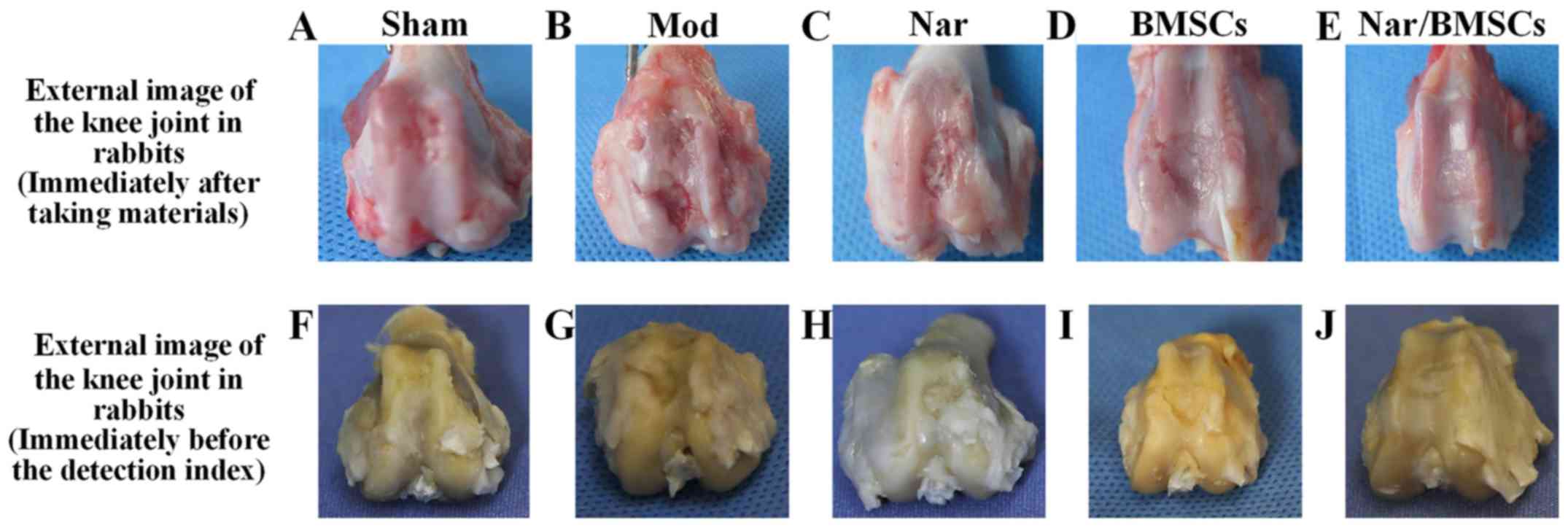
|
1
|
Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY,
Wang Y and Peng J: Basic science and clinical application of
platelet-rich plasma for cartilage defects and osteoarthritis: A
review. Osteoarthritis Cartilage. 21:1627–1637. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Browne JE and Branch TP: Surgical
alternatives for the treatment of articular cartilage lesions. J Am
Acad Orthop Sur. 8:180–189. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Katagiri H, Mendes LF and Luyten FP:
Definition of a critical size osteochondral knee defect and its
negative effect on the surrounding articular cartilage in the rat.
Osteoarthritis Cartilage. 25:1531–1540. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang AT, Feng Y, Jia HH, Zhao M and Yu H:
Application of mesenchymal stem cell therapy for the treatment of
osteoarthritis of the knee: A concise review. World J Stem Cells.
11:14–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ebihara G, Sato M, Yamato M, Mitani G,
Kutsuna T, Nagai T, Ito S, Ukai T, Kobayashi M, Kokubo M, et al:
Cartilage repair in transplanted scaffold-free chondrocyte sheets
using a minipig model. Biomaterials. 33:3846–3851. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Armiento AR, Alini M and Stoddart MJ:
Articular fibrocartilage-Why does hyaline cartilage fail to repair?
Adv Drug Deliv Rev. 146:289–305. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bae DK, Yoon KH and Sang JS: Cartilage
healing after microfracture in osteoarthritic knees. Arthroscopy.
22:367–374. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Yuan M, Guo QY, Lu SB and Peng J:
Mesenchymal stem cells for treating articular cartilage defects and
osteoarthritis. Cell Transplant. 24:1661–1678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Che CT, Wong MS and Lam CW: Natural
products from chinese medicines with potential benefits to bone
health. Molecules. 21(239)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Su YX, Yan H, Chen BJ, Zahn Q, Wang YR, Lu
ML, Wang WT, He Z and Sheng L: Effect of naringin of Drynaria
Rhizome, a Chinese medical component of Zhuanggu Jianxi recipe
containing serum on Caveolin-p38MAPK signal pathway in IL-1β
induced rabbit degenerated chondrocytes. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 34:1492–1498. 2014.PubMed/NCBI(In Chinese).
|
|
12
|
Lo PC, Lin FC, Tsai YC and Lin SK:
Traditional Chinese medicine therapy reduces the risk of total knee
replacement in patients with knee osteoarthritis. Medicine
(Baltimore). 98(e15964)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Teixeira J, Santos MJ, Matos LC and
Machado JP: Evaluation of the effectiveness of acupuncture in the
treatment of knee osteoarthritis: A case study. Medicines (Basel).
5(18)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
An J, Hao D, Zhang Q, Chen B, Zhang R,
Wang Y and Yang H: Natural products for treatment of bone erosive
diseases: The effects and mechanisms on inhibiting
osteoclastogenesis and bone resorption. Int Immunopharmacol.
36:118–131. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of Naringin: An overview. Pharm Biol.
54:3203–3210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Farrell MJ, Shin JI, Smith LJ and Mauck
RL: Functional consequences of glucose and oxygen deprivation on
engineered mesenchymal stem cell-based cartilage constructs.
Osteoarthritis Cartilage. 23:134–142. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wolf D and Wolf AM: Mesenchymal stem cells
as cellular immunosuppressants. Lancet. 371:1553–1554.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Santo VE, Gomes ME, Mano JF and Reis RL:
Controlled release strategies for bone, cartilage, and
osteochondral engineering-Part II: Challenges on the evolution from
single to multiple bioactive factor delivery. Tissue Eng Part B
Rev. 19:327–352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cha BH, Kim JH, Kang SW, Do HJ, Jang JW,
Choi YR, Park H, Kim BS and Lee SH: Cartilage tissue formation from
dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9
genes encoding bicistronic vector. Cell Transplant. 22:1519–1528.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paul R, Haydon RC, Cheng H, Ishikawa A,
Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, et al:
Potential use of Sox9 gene therapy for intervertebral degenerative
disc disease. Spine (Phila Pa 1976). 28:755–763. 2003.PubMed/NCBI
|
|
21
|
Tew SR, Pothacharoen P, Katopodi T and
Hardingham TE: SOX9 transduction increases chondroitin sulfate
synthesis in cultured human articular chondrocytes without altering
glycosyltransferase and sulfotransferase transcription. Biochem J.
414:231–236. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li PY, Li CG, Ye C, Qu Y, Chen J, Zhu GQ
and Zhao H: Experimental study on Naringin decoction combined with
bone marrow mesenchymal stromal stem cells in repair of articular
cartilage defects in rabbits. J Liaoning Univ Tradit Chinese Med.
16:34–38. 2014.
|
|
23
|
Li CG, Qu Y, Ye C, Chen J, Wang FX, Li PY,
Li SH, Ren JP and Qi J: Histology research on repairing of rabbit
articular cartilage defects with Naringin and tissue engineering
cartilage. Chin J Tissue Eng Res. 18:3165–3171. 2014.
|
|
24
|
Cai YT, Liu TS, Fang F, Xiong CL and Shen
SL: Comparisons of mouse mesenchymal stem cells in primary adherent
culture of compact bone fragments and whole bone marrow. Stem Cells
Int. 2015(708906)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Parodi AL: Ethical issue in animal
experimentation. Bull Acad Natl Med. 193:1737–1745. 2009.PubMed/NCBI(In French).
|
|
26
|
Wei W, Wu XM and Li YJ: Pharmacological
Experimental Methodology. 4th edition. People's Medical Publishing
House, pp69-73, 2010.
|
|
27
|
Gao XM: Science of Chinese Materia Medica.
2nd edition. China Press of Traditional Chinese Medicine,
pp335-336, 2007.
|
|
28
|
Smith GD, Taylor J, Almqvist KF, Erggelet
C, Knutsen G, Garcia Portabella M, Smith T and Richardson JB:
Arthroscopic assessment of cartilage repair: A validation study of
2 scoring systems. Arthroscopy. 21:1462–1467. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van den Borne MP, Raijmakers NJ, Vanlauwe
J, Victor J, de Jong SN, Bellemans J and Saris DB: International
Cartilage Repair Society. International Cartilage Repair Society
(ICRS) and Oswestry macroscopic cartilage evaluation scores
validated for use in Autologous Chondrocyte Implantation (ACI) and
microfracture. Osteoarthritis Cartilage. 15:1397–1402.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mainil-Varlet P, Aigner T, Brittberg M,
Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker
K, Roberts S, et al: Histological assessment of cartilage repair: A
report by the histology endpoint committee of the international
car-tilage repair society (ICRS). J Bone Joint Surg Am. 85A (Suppl
2):S45–S57. 2003.PubMed/NCBI
|
|
31
|
O'Driscoll SW, Keeley FW and Salter RB:
The chondrogenic potential of free autogenous periostal grafts for
biological resurfacing of major full-thickness defects in joint
surfaces under the influence of continuous passive motion. An
experimental investigation in the rabbit. J Bone Joint Surg Am.
68:1017–1035. 1986.PubMed/NCBI
|
|
32
|
Coricor G and Serra R: TGF-β regulates
phosphorylation and stabilization of Sox9 protein in chondrocytes
through p38 and Smad dependent mechanisms. Sci Rep.
6(38616)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ni Q, Lu K, Li J, Tan Y, Qin J, Magdalou
J, Chen L and Wang H: Role of TGFβ signaling in maternal
ethanol-induced fetal articular cartilage dysplasia and adult onset
of osteoarthritis in male rats. Toxicol Sci. 164:179–190.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Johnson K, Zhu S, Tremblay MS, Payette JN,
Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X and Schultz
PG: A stem cell-based approach to cartilage repair. Science.
336:717–721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bekkers JE, Creemers LB, Tsuchida AI, van
Rijen MH, Custers RJ, Dhert WJ and Saris DB: One-stage focal
cartilage defect treatment with bone marrow mononuclear cells and
chondrocytes leads to better macroscopic cartilage regeneration
compared to microfracture in goats. Osteoarthritis Cartilage.
21:950–956. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Caron MM, Emans PJ, Coolsen MM, Voss L,
Surtel DA, Cremers A, van Rhijn LW and Welting TJ:
Redifferentiation of dedifferentiated human articular chondrocytes:
Comparison of 2D and 3D cultures. Osteoarthritis Cartilage.
20:1170–1178. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu F, Zeng H, Yu HY, Lei M, Yuan H and
Xiao DM: Comparison of different special staining techniques of
chondrocytes and their application values. Chinese J Comp Med.
8:58–61. 2015.
|
|
38
|
Yu F, Lei M, Zeng H, Cao JF and Xiao DM:
Different special staining methods on cartilage tissue of
osteoarthritis morphology: A comparison study. Orthop J China.
19:79–85. 2015.
|
|
39
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2010.
|
|
40
|
Kock L, Donkelaar CCV and Ito K: Tissue
engineering of functional articular cartilage: The current status.
Cell Tissue Res. 347:613–627. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Caplan AI: Review: Mesenchymal stem cells:
Cell-based reconstructive therapy in orthopedics. Tissue Eng.
11:1198–1211. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Anraku Y, Mizuta H, Sei A, Kudo S,
Nakamura E, Senba K, Takagi K and Hiraki Y: The chondrogenic repair
response of undifferentiated mesenchymal cells in rat
full-thickness articular cartilage defects. Osteoarthritis
Cartilage. 16:961–964. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kurth TB, Dell'Accio F, Crouch V, Augello
A, Sharpe PT and De Bari C: Functional mesenchymal stem cell niches
in adult mouse knee joint synovium in vivo. Arthritis Rheumatol.
63:1289–1300. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Manna K, Das U, Das D, Kesh SB, Khan A,
Chakraborty A and Dey S: Naringin inhibits gamma radiation-induced
oxidative DNA damage and inflammation, by modulating p53 and NF-κB
signaling pathways in murine splenocytes. Free Radical Res.
49:422–439. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao Y, Zhong L, Wang W, Zhang H, Chen J,
Su P, Liu L and Li W: Naringin protects against cartilage
destruction in osteoarthritis through repression of NF-κB signaling
pathway. Inflammation. 39:385–392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xu Q, Zhang ZF and Sun WX: Effect of
naringin on monosodium iodoacetate-induced osteoarthritis pain in
rats. Med Sci Monitor. 23:3746–3751. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cao X, Lin W, Liang C, Zhang D, Yang F,
Zhang Y, Zhang X, Feng J and Chen C: Naringin rescued the
TNF-α-induced inhibition of osteogenesis of bone marrow-derived
mesenchymal stem cells by depressing the activation of NF-кB
signaling pathway. Immunol Res. 62:357–367. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kawaguchi K, Maruyama H, Hasunuma R and
Kumazawa Y: Suppression of inflammatory responses after onset of
collagen-induced arthritis in mice by oral administration of the
Citrus flavanone naringin. Immunopharm Immunot. 33:723–729.
2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ahmad SF, Zoheir KM, Abdelhamied HE,
Ashour AE, Bakheet SA, Attia SM and Abd-Allah AR: Amelioration of
autoimmune arthritis by Naringin through modulation of T regulatory
cells and Th1/Th2 cytokines. Cell Immunol. 287:112–120.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ashraf S, Cha BH, Kim JS, Ahn J, Han I,
Park H and Lee SH: Regulation of senescence associated signaling
mechanisms in chondrocytes for cartilage tissue regeneration.
Osteoarthritis Cartilage. 24:196–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Feng XH and Derynck R: Specificity and
versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev
Bi. 21:659–693. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dong R, Ying J, Xu TT, Hu SF, Zhang P, Xia
CJ, Fang L, Jin HT and Wang PG: Bushenhuoxue formula facilitates
articular cartilage repair and attenuates matrix degradation by
activation of TGF-β signaling pathway. Evid Based Complement
Alternat Med. 2018(2734581)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Patil AS, Sable RB and Kothari RM: An
update on transforming growth factor-β (TGF-β): Sources, types,
functions and clinical applicability for cartilage/bone healing. J
Cell Physiol. 226:3094–3103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Grimaud E, Heymann D and Rédini F: Recent
advances in TGF-beta effects on chondrocyte metabolism. Potential
therapeutic roles of TGF-beta in cartilage disorders. Cytokine
Growth Factor Rev. 13:241–257. 2002.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Blaney Davidson EN, van der Kraan PM and
van den Berg WB: TGF-beta and osteoarthritis. Osteoarthritis
Cartilage. 15:597–604. 2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li YP, Wei XC, Zhou JM and Wei L: The
age-related changes in cartilage and osteoarthritis. Biomed Res
Int. 2013(916530)2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mackay AM, Beck SC, Murphy JM, Barry FP,
Chichester CO and Pittenger MF: Chondrogenic differentiation of
cultured human mesenchymal stem cells from marrow. Tissue Eng.
4:415–428. 1998.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lu CH, Lin KJ, Chiu HY, Chen CY, Yen TC,
Hwang SM, Chang YH and Hu YC: Improved chondrogenesis and
engineered cartilage formation from TGF-β3-expressing
adipose-derived stem cells cultured in the rotating-shaft
bioreactor. Tissue Eng Part A. 18:2114–2124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Furumatsu T, Tsuda M, Taniguchi N, Tajima
Y and Asahara H: Smad3 induces chondrogenesis through the
activation of SOX9 via CREB-binding protein/p300 recruitment. J
Biol Chem. 280:8343–8350. 2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sha'ban M, Cassim SO, Yahya NM, Saim A and
Idrus R: Sox-9 Transient transfection enhances chondrogenic
expression of osteoarthritic human articular chondrocytes in vitro:
Preliminary analysis. J Tissue Eng Regen. 8:32–41. 2011.
|
|
61
|
Liu CF and Lefebvre V: The transcription
factors SOX9 and SOX5/SOX6 cooperate genome-wide through
super-enhancers to drive chondrogenesis. Nucleic Acids Res.
43:8183–8203. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kawakami Y, Rodriguez-León J and Izpisúa
Belmonte JC: The role of TGFbetas and Sox9 during limb
chondrogenesis. Curr Opin Cell Biol. 18:723–729. 2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lefebvr V, Huang W, Harley VR, Goodfellow
PN and De CB: SOX9 is a potent activator of the
chondrocyte-specific enhancer of the pro alpha1(II) collagen gene.
Mol Cell Biol. 17:2336–2346. 1997.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang Z, Li K, Sun H, Wang J, Fu Z and Liu
M: Icariin promotes stable chondrogenic differentiation of bone
marrow mesenchymal stem cells in self-assembling peptide nanofiber
hydrogel scaffolds. Mol Med Rep. 17:8237–8243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yoon BS, Ovchinnikov DA, Yoshii I, Mishina
Y, Behringer RR and Lyons KM: Bmpr1a and Bmpr1b have overlapping
functions and are essential for chondrogenesis in vivo. Proc Natl
Acad Sci USA. 102:5062–5067. 2005.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lefebvre V and Dvir-Ginzberg M: SOX9 and
the many facets of its regulation in the chondrocyte lineage.
Connect Tissue Res. 58:2–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lee YE, Liu HC, Lin YL, Liu SH, Yang RS
and Chen RM: Drynaria fortune J. Sm. improves the bone mass
of ovariectomized rats through osteocalcin-involved endochondral
ossification. J Ethnopharmacol. 158:94–101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hunziker EB, Lippuner K, Keel MJ and
Shintani N: An educational review of cartilage repair: Precepts and
practice - myths and misconceptions - progress and prospects.
Osteoarthritis Cartilage. 23:334–350. 2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Huey DJ, Hu JC and Athanasiou KA: Unlike
bone, cartilage regeneration remains elusive. Science. 338:917–921.
2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hara ES, Ono M, Hai PT, Sonoyama W, Kubota
S, Takigawa M, Matsumoto T, Young MF, Olsen BR and Kuboki T:
Fluocinolone acetonide is a potent synergistic factor of
TGF-β3-associated chondrogenesis of bone marrow-derived mesenchymal
stem cells for articular surface regeneration. J Bone Miner Res.
30:1585–1596. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Tew SR, Li Y, Pothacharoen P, Tweats LM,
Hawkins RE and Hardingham TE: Retroviral transduction with SOX9
enhances re-expression of the chondrocyte phenotype in passaged
osteoarthritic human articular chondrocytes. Osteoarthritis
Cartilage. 13:80–89. 2005.PubMed/NCBI View Article : Google Scholar
|