower than the pre-operative values and the
values of the fellow eyes. Similarly, Kim et al (36) demonstrated that eyes with ERM
following surgery had a lower parafoveal VD and a smaller FAZ in
the superficial capillary plexus and deep capillary plexus compared
with the fellow eyes. The reasons for the decrease in SRCD and DRCD
remain elusive; however, the results indicate that surgery may
cause potential damage to retinal function.
A major concern over the use of core-PPV for the
treatment of ERM is its potential to induce post-operative
proliferative vitreoretinopathy (PVR). All patients underwent
B-mode ultrasound and UBM at 6 and 12 months, respectively, which
did not reveal any proliferative changes. In theory, two major
factors may lead to the onset of PVR: Epithelial-to-mesenchymal
transition of the retinal pigment epithelium cells and the
extensive secretion of inflammatory mediators, cytokines and growth
factors (37). Thus, undetected
tears are a risk factor. In the present study, precise
pre-operative fundus examination was performed to exclude retinal
tears and detachment. In the case of either, the affected patient
was removed from the study group and treated by retinal
photocoagulation or vitrectomy. 27G core-PPV is also able to
minimize invasiveness of the surgery and the low inflammatory
response of the operated eye markedly decreases the probability of
PVR.
During the follow-up period, one patient experienced
inferior retinal detachment at 3 months post-operatively.
Peripheral retinal tears were observed at the 6 o'clock position
through the second thorough vitrectomy. In subsequent surgeries,
examination of the peripheral retina was enhanced under a
wide-angle lens, which prevented the occurrence of similar
cases.
In conclusion, in the present study, a modified
surgical technique of 27G core-PPV was applied, which was combined
with ILM peeling for the treatment of IERM and achieved effective
clinical outcomes. Higher and faster visual recovery rates were
observed compared with conventional PPV post-operatively in the
first week. At 12 months post-surgery, the lens densities of the 38
patients were not significantly increased compared with the
pre-operative values. The mean post-operative FAZs were
significantly larger than the pre-operative values; however, they
still remained smaller than those of the fellow eyes. Following
surgery, eyes with ERM demonstrated a lower VD and a smaller FAZ
compared with the fellow eyes. Compared with conventional PPV (23G,
25G) reported in previous studies, better clinical outcomes were
achieved in the present study. However, the present study lacked
control groups (no surgery group and 27G conventional PPV group).
Further clinical studies are required to confirm the outcomes of
this modified surgery.
Acknowledgements
Not applicable.
Funding
This work was supported by Shanghai 10th People's
Hospital New Emerging Talent Program (grant no. 2018SYPDRC036).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and FW designed the study protocol, analyzed the
data and drafted the manuscript. FW was the only surgeon who
performed all the surgeries in the study. KK and LH contributed to
data acquisition and examination of the patients. All authors had
read and approved the final version of the manuscript and have full
responsibility for all primary data.
Ethics approval and consent to
participate
All patients or their guardians provided written
informed consent to participate in the present study and the
present study was approved by the institutional ethical review
board of Shanghai 10th People's Hospital affiliated to Tongji
University (approval no. SHSY-IEC-4.0/16-148/02; Shanghai,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seidel G, Weger M, Stadlmüller L, Pichler
T and Haas A: Association of preoperative optical coherence
tomography markers with residual inner limiting membrane in
epiretinal membrane peeling. PLoS One. 8(e66217)2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reilly G, Melamud A, Lipscomb P and
Toussaint B: Surgical outcomes in patients with macular pucker and
good preoperative visual acuity after vitrectomy with membrane
peeling. Retina. 35:1817–1821. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu HY, Hsieh YT and Yang CM: Paravascular
abnormalities in eyes with idiopathic epiretinal membrane. Graefes
Arch Clin Exp Ophthalmol. 254:1723–1729. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hejsek L, Stepanov A, Dusova J, Marak J,
Nekolova J, Jiraskova N and Codenotti M: Microincision 25G pars
plana vitrectomy with peeling of the inner limiting membrane and
air tamponade in idiopathic macular hole. Eur J Ophthalmol.
27:93–97. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Do DV, Gichuhi S, Vedula SS and Hawkins
BS: Surgery for post-vitrectomy cataract. Cochrane Database Syst
Rev. 12(CD006366)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mizutani Y, Sato Y and Shimda H: Outcome
of nonvitrectomizing vitreous surgery for epimacular membranes.
Nippon Ganka Kiyo. 52:302–306. 2001.
|
|
7
|
Oshima Y, Wakabayashi T, Sato T, Ohji M
and Tano Y: A 27-gauge instrument system for transconjunctival
sutureless microincision vitrectomy surgery. Ophthalmology.
117:93–102.e2. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chylack LT Jr, Wolfe JK, Singer DM, Leske
MC, Bullimore MA, Bailey IL, Friend J, McCarthy D and Wu SY: The
Lens Opacities Classification System III. The Longitudinal Study of
Cataract Study Group. Arch Ophthalmol. 111:831–836. 1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang WC, Lin C, Lee CH, Sung TL, Tung TH
and Liu JH: Vitrectomy with or without internal limiting membrane
peeling for idiopathic epiretinal membrane: A meta-analysis. PLoS
One. 12(e0179105)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Varma R, Richter GM, Torres M, Foong AW,
Choudhury F and Azen SP: Los Angeles Latino Eye Study Group.
Four-year incidence and progression of lens opacities: The Los
Angeles Latino Eye Study. Am J Ophthalmol. 149:728–34.e1, 2.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Osawa S and Oshima Y: Innovations in
27-Gauge vitrectomy for sutureless microincision vitrectomy
surgery. Rsetina. 4:42–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Osawa S and Oshima Y: 27-Gauge vitrectomy.
Dev Ophthalmol. 54:54–62. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim YJ, Park SH and Choi KS: Fluctuation
of infusion pressure during microincision vitrectomy using the
constellation vision system. Retina. 35:2529–2536. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yoneda K, Morikawa K, Oshima Y, Kinoshita
S and Sotozono C: Japan Microincision Vitrectomy Surgery Study
Group. Japan microincision vitrectomy surgery study group. Surgical
outcomes of 27-gauge vitrectomy for a consecutive series of 163
eyes with various vitreous diseases. Retina. 37:2130–2137.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sachdev R, Gupta A, Narula R and Deshmukh
R: Limited vitrectomy in phacomorphic glaucoma. Indian J
Ophthalmol. 65:1422–1424. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Higaki S, Fukuda M, Matsumoto C and
Shimomura Y: Results of penetrating keratoplasty triple procedure
with 25-gauge core vitrectomy. Cornea. 31:730–733. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Naser H, Koss MJ, Singh P and Koch F:
Combined pharmacosurgery as treatment for diabetic macular edema:
Core pars plana vitrectomy and intravitreal injection of
bevacizumab and triamcinolone. Klin Monatsbl Augenheilkd.
228:910–914. 2011.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
18
|
Rizzo S, Genovesi-Ebert F, Murri S,
Belting C, Vento A, Cresti F and Manca ML: 25-gauge, sutureless
vitrectomy and standard 20-gauge pars plana vitrectomy in
idiopathic epiretinal membrane surgery: A comparative pilot study.
Graefes Arch Clin Exp Ophthalmol. 244:472–479. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song SJ, Kuriyan AE and Smiddy WE: Results
and prognostic factors for visual improvement after pars plana
vitrectomy for idiopathic epiretinal membrane. Retina. 35:866–872.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gupta OP, Weichel ED, Regillo CD, Fineman
MS, Kaiser RS, Ho AC, McNamara JA and Vander JE: Postoperative
complications associated with 25-gauge pars plana vitrectomy.
Ophthalmic Surg Lasers Imaging. 38:270–275. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nam Y, Chung H, Lee JY, Kim JG and Yoon
YH: Comparison of 25- and 23-gauge sutureless microincision
vitrectomy surgery in the treatment of various vitreoretinal
diseases. Eye (Lond). 24:869–874. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saito Y, Lewis JM, Park I, Ikuno Y,
Hayashi A, Ohji M and Tano Y: Nonvitrectomizing vitreous surgery: A
strategy to prevent postoperative nuclear sclerosis. Ophthalmology.
106:1541–1545. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reibaldi M, Longo A, Avitabile T,
Bonfiglio V, Toro MD, Russo A, Viti F, Nicolai M, Saitta A,
Giovannini A, et al: Transconjunctival Nonvitrectomizing vitreous
surgery versus 25-gauge vitrectomy in patients with epiretinal
membrane: A Prospective Randomized Study. Retina. 35:873–879.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sakaguchi H, Oshima Y and Tano Y: 27-gauge
transconjunctival nonvitrectomizing vitreous surgery for epiretinal
membrane removal. Retina. 27:1302–1304. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sandali O, El Sanharawi M, Lecuen N,
Barale PO, Bonnel S, Basli E, Borderie V, Laroche L and Monin C:
25-, 23-, and 20-gauge vitrectomy in epiretinal membrane surgery: A
comparative study of 553 cases. Graefes Arch Clin Exp Ophthalmol.
249:1811–1819. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hikichi T, Matsumoto N, Ohtsuka H, Higuchi
M, Matsushita T, Ariga H, Kosaka S and Matsushita R: Comparison of
one-year outcomes between 23- and 20-gauge vitrectomy for
preretinal membrane. Am J Ophthalmol. 147:639–643.e1.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stalmans P, Feron EJ, Parys-Van
Ginderdeuren R, Van Lommel A, Melles GR and Veckeneer M: Double
vital staining using trypan blue and infracyanine green in macular
pucker surgery. Br J Ophthalmol. 87:713–716. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahn SJ, Ahn J, Woo SJ and Park KH:
Photoreceptor change and visual outcome after idiopathic epiretinal
membrane removal with or without additional internal limiting
membrane peeling. Retina. 34:172–181. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pournaras CJ, Emarah A and Petropoulos IK:
Idiopathic macular epiretinal membrane surgery and ILM peeling:
Anatomical and functional outcomes. Semin Ophthalmol. 26:42–46.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim TW, Song SJ, Chung H and Yu HG:
Internal Limiting Membrane Peeling In Surgical Treatment of Macular
Epiretinal Membrane. J Korean Ophthalmol Soc. 6:989–994.
2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jung JJ, Hoang QV, Ridley-Lane ML, Sebrow
DB, Dhrami-Gavazi E and Chang S: Long-term retrospective analysis
of visual acuity and optical coherence Topographic changes after
single versus double peeling during vitrectomy for macular
epiretinal membranes. Retina. 36:2101–2109. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deák GG, Bolz M, Ritter M, Prager S,
Benesch T and Schmidt-Erfurth U: Diabetic Retinopathy Research
Group Vienna. A systematic correlation between morphology and
functional alterations in diabetic macular edema. Invest Ophthalmol
Vis Sci. 51:6710–6714. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Laban KG, Scheerlinck LM and van Leeuwen
R: Prognostic Factors Associated with Visual Outcome after Pars
Plana Vitrectomy with Internal Limiting Membrane Peeling for
Idiopathic Epiretinal Membrane. Ophthalmologica. 234:119–126.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Muto T, Nishimura T, Yamaguchi T, Chikuda
M and Machida S: Refractive changes after lens-sparing vitrectomy
for macular hole and epiretinal membrane. Clin Ophthalmol.
11:1527–1532. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kitagawa Y, Shimada H, Shinojima A and
Nakashizuka H: Foveal avascular zone area analysis using optical
coherence tomography angiography before and after idiopathic
epiretinal membrane surgery. Retina. 39:339–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim YJ, Kim S, Lee JY, Kim JG and Yoon YH:
Macular capillary plexuses after epiretinal membrane surgery: An
optical coherence tomography angiography study. Br J Ophthalmol.
102:1086–1091. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang S, Yao H, Li M, Li H and Wang F: Long
Non-Coding RNA MALAT1 Mediates Transforming Growth Factor
Beta1-Induced Epithelial-Mesenchymal Transition of Retinal Pigment
Epithelial Cells. PLoS One. 11(e0152687)2016.PubMed/NCBI View Article : Google Scholar
|