Introduction
Secondary hyperparathyroidism (SHPT) is one of the
most frequent complications in patients with chronic kidney disease
(CKD) (1). SHPT is characterized by
increased parathyroid hormone (PTH), which may cause vascular
calcification, soft tissue calcification and bone fracture
(2-4).
High levels of PTH are associated with an increased risk of
mortality (5-9).
The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines
recommended ‘calcitriol, vitamin D analogs, calcimimetics or a
combination of these drugs’ to reduce PTH levels (4).
Calcitriol is a classic treatment to control PTH
levels in patients with SHPT (10).
However, certain patients with refractory SHPT are characterized by
high levels of PTH, hypercalcaemia and hyperphosphatemia. High
doses of calcitriol increase the risk of hypercalcaemia and
hyperphosphatemia, which may increase mortality. Patients with SHPT
have the lowest risk of mortality when their serum calcium and
phosphorus levels are in the normal range (4). Recently, paricalcitol, a selective
vitamin D analogue, was demonstrated to only have a minor effect on
vitamin D receptors in the intestine and bone (11). Paricalcitol has been proved to be an
effective treatment to control PTH levels and reduce absorption of
calcium and phosphorus (12,13). In addition, cinacalcet, a kind of
calcimimetic, also provides effective control of PTH levels and has
the additional effect of reducing calcium and phosphate levels
(14-17).
Thus far, various studies have been performed to compare the
effects of PTH and calcium and phosphorus metabolism between the
two novel drugs; however, it has remained uncertain which is the
better drug. Therefore, the present meta-analysis was performed to
evaluate the effects of PTH on calcium and phosphorus metabolism
between the two drugs in patients with SHPT.
Materials and methods
Search strategy
The present meta-analysis was reported in line with
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (18). The
PubMed, Cochrane Library and Embase databases were searched for
entries from inception to June 1, 2019. The combined text and MeSH
terms included the following: ‘Secondary hyperparathyroidism’,
‘Paricalcitol’, ‘Cinacalcet’, ‘Vitamin D analogues’ and
‘Calcimimetics’. In addition, the cited papers and relevant
references were searched manually to identify eligible studies.
There were no language restrictions.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Randomized controlled trials (RCTs), case-control or cohort
studies; ii) Haemodialysis patients with SHPT, PTH levels >300
pg/ml (reference range, 150-300 pg/ml); iii) Comparison of outcomes
between paricalcitol and cinacalcet; and iv) Reported outcomes
include PTH, calcium and phosphorus levels. The exclusion criteria
were as follows: i) Case series, reviews, comments; ii) Patients
with parathyroidectomy or kidney transplantation; and iii) Lack of
relevant outcome data.
Data extraction and quality
assessment
Two investigators (XWX and LFG) retrieved and
independently selected all eligible records. Disagreements were
resolved by discussion with a third investigator (LJK). Details
including the first author's name, year of publication, location of
the study, study design, sample size, sex, mean age, follow-up
period, the dose of medication and treatment outcomes were
extracted. The Cochrane assessment tool was used to assess the
quality of RCTs (19) and the
Newcastle-Ottawa scale (NOS) was used to assess the quality of
non-randomized studies (20).
Statistical analysis
Data analysis was performed using Review Manager
version 5.3 (Cochrane Collaboration). Treatment outcomes were
summarized as odds ratios (OR) for categorical variables.
Continuous data of outcomes are presented as the mean difference
(MD). P<0.05 was considered to indicate statistical
significance. Heterogeneity was assessed via I2
statistics. I2>50% and P<0.10 were considered to
imply significant heterogeneity. Data with insignificant
heterogeneity were analyzed using the fixed-effects model. For
heterogeneous data, the random-effects model was used. Subgroup
analysis or sensitivity analysis was used to assess publication
bias.
Results
Study selection and
characteristics
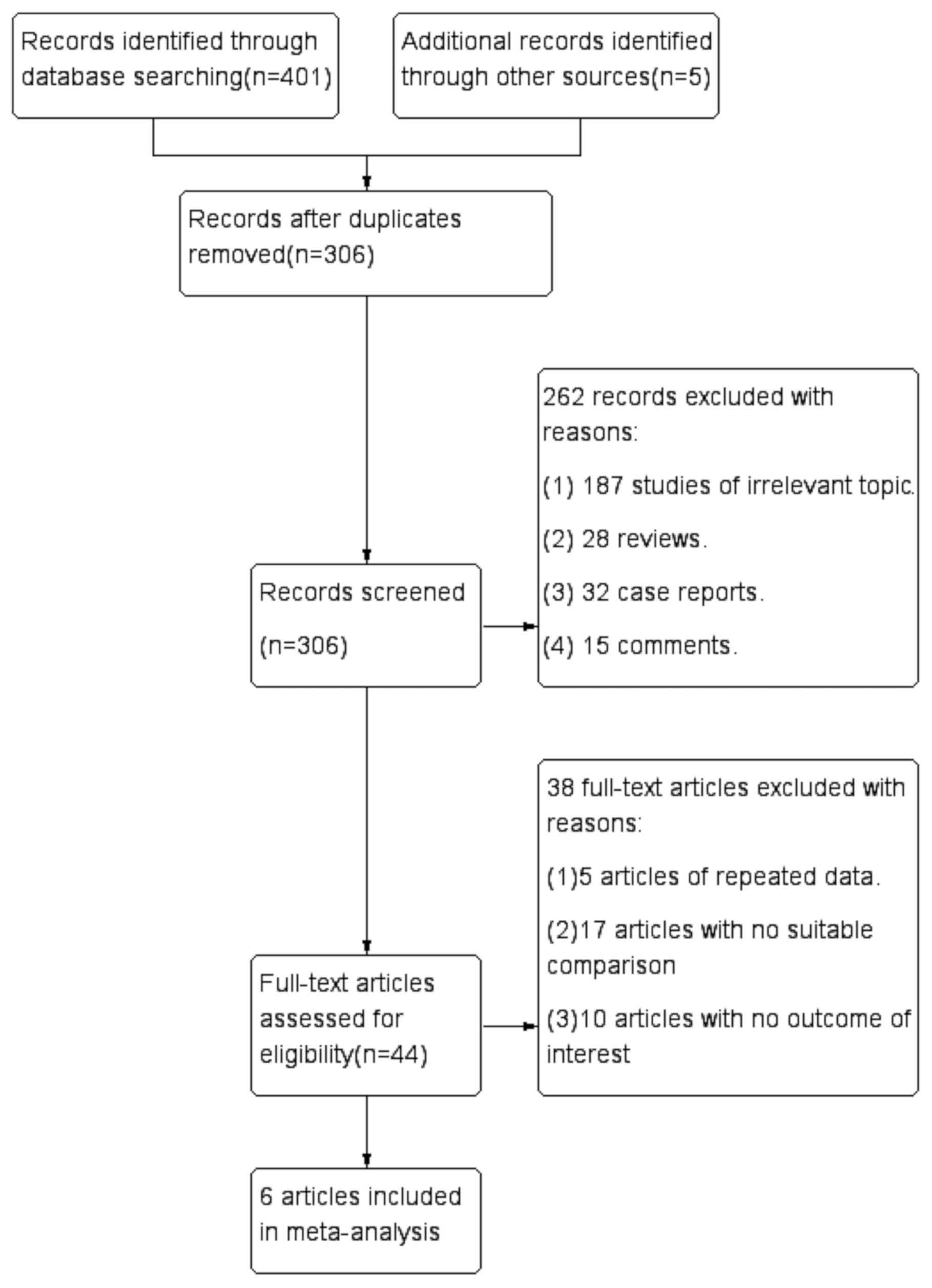
A total of 406 articles were initially selected.
After the exclusion of duplicated studies, 306 studies were
retained. Subsequently, 262 articles consisting of comments,
reviews, case reports and content unrelated to SHPT were removed by
analyzing the title and abstracts. A total of six studies (21-26)
were included in the final analysis after screening the full text
(Fig. 1). Of these six articles, one
from Ketteler et al (26)
involved two RCTs. The specific details of these two RCTs in the
article by Ketteler et al (26) are listed in Table I. In total, 456 patients were
included in the paricalcitol group and 412 patients were included
in the cinacalcet group. The shortest follow-up time among all
studies was 160 days and the longest was 12 months. The basic
characteristics of the six studies are listed in Table I. According to the NOS evaluation
criteria, the cohort studies scored an average of 6 points, with
medium quality (Table II). However,
the study by Kukavica et al (24) scored 5 points with low quality, where
the basic PTH level in the cinacalcet group (751.07±117.74) was
significantly lower compared with that in the paricalcitol group
(1040.31±79.56).
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| Author (year) | Country | Design | Follow-up period | Sample size | Mean age (years) | Males, n (%) | Creatinine
(mg/dl) | Calcium (mg/dl) | Phosphorus
(mg/dl) | PTH (pg/ml) | Dose of
medication | (Refs.) |
|---|
| Sharma (2014) | US | RCT | 28 wk | Paricalcitol: 51
Cinacalcet: 47 | 61.0±11.5
60.7±11.6 | 31 (60.8) 28
(59.6) | 8.1±2.3 9.0±2.5 | 9.0±0.5 9.1±0.4 | 4.8±1.0 5.0±1.0 | 516.6±147.9
524.3±149.7 | - | (21) |
| Zawierucha
(2019) | Poland | Cohort study | 52 wk | Paricalcitol (IV): 60
Cinacalcet (oral): 50 | 66 63 | 39 (65.0) 31
(62.0) | - | 8.5±0.9
7.1±3.2 | 5.1±1.4
5.7±1.5 | 1130 1271 | 6.76 µg/dialysis
0.6 mg/kg | (22) |
| Sprague (2015) | US | RCT | 12 m | Paricalcitol (IV):
157 Cinacalcet (oral): 155 | 55 53 | 95(61) 93(60) | - | 9.5±0.5
9.6±0.5 | 5.8±1.5 5.7
±1.6 | 815.7±427.9
845.7±431.3 | 21.4 ±1.5 µg/wk
85.6 ±5.4 mg/d | (23) |
| Kukavica
(2011) | Bosnia and
Herzegovina | Cohort study | 160 d | Paricalcitol: 41
Cinacalcet: 13 | 48.08±7.4 | - | - | - | - | 1040.31±79.56
751.07±117.74 | - | (24) |
| Kaperonis
(2012) | Greece | Cross-over
design | 6 m | Paricalcitol (IV):
13 Cinacalcet (oral): 13 | 57 | 11(84) | - | 9.3 9.4 | - | - | 13.2 µg/wk 40.4
mg/d | (25) |
| Ketteler
(2012) | 12 countries | RCT | 28 wk | Paricalcitol
(oral): 72 Cinacalcet (oral): 70 | 65.7±13.5
65.1±12.5 | 49 (68.1) 43
(61.4) | 8.9±2.6
8.4±2.6 | 9.0±0.6
9.0±0.7 | 4.9±1.1
4.4±1.1 | 494.8±170.3
509.5±138.5 | 3.5±3.5 µg/dialysis
31.8±28.7 mg/d | (26) |
| Ketteler
(2012) | 12 countries | RCT | 28 wk | Paricalcitol (IV):
62 Cinacalcet (IV): 64 | 61.2±12.7
59.9±12.0 | 38 (61.3) 38
(59.4) | 8.2±2.4
8.6±2.5 | 9.0±0.6
9.0±0.7 | 4.9±1.1
4.9±1.1 | 526.3±153.1
521.1±149.2 | 5.5±3.7 µg/dialysis
61.6±44.8 mg/d | (26) |
 | Table IIQuality assessment of cohort
studies. |
Table II
Quality assessment of cohort
studies.
| | Selection | Comparability | Outcome | |
|---|
| Author (year) | Representativeness
of the exposed group | Representativeness
of the non-exposed group | Ascertainment of
exposure | Demonstration that
outcome of interest was not present at start of study | Controls for the
most important factor | Controls for any
additional factors | Assessment of
outcome | Follow-up period
sufficient for the measurement of outcomes | Adequacy of
follow-up of cohorts | Score | (Refs.) |
|---|
| Zawierucha
(2019) | * | * | * | * | * | - | * | * | - | 7 | (22) |
| Kukavica
(2011) | * | - | - | * | * | - | * | * | - | 5 | (24) |
| Kaperonis
(2012) | * | * | - | * | * | - | * | * | - | 6 | (25) |
The risk of bias in the included RCTs were shown in
Table III. All RCTs were graded as
being of moderate quality. The method of random allocation was
mentioned in all RCTs. However, none of the RCTs elaborated on the
methods of random sequence generation, allocation concealment or
blinding. The completeness of the outcomes was unclear in the
studies by Sharma et al (21)
and Sprague et al (23).
 | Table IIIRisk of bias of randomized controlled
trialsa. |
Table III
Risk of bias of randomized controlled
trialsa.
| Author (year) | Random sequence
generation | Allocation
concealment | Blinding of
participants and personnel | Incomplete outcome
data | Selective
reporting | Other bias | (Refs.) |
|---|
| Sharma (2014) | ? | ? | ? | ? | + | ? | (21) |
| Sprague (2015) | ? | ? | ? | ? | + | ? | (23) |
| Ketteler
(2012) | ? | ? | ? | + | + | ? | (26) |
Meta-analysis results. PTH
Data regarding PTH levels were reported by six
trials included in five articles (22-26).
There was significant heterogeneity among the six trials
(P<0.10, I2=100%); therefore, the random-effects
model was used for the meta-analysis. There was no significant
difference between the paricalcitol and cinacalcet groups regarding
PTH levels (MD: 71.82, 95% CI: -185.20-328.85, P=0.58; Fig. 2).
A total of 3 trials included in two studies reported
data on the proportion of subjects with PTH levels of 150-300 pg/ml
at the end of follow-up (21,26). The
heterogeneity among these experiments was not significant (P=0.52,
I2=0%); therefore, the fixed-effects model was used for
the meta-analysis. The proportion of subjects treated with
paricalcitol who had PTH values of 150-300 pg/ml was significantly
greater (90/160, 56.3%) than the respective proportion in the
cinacalcet group (55/149, 37.0%). There was a statistically
significant difference between the two groups (OR: 2.19, 95% CI:
1.39-3.46, P<0.05; Fig. 3).
Calcium levels
Data regarding calcium levels were reported in five
trials included in four articles (22,23,25,26).
There was significant heterogeneity among these experiments
(P=0.02, I2=66%); therefore, the random-effects model
was ultimately used for the meta-analysis. The calcium levels of
the paricalcitol groups were higher than those of the cinacalcet
groups, and the difference was statistically significant (MD: 1.10,
95% CI: 0.92-1.28, P<0.05; Fig.
4).
Phosphorus levels
Data regarding phosphorus levels were reported in
five trials included in four studies (22-24,26).
There was significant heterogeneity among these studies (P<0.10,
I2=98%); therefore, the random-effects model was used
for the meta-analysis. There was no significant difference between
the groups regarding phosphorus levels (SMD: 0.59, 95% CI
-0.82-2.00, P=0.41; Fig. 5).
Sensitivity analyses and publication
bias
To evaluate the robustness of the estimated pooled
effect size for PTH, calcium, phosphorus levels and the rate of
reaching the PTH standard, a sensitivity analysis was performed by
sequentially deleting one study at a time and redetermining the
pooled effect size of the remaining studies. The results revealed
that none of the individual studies affected the overall results on
PTH, calcium, phosphorus levels and the rate of reaching the PTH
standard, which suggested that the pooled effects on PTH, calcium
and phosphorus levels were stable (Tables
SI-SIV).
Discussion
Vitamin D supplementation is the traditional
strategy for SHPT management. However, the application of vitamin D
is limited in certain patients due to hypercalcemia,
hyperphosphatemia and high levels of PTH (27,28). As
a novel selective vitamin D receptor agonist, paricalcitol is able
to effectively inhibit PTH synthesis and parathyroid hyperplasia,
but its effect on the intestine and bone is only 1/10 of that of
calcitriol (29). Thus, paricalcitol
is advantageous in reducing PTH levels and lowering the risk of
hypercalcemia and hyperphosphataemia. Furthermore, as a
calcimimetic, cinacalcet activates calcium-sensitive receptors of
the parathyroid (30). Certain
studies have suggested that cinacalcet is able to significantly
inhibit PTH secretion so as to reduce the number of
parathyroidectomy operations (31,32).
With the application of cinacalcet, the present analysis indicated
that there was a significant decreasing trend of serum calcium. The
present systematic review aimed to appraise the effects on PTH and
calcium and phosphorus metabolism between the two novel drugs for
SHPT.
The studies included in the present meta-analysis
indicated that paricalcitol and cinacalcet were both beneficial in
decreasing the PTH levels and the efficacy was not significantly
different. The Kidney Disease Outcomes Quality Initiative
recommends a PTH target of 150-300 pg/ml (33), while the KDIGO guidelines suggest
maintaining PTH levels in the range of 130-600 pg/ml (2-9X the
upper limit of normal) (4). The
present meta-analysis indicated that paricalcitol was more
effective than cinacalcet in achieving the target level of PTH
(150-300 pg/ml). Of note, the studies of Kukavica et al
(24) and Kaperonis et al
(25) reported a significant
advantage of cinacalcet treatment in decreasing the PTH levels
compared with that of paricalcitol treatment, but they did not
report any data on the proportion of subjects with PTH levels of
150-300 pg/ml. In addition, certain studies included suggested that
when the average baseline PTH levels were >800 pg/ml, both drugs
were able to decrease the PTH levels but did not decrease the
average PTH levels to 2-9X the upper limit of normal (4).
The present meta-analysis indicated that cinacalcet
significantly decreased the serum calcium levels compared with
paricalcitol. The KDIGO guidelines recommend avoiding hypercalcemia
(4). In all studies included, the
average serum calcium levels were still in the ideal range (8.4-10
mg/dl) (4) after paricalcitol
treatment. Only one study included reported on the incidence of
hypercalcaemia, which was significantly higher in the paricalcitol
group (12.7%) than in the cinacalcet group (0.7%). Although the
effect of paricalcitol on the intestine and bone is only 1/10 of
that of calcitriol, paricalcitol treatment still poses a risk of
hypercalcemia, which is proportional to the dosage of paricalcitol
(29). In three of the included
trials (22,26), the average serum calcium levels were
below the ideal range after cinacalcet treatment. Only one of the
articles included reported on the incidence of hypocalcemia, which
was significantly higher in the cinacalcet group (20.1%) than in
the paricalcitol group (0%). The reason is that cinacalcet mimics
the effect of Ca2+ on parathyroid cells so that it
reduces PTH and serum calcium (17,30).
However, cinacalcet combined with vitamin D reduces the incidence
of hypocalcemia (34).
The present results revealed that the serum
phosphate levels were relatively higher in the paricalcitol groups
than in the cinacalcet groups but there was no significant
difference. There is an increasing risk of mortality with
increasing levels of serum phosphate (4). In certain studies included, the average
serum phosphate levels of paricalcitol or cinacalcet were above the
upper limit of normal at the end of follow-up. In other words, both
drugs are associated with the risk of causing
hyperphosphataemia.
Only one of the studies included reported on the
incidence of adverse events, which were all higher in the
cinacalcet group (26). Therefore,
only a descriptive analysis was performed in the present study. The
incidence rates in the cinacalcet group were as follows: Nausea
(6.7%), vomiting (4.5%), constipation (3.0%) and muscle spasms
(2.2%). The incidence rates in the paricalcitol group were as
follows: Nausea (0%), vomiting (1.5%), constipation (0%) and muscle
spasms (0%). In addition, only one included article from the US
reported on the cost of treatment (26). The study revealed that paricalcitol
was more cost-effective than cinacalcet and that paricalcitol was
simultaneously more effective in achieving the target levels of
PTH. In a word, paricalcitol appeared to have an advantage in terms
of adverse events and cost.
There were certain limitations to the present
meta-analysis. First, only one study compared paricalcitol and
cinacalcet in terms of hypocalcemia, hypercalcemia,
hyperphosphataemia, adverse events and cost; thus, it was not
possible to perform a meta-analysis for these points. Furthermore,
additional studies reporting on the proportion of subjects with PTH
levels in the target range after treatment with these two drugs are
required.
In conclusion, the present meta-analysis revealed
that paricalcitol and cinacalcet were effective for decreasing PTH
levels. There was no difference between the two novel drugs
concerning the management of PTH and phosphorus levels. Cinacalcet
significantly reduced the serum levels of calcium. To further
confirm these conclusions, further large multicenter RCTs comparing
these two drug treatments are necessary.
Supplementary Material
Sensitivity analyses of parathyroid
hormone levels.
Sensitivity analyses of the proportion
of subjects with parathyroid hormone levels 150-300 pg/ml.
Sensitivity analyses of calcium
levels.
Sensitivity analyses of phosphorus
levels.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX and WGT contributed to this work by designing the
study, reviewing literature, interpreting the analyses and writing
the manuscript. LFG and JKL contributed to the literature review,
data collection and analysis, and writing the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies EW, Matza LS, Worth G, Feeny DH,
Kostelec J, Soroka S, Mendelssohn D, McFarlane P and Belozeroff V:
Health state utilities associated with major clinical events in the
context of secondary hyperparathyroidism and chronic kidney disease
requiring dialysis. Health Qual Life Outcomes.
13(90)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lewis R: Mineral and bone disorders in
chronic kidney disease: New insights into mechanism and management.
Ann Clin Biochem. 49:432–440. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wei Y, Lin J, Yang F, Li X, Hou Y, Lu R,
Shi X, Liu Z and Du Y: Risk factors associated with secondary
hyperparathyroidism in patients with chronic kidney disease. Exp
Ther Med. 12:1206–1212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Isakova T, Nickolas TL, Denburg M,
Yarlagadda S, Weiner DE, Gutiérrez OM, Bansal V, Rosas SE, Nigwekar
S, Yee J, et al: KDOQI US commentary on the 2017 KDIGO clinical
practice guideline update for the diagnosis, evaluation,
prevention, and treatment of chronic kidney disease-mineral and
bone disorder (CKD-MBD). Am J Kidney Dis. 70:737–751.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goettsch C, Iwata H and Aikawa E:
Parathyroid hormone: Critical brigde between bone metabolism and
cardiovascular disease. Arterioscler Thromb Vasc Biol.
34:1333–1335. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grams ME and Coresh J: Assessing risk in
chronic kidney disease: A methodological review. Nat Rev Nephrol.
9:18–25. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ye H, Ye P, Zhang Z, Hou A, Liang Z and
Kong Y: A bayesian network analysis on comparative efficacy of
treatment strategies for dialysis patients with secondary
hyperparathyroidism. Exp Ther Med. 17:531–540. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Naves-Díaz M, Passlick-Deetjen J,
Guinsburg A, Marelli C, Fernández-Martín JL, Rodríguez-Puyol D and
Cannata-Andía JB: Calcium, phosphorus, PTH and death rates in a
large sample of dialysis patients from Latin America. The CORES
Study. Nephrol Dial Transplant. 26:1938–1947. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Komaba H, Taniguchi M, Wada A, Iseki K,
Tsubakihara Y and Fukagawa M: Parathyroidectomy and survival among
Japanese hemodialysis patients with secondary hyperparathyroidism.
Kidney Int. 88:350–359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ifudu O: Care of patients undergoing
hemodialysis. N Engl J Med. 339:1054–1062. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Martin KJ, González EA, Gellens M, Hamm
LL, Abboud H and Lindberg J: 19-Nor-1-α-25-dihydroxyvitamin D2
(paricalcitol) safely and effectively reduces the levels of intact
parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol.
9:1427–1432. 1998.PubMed/NCBI
|
|
12
|
Ross EA, Tian J, Abboud H, Hippensteel R,
Melnick JZ, Pradhan RS, Williams LA, Hamm LL and Sprague SM: Oral
paricalcitol for the treatment of secondary hyperparathyroidism in
patients on hemodialysis or peritoneal dialysis. Am J Nephrol.
28:97–106. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mittman N, Desiraju B, Meyer KB,
Chattopadhyay J and Avram MM: Treatment of secondary
hyperparathyroidism in ESRD: A 2 -year-single-center cross-over
study. Kidney Int. (Suppl 8):S33–S36. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Block GA, Martin KJ, de Francisco AL,
Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa
AK, Messa P, et al: Cinacalcet for secondary hyperparathyroidism in
patients receiving hemodialysis. N Engl J Med. 350:1516–1525.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Block GA, Zeig S, Sugihara J, Chertow GM,
Chi EM, Turner SA and Bushinsky DA: TARGET Investigators. Combined
therapy with cinacalcet and low doses of vitamin D sterols in
patients with moderate to severe secondary hyperparathyroidism.
Nephrol Dial Transplant. 23:2311–2318. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fishbane S, Shapiro WB, Corry DB, Vicks
SL, Roppolo M, Rappaport K, Ling X, Goodman WG, Turner S and
Charytan C: Cinacalcet HCl and concurrent low-dose vitamin D
improves treatment of secondary hyperparathyroidism in dialysis
patients compared with vitamin D alone, the ACHIEVE study results.
Clin J Am Soc Nephrol. 3:1718–1725. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Block GA, Zaun D, Smits G, Persky M,
Brillhart S, Nieman K, Liu J and St Peter WL: Cinacalcet
hydrochloride treatment significantly improves all-cause and
cardiovascular survival in a large cohort of hemodialysis patients.
Kidney Int. 78:578–589. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ.
339(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions. Cochrane Book Series,
2008.
|
|
20
|
Wells GA, Shea B, O'Connell D, Petersen J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale(NOS) for
assessing the quality of nonrandomized studies in meta-analyses.
Ottawa Hospital Research Institute, Ottawa, 2015. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
|
|
21
|
Sharma A, Marshall TS, Khan SS and Johns
B: Cost effectiveness of paricalcitol versus cinacalcet with
low-dose vitamin D for management of secondary hyperparathyroidism
in haemodialysis patients in the USA. Clin Drug Investig.
34:107–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zawierucha J, Malyszko J, Malyszko JS,
Prystacki T, Marcinkowski WP and Dryl-Rydzynska T: Three
therapeutic strategies: Cinacalcet, paricalcitol or both in
secondary hyperparathyroidism treatment in hemodialysed patients
during 1-year observational study-A comparison. Front Endocrinol
(Lausanne). 10(40)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sprague SM, Wetmore JB, Gurevich K, Da
Roza G, Buerkert J, Reiner M, Goodman W and Cooper K: Effect of
cinacalcet and vitamin d analogs on fibroblast growth factor-23
during the treatment of secondary hyperparathyroidism. Clin J Am
Society Nephrol. 10:1021–1030. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kukavica N, Resic H, Ajanović S, Groša E,
Prohić N, Ćorić A and Mašnić F: Comparative effectiveness of
cinacalcet (sensipar/mimpara) versus paracalcitol in treatment of
secondary hyperparathyroidism in patients on hemodialysis. Acta Med
Croatica. 65:128–129. 2011.
|
|
25
|
Kaperonis N, Kourvelou C, Sgantzos1 A,
Nastou1 D, Ntatsis G, Ziakka S, Karakasis F, Nikolopoulos V,
Zoubaniotou V, Koutsovasili A, et al: Cinacalcet vs. paricalcitol
in hemodialysis patients. Nephrol Dial Transplant. 27:497–498.
2012.
|
|
26
|
Ketteler M, Martin KJ, Wolf M, Amdahl M,
Cozzolino M, Goldsmith D, Sharma A, Marx S and Khan S: Paricalcitol
versus cinacalcet plus low-dose vitamin D therapy for the treatment
of secondary hyperparathyroidism in patients receiving
haemodialysis: Results of the IMPACT SHPT study. Nephrol Dial
Transplant. 27:3270–3278. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Andress D: Nonclassical aspects of
differential vitamin D receptor activation, implications for
survival in patients with chronic kidney disease. Drugs.
67:1999–2012. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dusilová-Sulková S: Vitamin D metabolism
and vitamin D traditional and nontraditional, target organs,
implications for kidney patients. J Ren Care. 35:39–44.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matuszkiewicz-Rowińska J and Żebrowski P:
Paricalcitol-a selective vitamin D receptor activator for secondary
hyperparathy-roidism in patients with chronic kidney disease. Wiad
Lek. 69:756–759. 2016.PubMed/NCBI
|
|
30
|
Zawierucha J, Małyszko J, Małyszko J,
Prystacki T, Marcinkowski W and Dryl-Rydzyńska T: Treatment of
secondary hyperparathyroidism in hemodialysed patients-paricalcitol
with or without cinacalcet. Przegl Lek. 73:229–232. 2016.PubMed/NCBI(In Polish).
|
|
31
|
Soliman AR, Maamoun HA, Soliman MA,
Darwish H and Elbanna E: Cinacalcet versus parathyroidectomy in the
treatment of secondary hyperparathyroidism post renal
transplantation. Rom J Intern Med. 54:184–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Block GA, Bushinsky DA, Cheng S,
Cunningham J, Dehmel B, Drueke TB, Ketteler M, Kewalramani R,
Martin KJ, Moe SM, et al: Effect of etelcalcetide vs. cinacalcet on
serum parathyroid hormone in patients receiving hemodialysis with
secondary hyperparathyroidism: A randomized clinical trial. JAMA.
317:156–164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
National Kidney Foundation. K/DOQI
clinical practice guidelines for bone metabolism and disease in
chronic kidney disease. Am J Kidney Dis. 42 (4 Suppl 3):S1–S201.
2003.PubMed/NCBI
|
|
34
|
Ureña-Torres P, Bridges I, Christiano C,
Cournoyer SH, Cooper K, Farouk M, Kopyt NP, Rodriguez M, Zehnder D
and Covic A: Efficacy of cinacalcet with low-dose vitamin D in
incident haemodialysis subjects with secondary hyperparathyroidism.
Nephrol Dial Transplant. 28:1241–1254. 2013.PubMed/NCBI View Article : Google Scholar
|