Introduction
Carcinoid tumors are part of the neuroendocrine
tumors APUD (Amine Precursor Uptake and Decarboxylation)
originating in the Kulchitsky cells (agent affine cells). Pulmonary
carcinoid tumors are part of bronchopulmonary neuroendocrine
neoplasms (bpNETs) along with large and small cell pulmonary
carcinoma (1). The first resection
of this type of tumor was performed by Eloesser (2) through bronchotomy in 1939. The
clinical syndrome associated with bronchial carcinoid tumors was
first described by Stanford et al (3) in 1958. Arrigoni et al (4) described the atypical carcinoid as a
histopathological form of bronchial carcinoid. Being a
neuroendocrine tumor the bronchial carcinoid is capable of
producing a variety of peptides and biological active hormones
(5) thus leading to several
endocrine syndromes.
Carcinoid syndrome is determined by secretion of
serotonin along with histamine and bombesin. It can present with
bronchoconstriction, flush, hemodynamic instability, tachycardia
and accelerated digestive transit (6). Cushing syndrome is the second syndrome
caused by carcinoid tumors and is determined by the ectopic
production of ACTH (adrenocorticotropic hormone) (7). Another syndrome associated with
bronchial carcinoid tumors is that of an inadequate secretion of
AVP (arginine vasopressin) which may lead to sodium depletion and
water retention. Inadequate secretion of ADH (syndrome of
inappropriate antidiuretic hormone secretion) or inadequate
secretion of MSH (melanocyte stimulating hormone) is two other
examples of endocrine syndromes which occur in bronchial carcinoid
tumors. Bronchial carcinoid tumors make up 10% of all carcinoid
tumors and represent 4% of pulmonary tumors (8).
From a histological view there are two types of
carcinoids: Typical and atypical carcinoids. Typical carcinoids are
well defined and differentiated, less aggressive tumors, which are
commonly located in a larger bronchus. Atypical carcinoids are more
aggressive, less differentiated lesions, having a tendency to
metastasize at the level of the local lymph nodes, liver, bones or
brain. Atypical carcinoids are more common in elderly patients and
most often located in the periphery of the lung. Staging of the
bronchial carcinoid is done in the same manner as bronchopulmonary
cancer using the TNM (tumor, node, metastasis) system. When it
comes to typical carcinoids, which are considered less aggressive
lesions, a clinical staging is used, the TNM system being only
utilized in atypical pulmonary carcinoids.
The present study was approved by the Thoracic
Surgery Clinic of the ‘Marius Nasta’ Institute of Pneumophtisiology
(Bucharest, Romania). The aim of this study was to determine the
frequency of bronchial carcinoid tumors associated with
neuroendocrine syndrome as well as the frequency of symptoms
associated with this type of pathology.
Patients and methods
A descriptive and retrospective study on a period of
five years (2014-2018) was realized; the study included 98 patients
who underwent surgery in the clinic with a confirmed diagnosis of
bronchial carcinoid tumor. All patients included in the present
study presented specific clinical syndromes (carcinoid or Cushing
syndrome) and were submitted to paraclinical laboratory tests
consisting of specific hormones urinary and plasmatic dosage,
imagistic investigations (thoracic computed tomography-CT, brain
magnetic resonance imaging-MRI, chest X-ray), bronchoscopy and
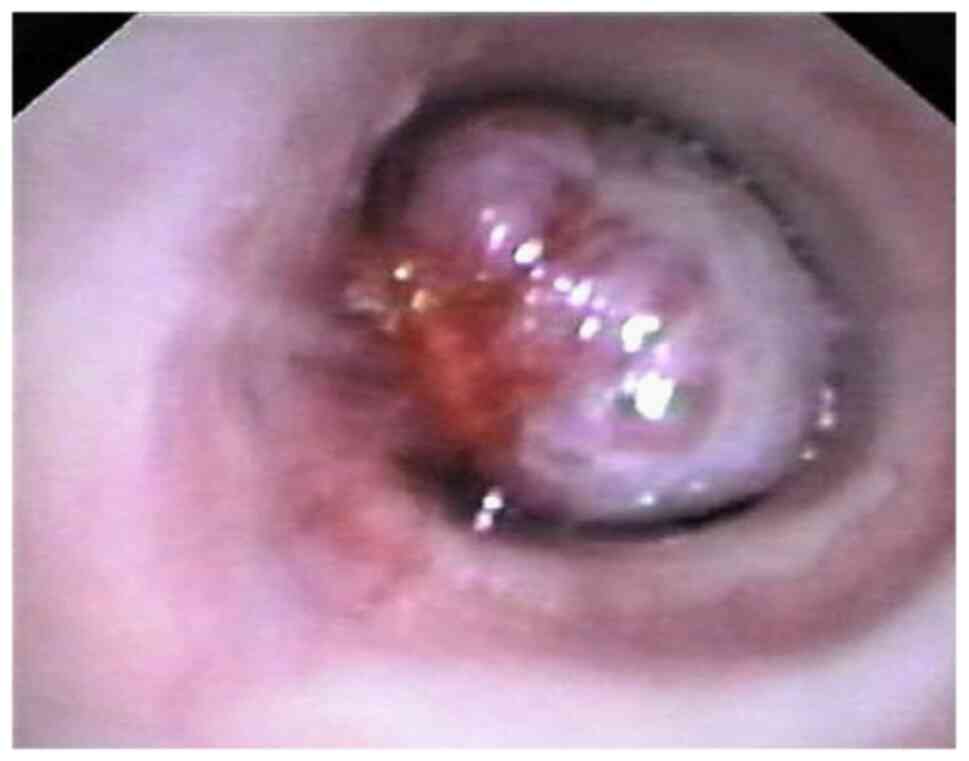
proved to have a histopathological diagnosis of typical or atypical
carcinoid (Figs. 1 and 2).
The final diagnostic of carcinoid tumor was
established on the specimens retrieved during bronchoscopy or on
those which were obtained after surgical resection.
The most commonly encountered clinical
manifestations were also monitored and correlated to the surgical
procedure performed. In patients with neuroendocrine syndrome an
ectopic secretion of ACTH was also investigated by dosing the
plasmatic levels of ACTH, plasmatic and urinary cortisol; a
suppression test with dexamethasone was also associated. In cases
presenting carcinoid syndrome dosing of neuron-specific enolase
marker, plasmatic and urinary serotonin were also performed.
Diagnosis was further confirmed by several investigations: Chest
Χ-ray, bronchoscopy, thoracic and superior abdomen CT, positron
emission tomography (PET-CT) and pituitary gland MRI.
Results
Of the 98 patients 39 (39.79%) were female and 59
(60.20%) male. Patients were aged between 31 and 85 years with an
average of 59.89 years. Distribution on age groups is as follows:
30-39 years, 4 cases (4.08%), 40-49 years, 12 cases (12.24%), 50-59
years, 24 cases (24.48%), 60-69 years, 39 cases (39.79%), 70-79
years, 15 cases (15.30%), 80-89 years, 4 cases (4.08%).
From a histological point of view 68 cases (69.38%)
were typical carcinoids, of which 26 in women and in 42 men, and 30
cases (30.61%) were atypical carcinoids of which 13 cases in women
and 17 in men. Bronchoscopy revealed a normal bronchial tree in 9
cases (9.18%) while in the remaining 89 cases (90.81%) bronchial
lesions specific to carcinoid tumors were found; in 29 out of the
total number of cases (29.59%) a bronchial biopsy was performed
(Fig. 2). Neuroendocrine syndrome
was found only in 5 out of the 30 cases of atypical carcinoids
(16.66%). When compared with the total number of cases of both
typical and atypical carcinoid tumors, neuroendocrine syndrome was
present in 5.1% of cases, in 4 females and 1 male. From these, 2
cases presented clinical manifestations of Cushing syndrome (two
women aged 64 and 70 years, 2.04%) and 3 cases with symptoms
specific to carcinoid syndrome (2 women, 1 male). In 17 patients
(17.34%) the diagnosis was confirmed preoperative through
bronchoscopy and biopsy of the tumor while in the remaining 81
patients (82.65%) the diagnosis was established by the pathology
examination of the excised specimen obtained after surgery. The
most commonly encountered symptoms were represented by: Cough, 90
cases, dyspnea, 45 cases, recurrent pulmonary infections, 32 cases,
hemoptysis, 27 cases, bronchospasm, 1 case, carcinoid syndrome, 3
cases, Cushing syndrome, 2 cases (Table
I).
 | Table IDistribution of clinical
manifestations in patients with carcinoid tumors. |
Table I
Distribution of clinical
manifestations in patients with carcinoid tumors.
| Cough | 90 |
| Dyspnea | 45 |
| Recurrent pulmonary
infections | 32 |
| Hemoptisis | 27 |
| Carcinoid
syndrome | 3 |
| Cushing
syndrome | 2 |
Association of two or more symptoms was also
encountered: Cough with dyspnea and recurrent pulmonary infections
in 32 cases and cough, dyspnea and hemoptysis in 27 cases.
Most bronchial carcinoid tumors were filed under
stage Ia, 19 cases, 19.38% (tumor <3 cm, with no pleural
invasion and without implication of the main bronchus, N0M0). 11
cases, 11.22% were stage IIb (tumor <5 cm, N1-mediastinal lymph
nodes present, M0). Preoperatively only the patient with Cushing
syndrome was staged as IIb, but after surgery N1 was infirmed and
the patient restaged to stage II a (T2bN0M0). The remaining cases
of atypical carcinoid were staged as Ia and Ib.
Cushing syndrome was present in a 64-year-old woman,
smoker, from an urban area who was investigated for persistent
cough and severe dyspnea. The patient was obese and presented with
typical clinical signs: Purple stretch marks on the lower abdomen
and thighs, thin skin. Imagistic investigations (chest X-ray and CT
scan) revealed the presence of a ¾ cm mass in the right upper lobe
associated with enlarged lymph nodes in the mediastinum (measuring
2.5 cm in diameter). Blood cortisol level was repeatedly measured
at 00:00 and 9:00 a.m. with values between 700 and 900 nmol/l
(reference value 130-600 nmol/l). Also 24 h urinary free cortisol
was measured and demonstrated the presence of elevated values 2,350
nmol/24 h (reference value 100-380 nmol/24 h). Plasmatic ACTH was
dosed in the same way and demonstrated the presence of elevated
values 450 and 422 pmol/l (reference value: 2.2-17 pmol/l).
Dexamethasone test was negative. A pituitary MRI was performed in
order to exclude the presence of a central cause for the hormonal
imbalance but showed no pathological modifications. The final
diagnosis was of a lung tumor in the right superior lobe associated
with Cushing syndrome, classifying the lesion as a neuroendocrine
tumor with ectopic secretion of ACTH.
Treatment was surgical and consisted in a right
upper lobectomy with hilar and mediastinal lymphadenectomy.
Pathology examination of the lobe identified a pulmonary atypical
carcinoid tumor with no metastases in the lymph nodes.
Postoperatively the serum hormonal levels normalized.
The second patient with Cushing syndrome was a
70-year-old woman with no smoking history, who presented for severe
dyspnea and hemoptysis. Patient was obese and also associated skin
hyperemia. The thoracic CT scan in association with bronchoscopy
established the final diagnosis of bronchial carcinoid tumor within
the inferior bronchus. Both morning and evening cortisol levels
were elevated (800-1,100 nmol/l) as well as urinary cortisol, with
values of 2,400 nmol/ h. Plasmatic ACTH was highly elevated: 743
pmol/l. Surgery consisted in left inferior lobectomy with
mediastinal lymphadenectomy; at one year follow-up the ACTH serum
level was normalized.
Carcinoid syndrome was found in 3 cases (3.06%),
patients presenting with tachycardia, high blood pressure, flush,
bronchospasm and an accelerated digestive transit. Diagnosis was
further confirmed by 5-hydroxyindoleacetic acid dosing (serotonin
metabolite). This metabolite was found to be elevated between 25
and 43 mg/24 h (reference value 2-9 mg/24 h). A 24 h urinary
serotonin dosing also showed elevated values of up to 500 µ/24 h
(reference value 50-250 µ/24 h). Plasmatic serotonin levels were
repeatedly dosed and were also found elevated (600-850 µ/l) with a
reference value of 80-400 µ/l. In all 3 cases neuron-specific
enolase was also dosed and presented twice the reference values
(reference value <16 µ/ml). All the laboratory findings together
with imagistic investigations and bronchoscopy led us to the
diagnosis of neuroendocrine pulmonary tumor with typical carcinoid
syndrome manifestations. From a histological point atypical
carcinoid was found in 30 patients (30.61%) of which 5 (5.1%) were
associated with neuroendocrine syndrome.
All of the examined patients underwent surgery.
Surgical treatment consisted of pulmonary resections ranging from
wedge resection (4 cases, 4.08%), lobectomy (79 cases, 80.61%) of
which: Lobectomy with lymphadenectomy (6 cases), bronchial sleeve
resections (21 cases), bilobectomy (5 cases, 5.1%), and
pneumonectomy (10 cases, 10.2%). Postoperatively in all cases the
neuroendocrine syndrome disappeared at one month follow up. The
long-term follow-up included performing chest X ray, thoracic CT
and bronchoscopy at every six months while in cases diagnosed with
atypical carcinoids with neuroendocrine syndromes PET CT was
associated.
Discussion
Bronchial carcinoid tumors have a rate of occurrence
of 10-20% out of all carcinoids, being rarer when compared with
intestinal carcinoids (9). Atypical
carcinoid tumor appears in 2% of all cases (10,11),
the mean age at diagnostic being 45-55 years. In our patients the
average age was 59.89 years, however, for atypical carcinoids
(30.61% of cases) the average age was 51.5 years. Data from
literature indicates that it occurs more often in females. In our
group of patients the ratio of female to male was 1:2.26. In our
study, bronchial carcinoid tumors were more often found in male
patients rather than in female patients, as opposed to data found
in literature. Location of bronchial carcinoids in 75% of cases is
in the main respiratory tract (12).
In literature among the risk factors for developing
a carcinoid tumor, smoking is mentioned (13). Of our patients 55 were smokers
(56.12%), but we cannot state with certainty that smoking would
contribute to the development of bronchial carcinoid. It has been
observed that this type of tumor is more common in Caucasians
compared with other ethnic groups (14).
The differentiation of carcinoids in our group of
patients was made by the pathology examination. An important
parameter for differentiating carcinoids is considered to be the
distribution of tumoral cells in the ‘peritumoral aerian space’
(STAS, spread through air spaces). According to this the presence
of even one cell beyond the margins of the tumor would be
suggestive. In typical carcinoids this phenomenon (STAS) appears in
48% of cases, whereas in atypical carcinoids it is present in 88%
of cases (15).
In order to establish the diagnosing of carcinoid
tumors an important role is played by immunohistochemistry,
especially by Chromogranin A and Synaptophysin (16). Regarding this, some authors proposed
the determination of a specific tumoral marker: Orthopedia homebox
protein which would be specific for bronchial carcinoid, both
typical and atypical (17). Another
factor which may be considered a tumoral marker for cell
proliferation is Ki-67, thus having an important role in
establishing an outcome (18). This
factor is a mitotic index and a high number of >10 mitosis/2
mm2 suggests that the tumor is a neuroendocrine
carcinoid (17), whereas a number
<4 mitosis/2 mm2 would be suggestive of a typical
carcinoid while a value >4 mitosis/2 mm2 for atypical
carcinoids. Previously atypical carcinoids were studied and
alteration of the cellular genome (3 transcriptional subtypes with
a specific genome alteration) similar to large cell
bronchopulmonary carcinoma was observed. In carcinoid cells a
degradation of the ratio between KEAP1 and NFE2L2 genes appears due
to a promoter KEAP1 with effects on the hypermethylation process
(19). It is known that KEAP1
during its interaction with NFE2 would dampen the oxidative stress
of the cell (19). Also, in
non-small cell bronchopulmonary carcinomas as well as pulmonary
neuroendocrine tumors, an elevated level of Pro-GRP (progastrin
releasing peptide) is found (11,20).
A case of a bronchial carcinoid associated with
Cushing syndrome and elevated Pro-GRP in relation with V1b
(negative vasopressin receptor) was reported. With this data the
authors proposed a new subtype of typical bronchial carcinoid
(7). The American Clinical
Oncological Society proposed the association of all neuroendocrine
tumors, benign and malignant, under a new concept-NET
(neuroendocrine tumor). There are 3 types of NETs in the lung:
Carcinoid (typical and atypical), small and large cell
bronchopulmonary carcinomas (21).
Carcinoid syndrome is found in 1-5% of cases and
Cushing syndrome in 4% of cases of bronchial carcinoid (18,22,23).
In our study, carcinoid syndrome was found in 3 patients (3.06%)
and Cushing syndrome in two cases (2.04%) of all bronchial
carcinoids. The ectopic secretion of ACTH in cases with Cushing
syndrome would be responsible for 10-15% of neurosecretant tumors
(24,25). Both Cushing and carcinoid syndromes
appear in patient in the presence of liver metastasis when they are
capable of ectopic hormonal production (4,10).
Treatment for bronchial carcinoid tumors is complex,
depending mainly on the histological type but also on the TNM
stage. Most authors agree that if the tumor is resectable, surgical
treatment is enough (26). Others
consider that patients with mediastinal lymph node involvement must
receive chemotherapy and radiotherapy in association with surgical
resection (4,27).
In our series of patients, surgical treatment varied
and the type of performed resection was chosen based on location of
the tumor, on the presence of secondary pulmonary suppurative
reaction, associated neuroendocrine syndromes as well as on the
presence of mediastinal lymph nodes. Therefore, anatomical
resections were performed in 95.91% of cases while wedge resections
were performed in only 4.08%, this approach being allowed by the
peripheral development of the tumor.
Regarding atypical carcinoids, we performed
anatomical resections in 6 cases and wedge resections in 2 cases.
The wedge resections where performed for tumors located
peripherally with uncertain macroscopical or intraoperative
histological examination. On the contrary the treatment for
secretant atypical carcinoid tumors consist of anatomical resection
associated with mediastinal lymphadenectomy (27).
Patients with stage III atypical carcinoid tumors
must go through induction chemo- and radiotherapy. Bronchoscopy
resections of endobronchial carcinoid tumors are only viable in
selected cases while palliative treatment is reserved only for
unresectable or metastatic tumors; in such cases chemotherapy and
somatostatin administration might be taken into consideration
(28,29) Octreotide or Lanreotide might also be
administered when the bronchial carcinoid has somatostatin
receptors or if the patient presents hormonal symptoms.
Somatostatin receptors may be observed through somatostatin
receptor scintigraphy (SRS) (30,31).
Previously, a Nd-Yag LASER was used for
endobronchial resection of a typical bronchial carcinoid tumor
located at the origin of the superior right bronchus (31); however, one year later surgical
resection in the form of a bronchial sleeve lobectomy was
performed.
In our series follow-up was done using mainly
imagistic investigations (chest X-ray and CT) and also through
bronchoscopy. In two cases PET-CT was used for patient that had
atypical carcinoids with neuroendocrine syndromes. Recent studies
have demonstrated that the factors which lead to a negative outcome
are lymph node metastasis, the histological differentiation degree,
location of the tumor (central or peripheral) as well as tumor size
(32).
There are patients with bronchial carcinoids which
presented with partial tumoral regression with no obvious
explanation (33,34).
In patients with carcinoid tumors in the absence of
metastatic disease the 5 year survival rate is very high at 97%,
much higher than in other pulmonary tumors such as non-small lung
cancer (35-37).
In our study there were no recurrences or metastasis while
paraneoplastic syndrome disappeared in all cases within the first
month after resection.
Bronchial carcinoid tumors associated with
neuroendocrine syndrome must be given surgical treatment similar to
bronchopulmonary carcinomas no matter where they are located
(central or periphery). In the short-term outcomes, neuroendocrine
syndrome is expected to disappear shortly after surgery while a
normalization of the serum markers is to be expected; in for the
long-term outcomes, this histopathological subtype seems to be
associated with a significantly better survival when compared with
other histopathological subtypes of bronchopulmonary
malignancies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CoS performed the surgical procedures. AM, JLL and
NB were part of the surgical team. AM, JLL, GG and IB prepared the
manuscript. CM, OS, OB and CaS performed data analysis. CD and LI
preoperatively examined the patients. GG, IB and EB conceived the
study and drafted the manuscript. EB advised on the oncological
outcome. CoS and NB revised the final draft of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients read and signed the informed consent
before participating in the study and before the publication of
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peri M, Botteri E, Pisa E, De Marinis F,
Ungaro A, Spada F, Grana CM, Gasparri R, Spaggiari L, Romentz N,
Badalamenti G, Russo A and Fazio N: A single-institution
retrospective analysis of metachronous and synchronous metastatic
bronchial neuroendocrine tumors. J Thorac Dis. 10:3928–3939.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eloesser L: Transthoracic bronchotomy for
removal of benign tumors of the bronchi. Ann Surg. 112:1067–1070.
1940.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stanford WR, Davis JE, Gunter JU and
Hobart SG Jr: Bronchial adenoma (carcinoid type) with solitary
metastasis and associated functioning carcinoid syndrome. South Med
J. 51:449–454. 1958.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arrigoni MG, Woolner LB and Bernatz PE:
Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg.
64:413–421. 1972.PubMed/NCBI
|
|
5
|
Baudin E, Hayes AR, Scoazec JY, Filosso
PL, Lim E, Kaltsas G, Frilling A, Chen J, Kos-Kudla B, Gorbunova V,
et al: Unmet medical needs in pulmonary neuroendocrine (Carcinoid)
neoplasms. Neuroendocrinology. 108:7–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
La Rosa S, Volante M, Uccella S,
Maragliano R, Rapa I, Rotolo N, Inzani F, Siciliani A, Granone P,
Rindi G, et al: ACTH-producing tumorlets and carcinoids of the
lung: Clinico-pathologic study of 63 cases and review of the
literature. Virchows Arch. 475:587–597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamamuro T, Inoue K, Nagai Y, Azuma D,
Yamamoto A, Hara K, Kohara M, Iwata T, Nakatsuka S, Morii E and
Yamamoto T: A case of ectopic ACTH syndrome due to DDAVP-sensitive
but V1b receptor-negative bronchial typical carcinoid with
lymphatic metastasis and plasma ProGRP elevation. Endocr J.
65:1161–1169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Warren WH, Gould VE, Faber LP, Kittle CF
and Memoli VA: Neuroendocrine neoplasms of the bronchopulmonary
tract. A classification of the spectrum of carcinoid to small cell
carcinoma and intervening variants. J Thorac Cardiovasc Surg.
89:819–825. 1985.PubMed/NCBI
|
|
9
|
Guenter RE, Aweda T, Carmona Matos DM,
Whitt J, Chang AW, Cheng EY, Liu XM, Chen H, Lapi SE and
Jaskula-Sztul R: Pulmonary carcinoid surface receptor modulation
using histone deacetylase inhibitors. Cancers (Basel).
11(767)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Garg R, Kumar R, Singh P and Kshetrimayum
S: Atypical carcinoid tumor of the lung: A rare entity. Lung India.
36:236–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grigoroiu M, Tagett R, Draghici S, Dima S,
Nastase A, Florea R, Sorop A, Ilie V, Bacalbasa N, Tica V, et al:
Gene-expression profiling in non-small cell lung cancer with
invasion of mediastinal lymph nodes for prognosis evaluation.
Cancer Genomics Proteomics. 12:231–242. 2015.PubMed/NCBI
|
|
12
|
Betser L, De Wolf J, Glorion M and
Chapelier A: Use of 3-dimensional computed tomography for planning
a complex sleeve bronchoplasty with total parenchyma-sparing
resection of a carcinoid tumour in the right main bronchus.
Interact Cardiovasc Thorac Surg. 29:638–640. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scoazec JY: Lung and digestive
neuroendocrine neoplasms From WHO classification to biomarker
screening: Which perspectives? Ann Endocrinol (Paris). 80:163–165.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zabaleta J, Aguinagalde B, Lopez I, Laguna
SM, Mendoza M, Galardi A, Matey L, Larranaga A, Baqueriza G and
Izeta A: Creation of a multidisciplinary and multicenter study
group for the use of 3D printing in general thoracic surgery:
Lessons learned in our first year experience. Med Devices (Auckl).
12:143–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Altinay S, Metovic J, Massa F, Gatti G,
Cassoni P, Scagliotti GV, Volante M and Papotti M: Spread through
air spaces (STAS) is a predictor of poor outcome in atypical
carcinoids of the lung. Virchows Arch. 475:325–334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alrajhi NN, Paramasivam MP, Alboukai AA,
Alrikabi AC and Alhamad EH: Diffuse idiopathic pulmonary
neuroendocrine cell hyperplasia: Unusual presentation. Ann Thorac
Med. 14:161–163. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Viswanathan K, Borczuk AC and Siddiqui MT:
Orthopedia homeobox protein (OTP) is a sensitive and specific
marker for primary pulmonary carcinoid tumors in cytologic and
surgical specimens. J Am Soc Cytopathol. 8:39–46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Grondahl V, Binderup T, Langer SW,
Petersen RH, Nielsen K, Kjaer A, Federspiel B and Knigge U:
Characteristics of 252 patients with bronchopulmonary
neuroendocrine tumours treated at the Copenhagen NET Centre of
excellence. Lung Cancer. 132:141–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sparaneo A, Fabrizio FP, la Torre A,
Graziano P, Di Maio M, Fontana A, Bisceglia M, Rossi A, Pizzolitto
S, De Maglio G, et al: Effects of KEAP1 silencing on the regulation
of NRF2 activity in neuroendocrine lung tumors. Int J Mol Sci.
20(2531)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tutar N, Yetkin NA, Yazici C, Onal O,
Kontas O and Kelestemur F: Clinical significance of
progastrin-releasing peptide, neuron-specific enolase, chromogranin
a, and squamous cell cancer antigen in pulmonary neuroendocrine
tumors. Turk J Med Sci. 49:774–781. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tastepe AI, Kurul IC, Demircan S, Liman
ST, Kaya S and Cetin G: Long-term survival following bronchotomy
for polypoid bronchial carcinoid tumours. Eur J Cardiothorac Surg.
14:575–577. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shariff MZ, Curras-Martin D, Campbell N,
Gupta V, Mikhail JD, Levitt MJ and Hossain MA: Carcinoid tumor of
lung and BRCA mutation: A case report. J Med Case Rep.
13(132)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cattoni M, Vallieres E, Brown LM,
Sarkeshik AA, Margaritora S, Siciliani A, Filosso PL, Guerrera F,
Imperatori A, Rotolo N, et al: Sublobar resection in the treatment
of peripheral typical carcinoid tumors of the lung. Ann Thorac
Surg. 108:859–865. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wegner RE, Abel S, Hasan S, Horne ZD,
Colonias A, Weksler B and Verma V: The role of adjuvant therapy for
atypical bronchopulmonary carcinoids. Lung Cancer. 131:90–94.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Benito-Martinez E, Galeano-Valle F,
Gonzalez A, Edgar MA, Oprea-Ilies G, Ioachimescu AG and Pasquel FJ:
Ectopic ACTH syndrome with association of multiple pulmonary
sclerosing pneumocytomas and multiple carcinoid tumorlets. J Endocr
Soc. 3:937–942. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dermawan JK and Farver CF: The prognostic
significance of the 8th edition TNM staging of pulmonary carcinoid
tumors: A single institution study with long-term follow-up. Am J
Surg Pathol. 43:1291–1296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Adrega T, Monteiro JP, Lareiro S, Guerra M
and Vouga L: Surgical treatment of concomitant severe heart disease
and lung cancer. Rev Port Cir Cardiotorac Vasc. 26:27–30.
2019.PubMed/NCBI
|
|
28
|
Pelosi G, Massa F, Gatti G, Righi L,
Volante M, Birocco N, Maisonneuve P, Sonzogni A, Harari S, Albini A
and Papotti M: Ki-67 evaluation for clinical decision in metastatic
lung carcinoids: A proof of concept. Clin Pathol.
12(2632010X19829259)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Torniai M, Scortichini L, Tronconi F,
Rubini C, Morgese F, Rinaldi S, Mazzanti P and Berardi R: Systemic
treatment for lung carcinoids: From bench to bedside. Clin Transl
Med. 8(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rekhtman N, Desmeules P, Litvak AM,
Pietanza MC, Santos-Zabala ML, Ni A, Montecalvo J, Chang JC, Beras
A, Preeshagul IR, et al: Stage IV lung carcinoids: Spectrum and
evolution of proliferation rate, focusing on variants with elevated
proliferation indices. Mod Pathol. 32:1106–1122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Inada H, Miyajima K, Imai K, Maeda J,
Hagiwara M, Ito T and Ikeda N: Bronchial typical carcinoid
requiring left upper sleeve lobectomy after rigid bronchoscopic
intervention. Kyobu Geka. 71:1097–1101. 2018.PubMed/NCBI(In Japanese).
|
|
32
|
Reuling EMBP, Dickhoff C, Plaisier PW,
Bonjer HJ and Daniels JMA: Endobronchial and surgical treatment of
pulmonary carcinoid tumors: A systematic literature review. Lung
Cancer. 134:85–95. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kuwal A, Chauhan N, Dutt N, Elhence P,
Advani M and Kumar S: Spontaneous partial regression of a carcinoid
tumor: Radiology may not capture the real picture. Turk Thorac J.
20:153–156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Uchida T, Matsubara H, Sugimura A,
Matsuoka H, Ichihara T and Nakajima H: Spontaneous regression of a
carcinoid tumor that required resection owing to its reappearance
and subsequent enlargement after 2 years: A case report. Int Cancer
Conf J. 8:58–60. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Brcic L, Heidinger M, Sever AZ, Zacharias
M, Jakopovic M, Fediuk M, Maier A, Quehenberger F, Seiwerth S and
Popper H: Prognostic value of cyclin A2 and B1 expression in lung
carcinoids. Pathology. 51:481–486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dediu M, Horvat T, Tarlea A, Anghel R,
Cordos I, Miron G, Iorga P, Alexandru A, Nistor C, Grozavu C and
Savu C: Adjuvant chemotherapy for radically resected non-small cell
lung cancer: A retrospective analysis of 311 consecutively treated
patients. Lung Cancer. 47:93–101. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Galie N, Vasile R, Savu C, Petreanu C,
Grigorie V and Tabacu E: Superior vena cava syndrome -surgical
solution case report. Chirurgia (Bucur). 105:835–838.
2010.PubMed/NCBI(In Romanian).
|