Introduction
The incidence of spontaneous abortion (SA) is 10-15%
in clinical pregnancies globally (1). The definition of SA is clinically
confirmed pregnancy loss within 24 weeks of pregnancy (2,3). The
etiology of pregnancy loss is complicated, and genetic factors such
as chromosomal abnormalities and single gene disorders,
endocrinological factors such as diabetes mellitus and thyroid
disease, environmental factors such as alcohol and tobacco and
infectious factors such as viral and bacterial factors are all the
risk factors of SA (4,5). However, there may still be other
unknown factors contributing to SA, therefore further investigation
is needed.
The cellular mechanisms underlying recurrent
abortion are the proliferation and apoptosis of cytotrophoblasts
and human decidual cells (6). Zhang
et al (7) reported that
microRNA (miR)-184 is highly expressed in decidual stromal cells,
decidual immune cells and peripheral blood of patients who
experience recurrent spontaneous abortion (RSA). Mechanically,
miR-184 promotes apoptosis and inhibits the proliferation of
trophoblast cells by targeting and regulating WIG1(7). miR-520 also enhances the apoptosis of
trophoblast cells by targeting poly (ADP-ribose) polymerase (PARP)1
in human-trophoblast-derived HTR-8/SVneo cells (8). Ferroptosis is form of programed cell
death that is different from apoptosis both in morphology and
biochemical levels such as caspase-3 activation, and is
characterized by the accumulation of reactive oxygen species (ROS)
as a result of iron accumulation and lipid peroxidation (9). Ferroptosis is involved in several
diseases, such as ischemia/reperfusion-induced organ injury, stroke
and cancer, and ferroptosis inhibition is effective in treating
ischemia/reperfusion-induced organ injury and stroke in a number of
experimental models in vivo (10-12).
However, whether ferroptosis is involved in the process of SA is
still unknown.
Long non-coding RNAs (lncRNAs), which are >200
nucleotides in length, have important roles in a number of cellular
processes, including posttranslational regulation and
carcinogenesis (13,14). H19 is a lncRNA that is 2.3 kb long
and is primarily located in cytoplasm. During embryonic
development, H19 is highly expressed; however, after birth, the
expression of H19 is decreased (15). Most studies investigating H19 have
focused on its role in carcinogenesis, including tumor growth and
metastasis (16,17), and although several studies have
analyzed the expression and methylation status of H19 in SA
(18,19), whether H19 regulates apoptosis and
ferroptosis in SA is unknown.
The present study analyzed the expression levels of
H19 and the apoptosis- and ferroptosis-associated genes
Bcl-2, Bax and phospholipid hydroperoxide glutathione
peroxidase (GPX4) in SA tissues. Importantly, the current
study also further evaluated the regulatory correlations between
H19 and Bcl/GPX4/Bax both at the mRNA and
protein levels.
Materials and methods
Patient sample collection
The placental villi tissues from patients with SA
(SA group) and clinically normal pregnancies terminated for
non-medical reasons (control group) were collected. The SA group
consisted of 30 women aged 21-38 years. The criteria for inclusion
in SA group were as follows: i) Normal chromosome number and
structure of both the mother and father, ii) no thyroid
dysfunction, diabetes and other systemic diseases, iii) no
reproductive endocrine disease, iv) no deformity of genital tract
and uterus and v) the activity and quality of sperm of the father
were normal. The control group included 30 women aged 21-38 years
who requested termination due to an unplanned pregnancy. The
criteria for control group were as follows: i) No vaginal bleeding,
ii) no abdominal pain. All women in these two groups were between
gestational weeks six to nine. The present study was approved by
The Medical Ethics Committee of the First People's Hospital of
Yunnan Province (approval no. 2018-012, Kunming, China) and written
informed consent was provided by each patient.
Cell culture
HTR-8/SVneo cells (henceforth referred to as HTR-8)
were purchased from the American Type Culture Collection and
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml in
CO2 at 37˚C.
Cell transfection
A H19 knockdown lentiviral vector
(pGLVU6-puro-H19-shRNA) and negative vector (non-targeting control,
pGLVU6-puro-NC-shRNA) were constructed by Shanghai GenePharma Co.,
Ltd. 3 µg H19 knockdown lentiviral vector/negative vector were
co-transfected with 2.5 µg pMD2.G and 7.5 µg psPAX2 plasmids into
293T cells (75 cm2 flasks) for lentivirus packaging with
Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.). The
sequences are as follows: H19 short hairpin (sh)RNA,
5'-CCGGCAGCCTTCAAGCATTCCATTACTCGAGTTTTTG-3'; NC shRNA,
5'-CCGGTTCTCCGAACGTGTCACGTTTTTTG-3'. Lentivirus infection was
applied to HTR-8 cells with 50-60% confluence. Reverse
transcription-quantitative (RT-q)PCR was used to examine the
efficiency of H19 knockdown after 72 h of infection.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA of tissues
and cells according to the protocol of the manufacturer. A
QuantiTect Reverse Transcription kit (Qiagen China Co., Ltd.) was
used to synthesize cDNA by incubating at 42˚C for 15
min. H19, Bcl-2, Bax and GPX4 levels
were detected by using a SYBR Green RT-qPCR assay kit (Takara
Biotechnology Co., Ltd.), and GAPDH was used as the endogenous
control. The PCR program was as follows: Stage 1, 95˚C
for 30 sec; stage 2, 95˚C for 5 sec and 60˚C
for 34 sec, for 40 cycles. The primer sequences were as follows:
Bcl-2, forward, 5'-GGTGGGGTCATGTGTGTGG-3' and reverse,
5'-CGGTTCAGGTACTCAGTCATCC-3'; Bax, forward,
5'-CCCGAGAGGTCTTTTTCCGAG-3' and reverse,
5'-CCAGCCCATGATGGTTCTGAT-3'; GPX4, forward,
5'-GAGGCAAGACCGAAGTAAACTAC-3' and reverse,
5'-CCGAACTGGTTACACGGGAA-3'; H19, forward,
5'-CGTGACAAGCAGGACATGACA-3' and reverse, 5'-CCATAGTGTGCCGACTCCG-3';
GAPDH, forward, 5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'. Quantitative measurements were
evaluated using the 2-ΔΔCq method (20). The mRNA levels of H19, Bcl-2, Bax
and GPX4 in shCON group were normalized to 1.
Western blotting
HTR-8 cells were collected and lysed using RIPA
lysis buffer (Beyotime Institute of Biotechnology) for 25 min on
ice. Protein concentrations were evaluated using the BCA method.
Equal amounts of proteins (7-10 µg per sample) were loaded per lane
on a 12% gel, resolved using SDS-PAGE and subsequently transferred
to PVDF membranes. Then the membranes were blocked with 5% non-fat
milk for 1 h at the room temperature. The membranes were treated
with primary antibodies overnight at 4˚C. Next, the membranes were
treated with the secondary antibodies for 1 h at room temperature.
The protein signals were detected using ECL Detection Reagent
(Beijing Solarbio Science & Technology Co., Ltd.). The primary
antibodies including anti-Bcl-2 (1:1,000; cat. no. 15071), anti-Bax
(1:1,000; cat. no. 5023) and anti-β-actin (1:5,000; cat. no. 3700)
antibodies were purchased from Cell Signaling Technology, Inc., and
anti-GPX4 (1:1,000; cat. no. ab125066) was purchased from Abcam.
The secondary antibodies including anti-mouse IgG, HRP-linked
antibody (1:3,000; cat. no. 7076) and anti-rabbit IgG, HRP-linked
antibody (1:3,000; cat. no. 7074) were purchased from Cell
Signaling Technology, Inc. β-actin was used as the internal
control.
Cell viability analysis
After H19 shRNA infection for 48 h, HTR-8 cells were
seeded in 96-well plates. The cells were then divided into six
groups, the shCON, shH19, shCON+Z-VAD-FMK, shH19+Z-VAD-FMK,
shCON+Ferrostatin-1 and the shH19+Ferrostatin-1 group. After
treatment for 48 h, cell viability was examined by using the Cell
Counting Kit-8 method as previously described (21). Z-VAD-FMK (Selleck Chemicals) was a
strong apoptosis inhibitor, and ferrostatin-1 (Selleck Chemicals)
was a ferroptosis inhibitor which could depress the cellular lipid
peroxidation (22).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS version 19.0 software
(IBM, Corp.). Data were analyzed using a two-tailed unpaired
Student's t-test (two groups) or analysis of variance (ANOVA) with
Tukey's post hoc test for multiple comparisons. The correlation
between Bax, Bcl-2 and GPX4 mRNA expression
with H19 levels was analyzed using Pearson's correlation. P<0.05
was considered to indicate a statistically significant difference.
All in vitro experiments were repeated in triplicate.
Results
Expression levels of H19 and
apoptosis- and ferroptosis-associated genes in the SA group
Sixty patients were included in the present study,
including 30 cases in the SA group and 30 cases in the control
group. RT-qPCR was used to detect the RNA expression levels of
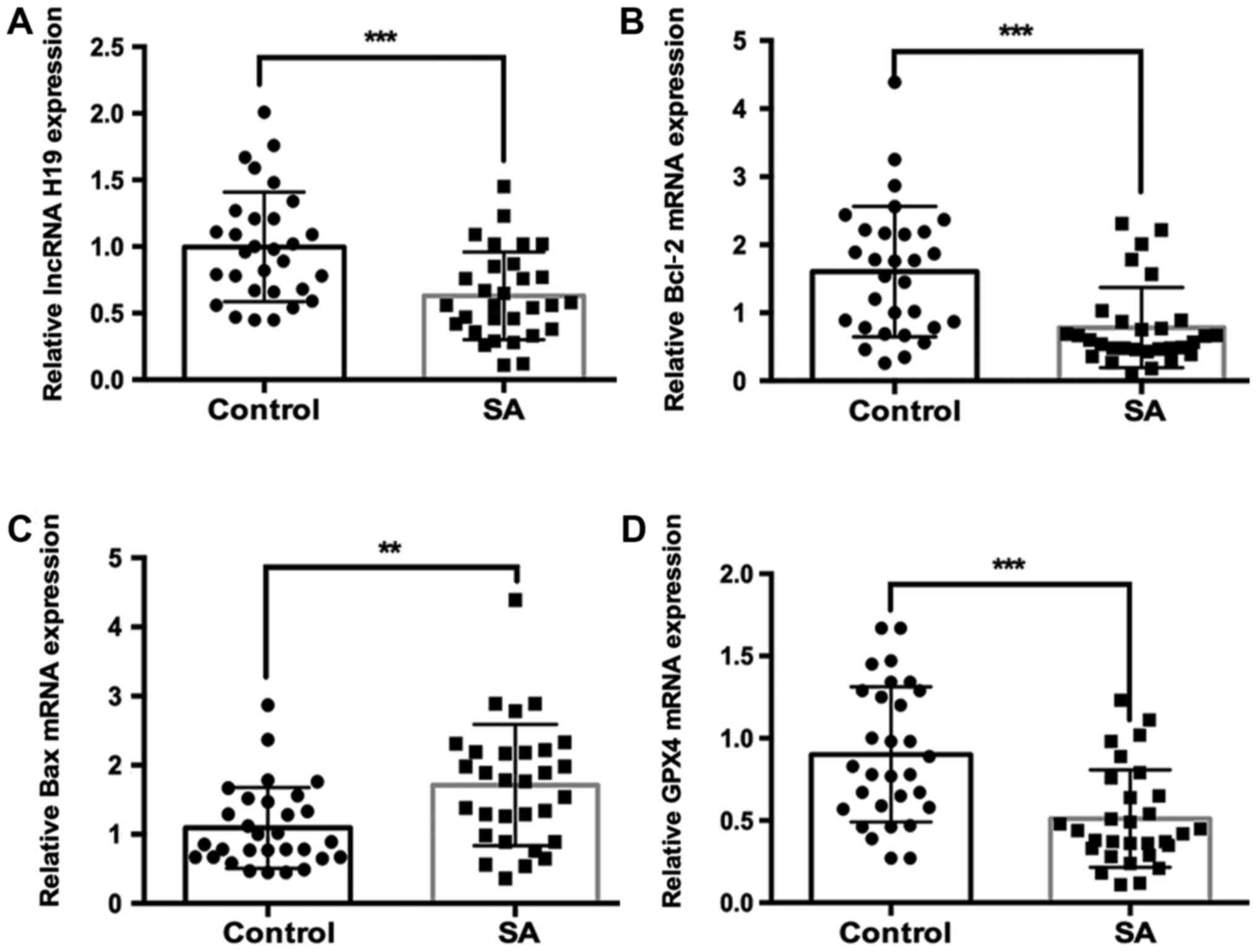
H19, Bcl-2, Bax and GPX4. The results showed that the
RNA expression levels of H19 were significantly lower in SA
group compared with those in the control group (Fig. 1A). It was further found that the
mRNA expression of anti-apoptosis gene Bcl-2 was
significantly lower, and the mRNA expression of proapoptotic gene
Bax was significantly higher in SA group compared with those
in control group (Fig. 1B and
C). Notably, the
ferroptosis-associated gene GPX4 was also significantly
downregulated in SA group compared with the control group (Fig. 1D).
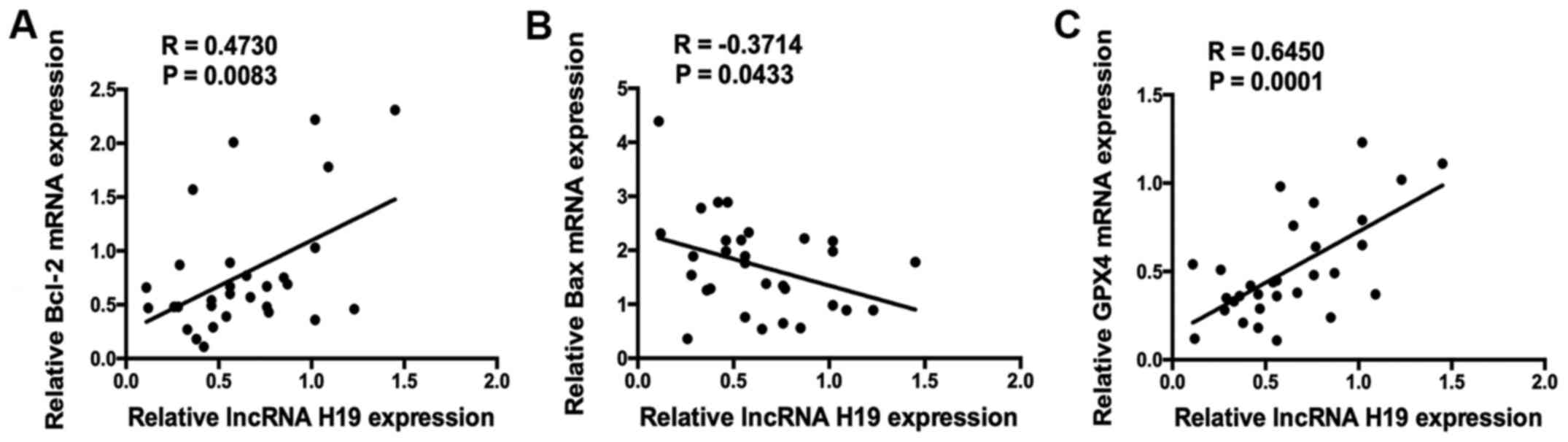
Correlation analysis was applied to evaluate
relationship between the RNA expression levels of H19 and
apoptosis- and ferroptosis-associated genes. lncRNA H19 expression
was significantly positively correlated with Bcl-2
expression (R=0.4730, P=0.0083), and significantly negatively
correlated with Bax expression (R=-0.3714, P=0.0433;
Fig. 2A and B). Notably, it was also demonstrated that
H19 expression was significantly positively correlated with
GPX4 expression (R=0.6450, P=0.0001; Fig. 2C).
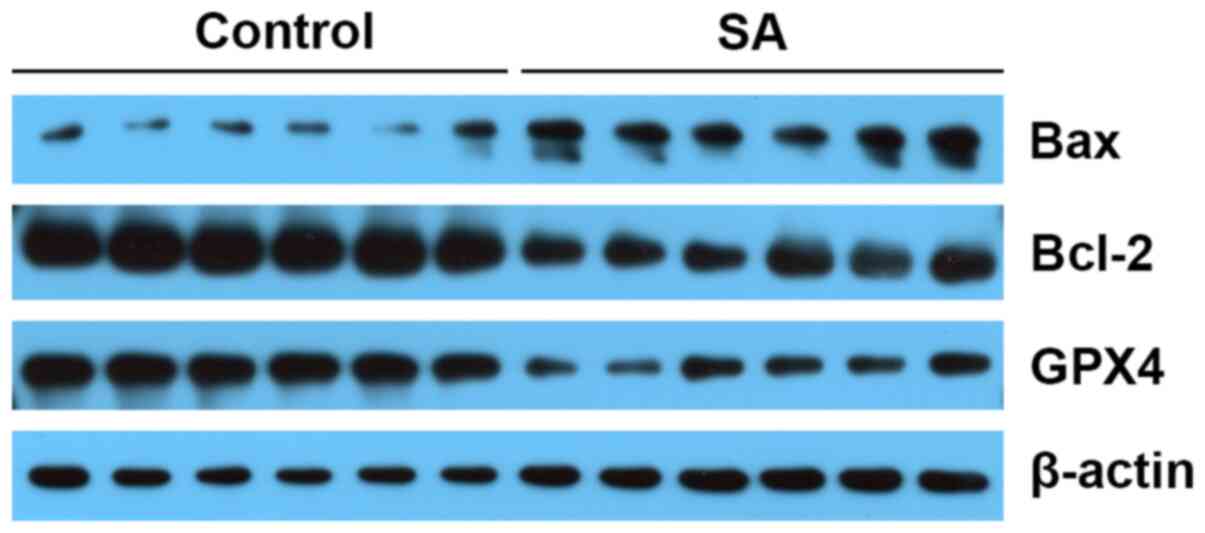
Western blotting was used to evaluate the expression
of apoptosis- and ferroptosis-associated proteins. The protein
levels of Bax were higher in SA group, and Bcl-2 levels were lower
in SA group compared those in the control group. In addition, GPX4
expression was downregulated in SA group compared with the control
group (Fig. 3).
Silencing H19 downregulates Bcl-2 and
GPX4 and upregulates Bax in HTR-8 cells
HTR-8 cells were transfected with the shH19
lentiviral construct, and the results showed that the expression of
H19 was significantly decreased by ~80% (Fig. 4A). Then, the expressions of
Bcl-2, Bax and GPX4 were evaluated using
RT-qPCR and western blotting. The results showed that silencing H19
significantly reduced expression of Bcl-2 and GPX4
and increased Bax expression at both the mRNA and protein
levels (Fig. 4B-E).
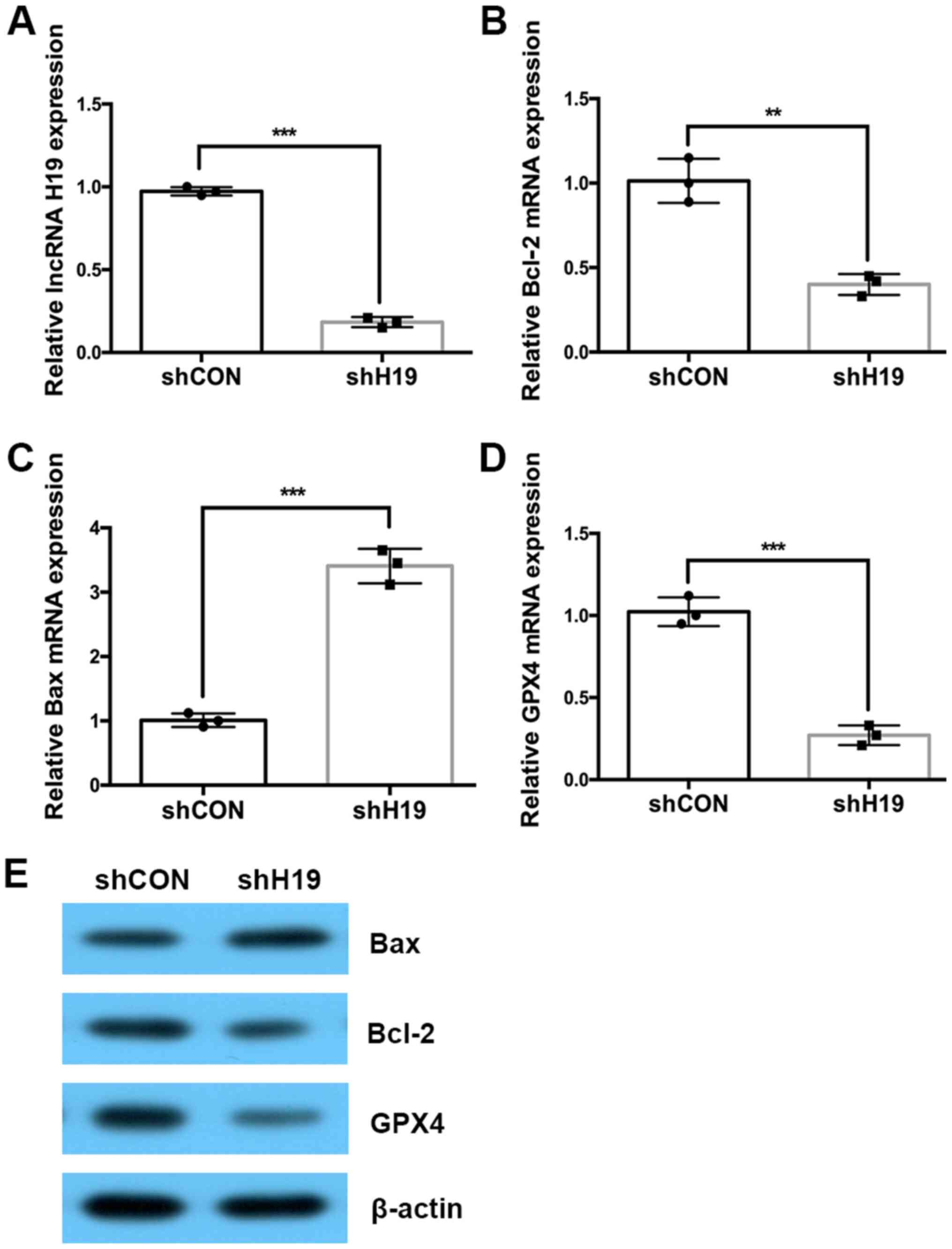 | Figure 4Silencing H19 downregulates
Bcl-2 and GPX4 expression and upregulates Bax
expression at both the mRNA and protein levels. (A-D) Reverse
transcription-quantitative PCR was used to detect the expression of
H19, Bcl-2, Bax and GPX4 in H19
silenced HTR-8 cells. (E) Western blotting was used to detect the
protein expression of Bcl-2, Bax and GPX4 in
the H19 silenced HTR-8 cells. The experiments were performed three
times. **P<0.01 and ***P<0.001 vs.
control. sh, short hairpin; shNC, negative control group; shH19,
H19 knockdown group; GPX4, phospholipid hydroperoxide glutathione
peroxidase; lncRNA, long non-coding RNA. Circle, expression data of
shCON group; square, expression data of shH19 group. |
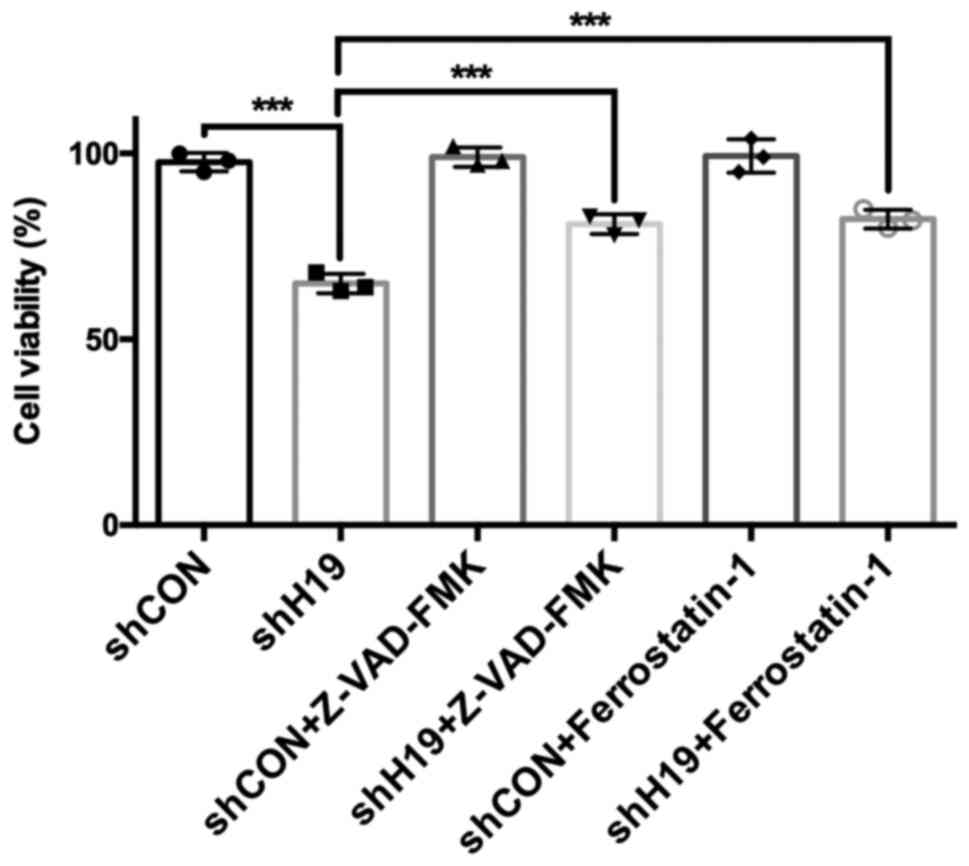
The viability of HTR-8 cells following H19 knockdown
and apoptosis or ferroptosis inhibitor treatment was further
examined. The results showed that H19 silencing significantly
reduced the viability of HTR-8 cells, and Z-VAD-FMK (an apoptosis
inhibitor) and Ferrostatin-1 (a ferroptosis inhibitor) (22) could partially rescue the decreased
viability of HTR-8 cells induced by H19 knockdown (Fig. 5).
Discussion
SA is the most frequently occurring pregnancy
disorder and is a serious threat to women's health (23). SA has been reported to be associated
with several risk factors, including endocrine disorders, anatomic
deformation, immunodeficiency and chromosomal abnormalities
(24-27).
However, other risk factors and underlying molecular mechanisms of
SA still need further investigation.
H19, a lncRNA, is involved in the pathogenesis of
several diseases, including lung cancer and pre-eclampsia (28,29).
Although a previous study reported that the methylation of H19
differentially methylated regions is increased in the placental
samples of SA cases (23), the role
and underlying mechanism of H19 in SA are still largely unclear.
The present study revealed that the expression of H19 was lower in
SA group compared with that in control group.
Trophoblast cells participate in the formation of
placenta and have important functions in embryo implantation
(30). Apoptosis of trophoblast
cells is involved in SA (31). The
apoptotic rate and mRNA and protein expression of Fas and
FasL were significantly higher in recurrent spontaneous
abortion compared with the abortion group (32). Li et al (33) reported that knockdown of
Storkhead-box protein 1 induced apoptosis and decreased the
migration and proliferation of HTR-8/SVneo cells via mediating the
PI3K/AKT signaling pathway (33). A
previous study also reported that miR-184, which is highly
expressed in RSA, repressed the proliferation and promoted the
apoptosis of trophoblast cells by targeting WIG1 and upregulating
its downstream molecule Fas (7).
Dong et al (8) also found
that miR-520, which is highly expressed in villi in RSA, enhanced
the apoptosis of human trophoblast cells by targeting and
regulating PARP1 expression. Increased cyclin-dependent
kinase inhibitor 1 and Bax expression was associated with
increased apoptosis of cytotrophoblasts and human decidual cells in
the RSA group (34). However, the
regulatory mechanisms underlying trophoblast apoptosis are still
largely unknown. The present results demonstrated that the mRNA and
protein expression of Bcl-2 was lower and the expression of
Bax was higher in SA compared with control tissues. Notably,
the expression of Bcl-2 was positively correlated with
H19 expression, and Bax expression was negatively
correlated with H19 levels. In vitro analysis
revealed that silencing H19 decreased Bcl-2 and upregulated
Bax expression at both the mRNA and protein levels.
Ferroptosis is a caspase-independent, non-apoptotic
and newly defined type of cell death characterized by high levels
of lipid peroxidation (35). GPX4
is the key regulator of ferroptosis by controlling the activity of
cyclooxygenase and lipid-modulating lipoxygenase enzymes (36). A recent study has reported that an
inactivating mutation of GPX4 has a dominant-negative effect
on male fertility (36). However,
the roles of ferroptosis and GPX4 in SA are still unknown.
The present findings reported that GPX4 expression was lower
in SA group compared with that in control group and was positively
correlated with H19 expression. In addition, silencing H19
downregulated GPX4 expression levels. Overall, these results
indicated that ferroptosis was involved in SA.
The mechanisms by which lncRNA H19 regulates
Bcl-2, Bax and GPX4 expression is still
unclear. A previous study reported that H19 could directly binds to
the miR-17-92 cluster and suppress STAT3 activity, decreasing the
expression of its target genes Bcl-2 and Bcl-2L1
(37). H19 is also reported to
positively regulate Bcl-2 expression by sponging miR-877-3p
in myocardial ischemia/reperfusion injury (I/RI) (38). Whether H19 regulates Bcl-2 by
these two pathways or other pathways in SA still needs to be
explored.
There are some limitations to the present study.
Firstly, the regulatory association between H19 and Bcl-2,
Bax and GPX4 was confirmed in only one cell line. In
the future, more cell lines should be used to verify the present
findings. Secondly, an Annexin V/PI and lipid peroxidation assays
should be used in the figure to investigate the regulatory
association between H19 and apoptosis and ferroptosis.
Overall, the present study demonstrated that
H19 was downregulated in SA cases, and H19 expression
was positively correlated with Bcl-2 and GPX4 levels,
and negatively correlated with Bax expression. In addition,
silencing H19 downregulated Bcl-2 and GPX4,
and upregulated Bax at both the mRNA and protein levels. The
results of the current study may provide a novel insight for the
pathogenesis for SA.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Open Project
Program of Yunnan Provincial Key Laboratory for Birth Defects and
Genetic Diseases (grant no. 2017ZDKFKT001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RXB and ZYT designed the study. RXB and ZYT
performed the experiments. ZYT wrote the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Medical Ethics
Committee of the First People's Hospital of Yunnan Province
(approval no. 2018-012, Kunming, China) and written informed
consent was provided by each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Den Berg MM, Van Maarie MC, Van Wely M
and Goddijn M: Genetics of early miscarriage. Biochim Biophys Acta.
1822:1951–1959. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tur-Torres MH, Garrido-Gimenez C and
Alijotas-Reig J: Genetics of recurrent miscarriage and fetal loss.
Best Pract Res Clin Obstet Gynaecol. 42:11–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Regan L and Rai R: Epidemiology and the
medical causes of miscarriage. Baillieres Best Pract Res Clin
Obstet Gynaecol. 14:839–854. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Christiansen OB, Steffensen R, Nielsen HS
and Varming K: Multifactorial etiology of recurrent miscarriage and
its scientific and clinical implications. Gynecol Obstet Invest.
66:257–267. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Warren JE and Silver RM: Genetics of
pregnancy loss. Clin Obstet Gynecol. 51:84–95. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cinar O, Kara F and Can A: Potential role
of decidual apoptosis in the pathogenesis of miscarriages. Gynecol
Endocrinol. 28:382–385. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Y, Zhou J, Li MQ, Xu J, Zhang JP and
Jin LP: MicroRNA-184 promotes apoptosis of trophoblast cells via
targeting WIG1 and induces early spontaneous abortion. Cell Death
Dis. 10(223)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dong X, Yang L and Wang H: miR-520
promotes DNA-damage-induced trophoblast cell apoptosis by targeting
PARP1 in recurrent spontaneous abortion (RSA). Gynecol Endocrinol.
33:274–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alim I, Caulfield JT, Chen Y, Swarup V,
Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie
EJ, et al: Selenium drives a transcriptional adaptive program to
block ferroptosis and treat stroke. Cell. 177:1262–1279, e1225.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cheng G, Song Z, Liu Y, Xiao H, Ruan H,
Cao Q, Wang K, Xiao W, Xiong Z, Liu D, et al: Long noncoding RNA
SNHG12 indicates the prognosis of prostate cancer and accelerates
tumorigenesis via sponging miR-133b. J Cell Physiol. 235:1235–1246.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matouk I, Raveh E, Ohana P, Lail RA,
Gershtain E, Gilon M, De Groot N, Czerniak A and Hochberg A: The
increasing complexity of the oncofetal H19 gene locus: Functional
dissection and therapeutic intervention. Int J Mol Sci.
14:4298–4316. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng ZH, Wu DM, Fan SH, Zhang ZF, Chen GQ
and Lu J: Upregulation of miR-675-5p induced by lncRNA H19 was
associated with tumor progression and development by targeting
tumor suppressor p53 in non-small cell lung cancer. J Cell Biochem.
120:18724–18735. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Si H, Chen P, Li H and Wang X: Long
non-coding RNA H19 regulates cell growth and metastasis via miR-138
in breast cancer. Am J Transl Res. 11:3213–3225. 2019.PubMed/NCBI
|
|
18
|
Hu L, Zeng H and Liu N: Expression of H19
long non-coding RNA and ZEB1 in the trophoblast of women with
spontaneous abortion. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
43:179–183. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
19
|
Liu Y, Tang Y, Ye D, Ma W, Feng S, Li X,
Zhou X, Chen X and Chen S: Impact of abnormal DNA methylation of
imprinted loci on human spontaneous abortion. Reprod Sci.
25:131–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu X, Wang J, Han K, Li S, Xu F and Yang
Y: Antimalarial drug mefloquine inhibits nuclear factor kappa B
signaling and induces apoptosis in colorectal cancer cells. Cancer
Sci. 109:1220–1229. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bai T, Wang S, Zhao YP, Zhu RT, Wang WJ
and Sun YL: Haloperidol, a sigma receptor 1 antagonist, promotes
ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res
Commun. 491:919–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vasconcelos S, Ramalho C, Marques CJ and
Doria S: Altered expression of epigenetic regulators and imprinted
genes in human placenta and fetal tissues from second trimester
spontaneous pregnancy losses. Epigenetics. 14:1234–1244.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Caseiro AL, Regalo A, Pereira E, Esteves
T, Fernandes F and Carvalho J: Implication of sperm chromosomal
abnormalities in recurrent abortion and multiple implantation
failure. Reprod Biomed Online. 31:481–485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li TC, Makris M, Tomsu M, Tuckerman E and
Laird S: Recurrent miscarriage: Aetiology, management and
prognosis. Hum Reprod Update. 8:463–481. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kaur R and Gupta K: Endocrine dysfunction
and recurrent spontaneous abortion: An overview. Int J Appl Basic
Med Res. 6:79–83. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patriarca A, Piccioni V, Gigante V and
Benedetto C: The use of intravenous immunoglobulin in sine causa or
alloimmune recurrent spontaneous abortion (RSA). Panminerva Med.
42:193–195. 2000.PubMed/NCBI
|
|
28
|
Shu C, Yan D, Chen C, Mo Y, Wu L, Gu J,
Shah NK, He J and Dong S: Metformin exhibits its therapeutic effect
in the treatment of pre-eclampsia via modulating the
Met/H19/miR-148a-5p/P28 and Met/H19/miR-216-3p/EBI3 signaling
pathways. Int Immunopharmacol. 74(105693)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liao S, Yu C, Liu H, Zhang C, Li Y and
Zhong X: Long non-coding RNA H19 promotes the proliferation and
invasion of lung cancer cells and regulates the expression of
E-cadherin, N-cadherin, and vimentin. Onco Targets Ther.
12:4099–4107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hustin J, Jauniaux E and Schaaps JP:
Histological study of the materno-embryonic interface in
spontaneous abortion. Placenta. 11:477–486. 1990.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xiang H, Yan H, Sun B, Feng F and Chen P:
Decreased expression of long non-coding RNA SNHG7 cause recurrent
spontaneous abortion through suppression proliferation and invasion
of trophoblast cells via miR-34a. Am J Transl Res. 11:463–472.
2019.PubMed/NCBI
|
|
32
|
Sun Q and Zhang XL: Research on apoptotic
signaling pathways of recurrent spontaneous abortion caused by
dysfunction of trophoblast infiltration. Eur Rev Med Pharmacol Sci.
21 (Suppl 3):12–19. 2017.PubMed/NCBI
|
|
33
|
Li Z, Zhou G, Jiang L, Xiang H and Cao Y:
Effect of STOX1 on recurrent spontaneous abortion by regulating
trophoblast cell proliferation and migration via the PI3K/AKT
signaling pathway. J Cell Biochem. 120:8291–8299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lv X, Cai Z and Li S: Increased apoptosis
rate of human decidual cells and cytotrophoblasts in patients with
recurrent spontaneous abortion as a result of abnormal expression
of CDKN1A and Bax. Exp Ther Med. 12:2865–2868. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Seiler A, Schneider M, Förster H, Roth S,
Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, et
al: Glutathione peroxidase 4 senses and translates oxidative stress
into 12/15-lipoxygenase dependent- and AIF-mediated cell death.
Cell Metab. 8:237–248. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ingold I, Aichler M, Yefremova E, Roveri
A, Buday K, Doll S, Tasdemir A, Hoffard N, Wurst W, Walch A, et al:
Expression of a catalytically inactive mutant form of glutathione
peroxidase 4 (Gpx4) confers a dominant-negative effect in male
fertility. J Biol Chem. 290:14668–14678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang A, Shang W, Nie Q, Li T and Li S:
Long non-coding RNA H19 suppresses retinoblastoma progression via
counteracting miR-17-92 cluster. J Cell Biochem. 119:3497–3509.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li X, Luo S, Zhang J, Yuan Y, Jiang W, Zhu
H, Ding X, Zhan L, Wu H, Xie Y, et al: lncRNA H19 alleviated
myocardial I/RI via suppressing miR-877-3p/Bcl-2-mediated
mitochondrial apoptosis. Mol Ther Nucleic Acids. 17:297–309.
2019.PubMed/NCBI View Article : Google Scholar
|