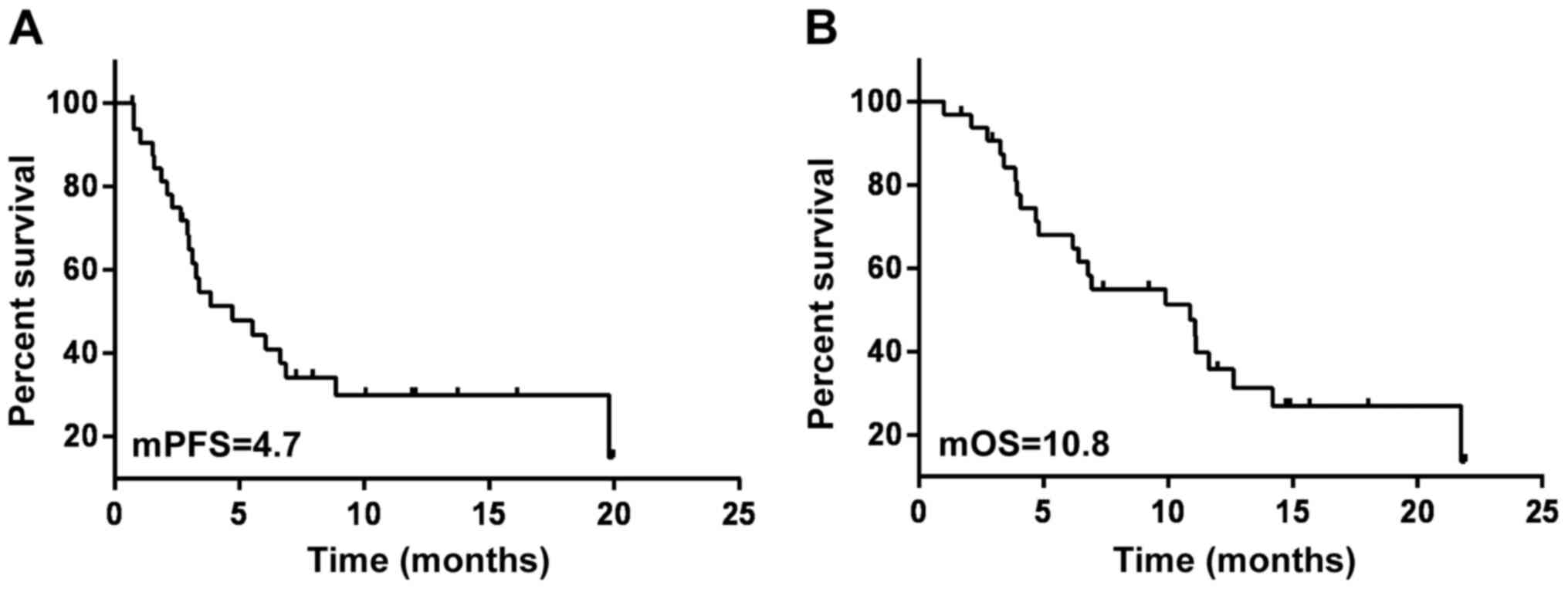
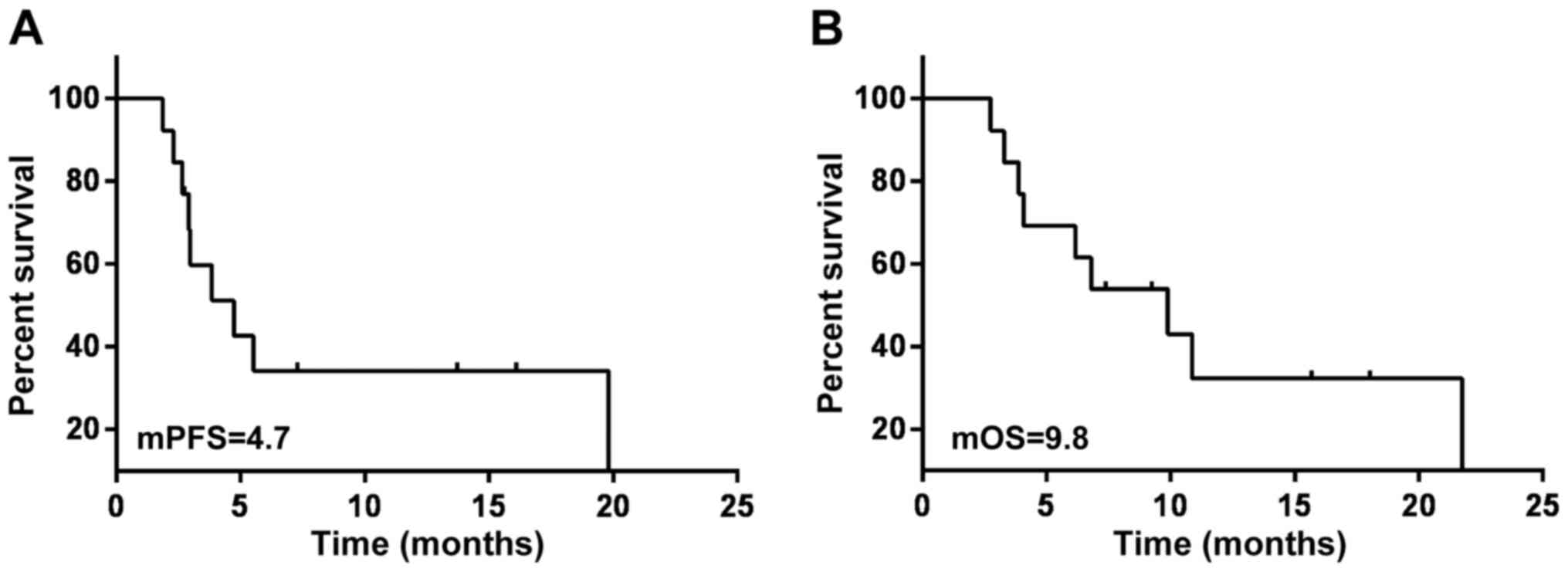
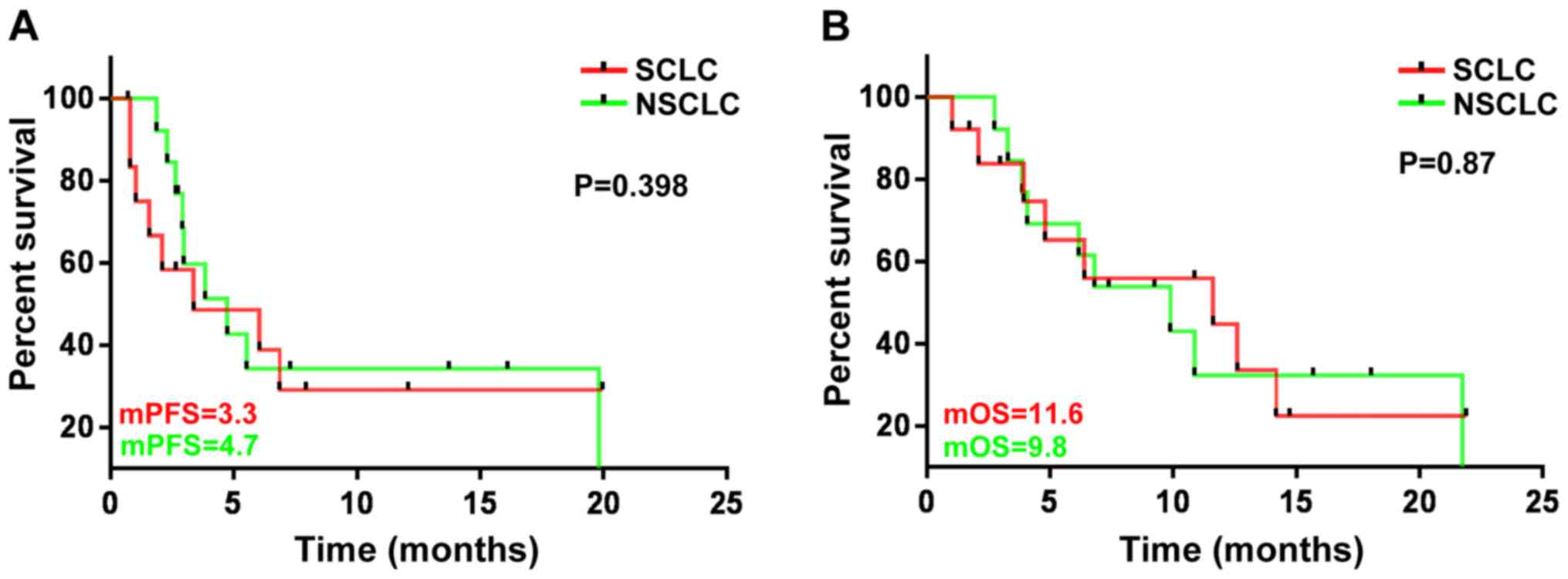
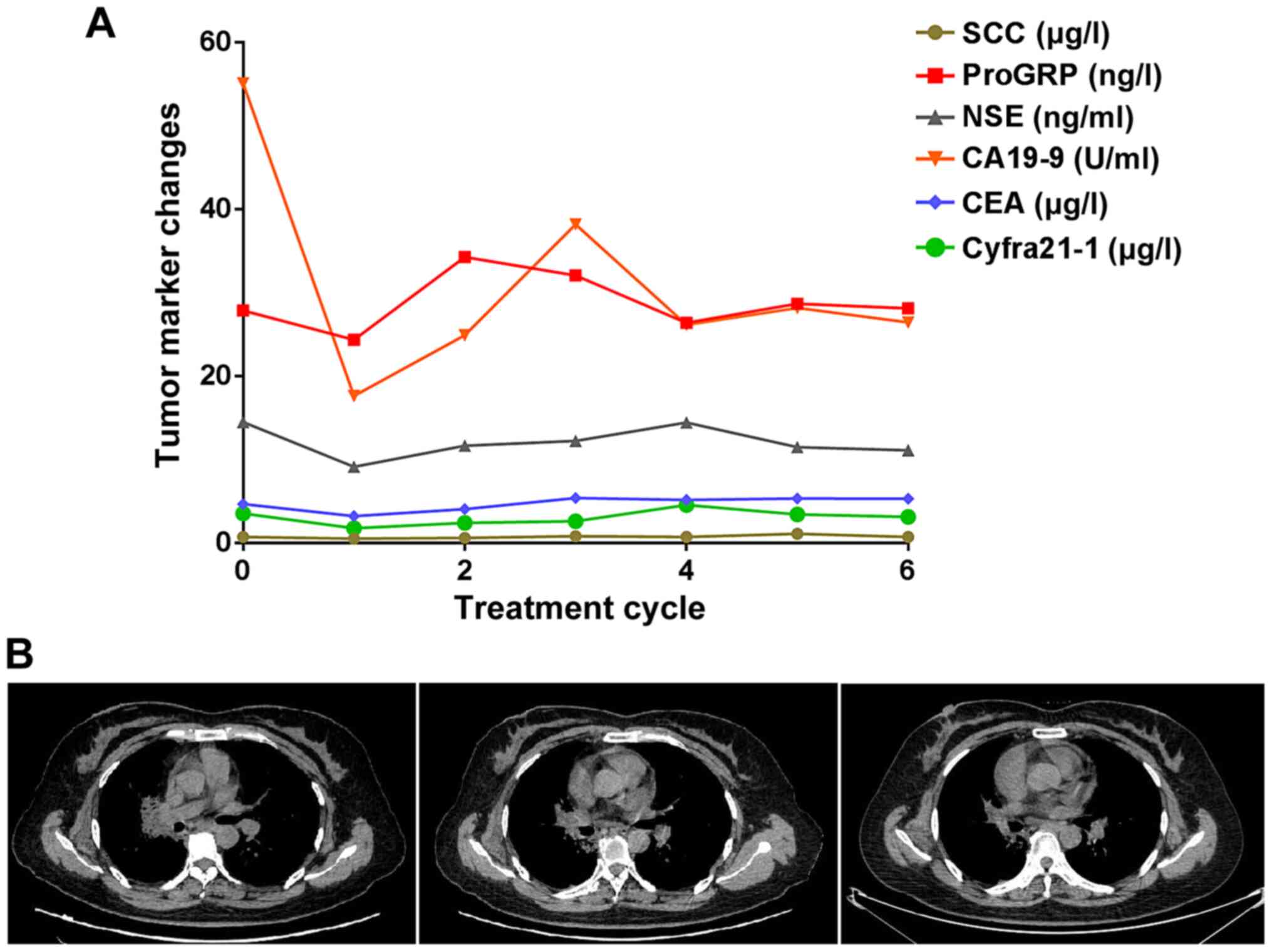
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song Z, Yu X, Lou G, Shi X and Zhang Y:
Salvage treatment with apatinib for advanced non-small-cell lung
cancer. Onco Targets Ther. 10:1821–1825. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Roudsari LC and West JL: Studying the
influence of angiogenesis in in vitro cancer model systems. Adv
Drug Deliv Rev. 97:250–259. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ebos JM, Bocci G, Man S, Thorpe PE,
Hicklin DJ, Zhou D, Jia X and Kerbel RS: A naturally occurring
soluble form of vascular endothelial growth factor receptor 2
detected in mouse and human plasma11ontario graduate scholarship in
science and technology (J.M.L. Ebos); sunnybrook trust for medical
research (G. Bocci); and NIH grant CA41223, Canadian institutes for
health research, and national cancer institute of Canada (R.S.
Kerbel). Mol Cancer Res. 2:315–326. 2004.
|
|
8
|
Geng R, Song L, Li J and Zhao L: The
safety of apatinib for the treatment of gastric cancer. Expert Opin
Drug Saf. 17:1145–1150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brower V: Apatinib in treatment of
refractory gastric cancer. Lancet Oncol. 17(e137)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ding L, Li QJ, You KY, Jiang ZM and Yao
HR: The use of apatinib in treating nonsmall-cell lung cancer: Case
report and review of literature. Medicine (Baltimore).
95(e3598)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li F, Liao Z, Zhao J, Zhao G, Li X, Du X,
Yang Y and Yang J: Efficacy and safety of Apatinib in stage IV
sarcomas: Experience of a major sarcoma center in China.
Oncotarget. 8:64471–64480. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scott A, Messersmith W and Jimeno A:
Apatinib: A promising oral antiangiogenic agent in the treatment of
multiple solid tumors. Drugs Today (Barc). 51:223–229.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J,
Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al: Multicenter phase
II study of apatinib, a novel VEGFR inhibitor in heavily pretreated
patients with metastatic triple-negative breast cancer. Int J
Cancer. 135:1961–1969. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J and Wang L: Efficacy and safety of
apatinib treatment for advanced esophageal squamous cell carcinoma.
Onco Targets Ther. 10:3965–3969. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li F, Zhu T, Cao B, Wang J and Liang L:
Apatinib enhances antitumour activity of EGFR-TKIs in non-small
cell lung cancer with EGFR-TKI resistance. Eur J Cancer.
84:184–192. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L,
Tong Z, Wang S, Li J, Wang Z, et al: Multicenter phase II study of
apatinib in non-triple-negative metastatic breast cancer. BMC
Cancer. 14(820)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shirasaka T, Nakano K, Takechi T, Satake
H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.PubMed/NCBI
|
|
19
|
Yumine K and Kawahara M: Phase II study of
S-1, a novel oral fluorouracil, in advanced non-small-cell lung
cancer. Gan To Kagaku Ryoho. 33 (Suppl 1):189–192. 2006.PubMed/NCBI(In Japanese).
|
|
20
|
Zhang H, Xiong J, Guo L, Patel N and Guang
X: Integrated traditional Chinese and western medicine modulator
for overcoming the multidrug resistance with carbon nanotubes. RSC
Adv. 5:71287–71296. 2015.
|
|
21
|
Wu P, Li S and Zhang H: Design real-time
reversal of tumor multidrug resistance cleverly with shortened
carbon nanotubes. Drug Des Devel Ther. 8:2431–2438. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP,
Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, et al: Apatinib
(YN968D1) reverses multidrug resistance by inhibiting the efflux
function of multiple ATP-binding cassette transporters. Cancer Res.
70:7981–7991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gotwals P, Cameron S, Cipolletta D,
Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S,
Sabatos-Peyton C, Petruzzelli L, et al: Prospects for combining
targeted and conventional cancer therapy with immunotherapy. Nat
Rev Cancer. 17:286–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang SY, Liu Z, Ou W, Li N, Wu HQ, Mao XY
and Yuan C: Apatinib monotherapy for advanced non-small cell lung
cancer after the failure of chemotherapy or other targeted therapy.
J Clin Oncol. 35 (15 Suppl)(e20626)2017.
|
|
25
|
Yan X, Wang Q, Wang H, Li P, Zhang G,
Zhang M, Zheng X, Yang J, Zhang X and Ma Z: Apatinib as maintenance
therapy in extensive-stage small-cell lung cancer: Results from a
single-center retrospective study. J Cancer Res Clin Oncol.
145:235–240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roskoski R Jr: Vascular endothelial growth
factor (VEGF) signaling in tumor progression. Crit Rev Oncol
Hematol. 62:179–213. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kamba T and McDonald D: Mechanisms of
adverse effects of anti-VEGF therapy for cancer. Br J Cancer.
96:1788–1795. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Scott LJ: Apatinib: A review in advanced
gastric cancer and other advanced cancer. Drugs. 78:747–758.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kilickap S, Abali Hs and Celik I:
Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol.
21:3542–3543. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Clark JW, Eder JP, Ryan D, Lathia C and
Lenz HJ: Safety and pharmacokinetics of the dual action Raf kinase
and vascular endothelial growth factor receptor inhibitor, BAY
43-9006, in patients with advanced, refractory solid tumors. Clin
Cancer Res. 11:5472–5480. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Strumberg D, Clark JW, Awada A, Moore MJ,
Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B, et
al: Safety, pharmacokinetics, and preliminary antitumor activity of
sorafenib: A review of four phase I trials in patients with
advanced refractory solid tumors. Oncologist. 12:426–437.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saeki T, Takashima S, Sano M, Horikoshi N,
Miura S, Shimizu S, Morimoto K, Kimura M, Aoyama H, Ota J, et al: A
phase II study of S-1 in patients with metastatic breast cancer-a
Japanese trial by the S-1 cooperative study group, breast cancer
working group. Breast Cancer. 11:194–202. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Goto A, Yamada Y, Yasui H, Kato K,
Hamaguchi T, Muro K, Shimada Y and Shirao K: Phase II study of
combination therapy with S-1 and irinotecan in patients with
advanced colorectal cancer. Ann Oncol. 17:968–973. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Okusaka T, Funakoshi A, Furuse J, Boku N,
Yamao K, Ohkawa S and Saito H: A late phase II study of S-1 for
metastatic pancreatic cancer. Cancer Chemother Pharmacol.
61:615–621. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takakuwa O, Oguri T, Maeno K, Ozasa H,
Iwashima Y, Miyazaki M, Kunii H, Takano Y, Mori T, Sato S and Ueda
R: Efficacy of S-1 monotherapy for non-small cell lung cancer after
the failure of two or more prior chemotherapy regimens. Oncol Lett.
1:147–150. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tatsumi K, Fukushima M, Shirasaka T and
Fujii S: Inhibitory effects of pyrimidine, barbituric acid and
pyridine derivatives on 5-fluorouracil degradation in rat liver
extracts. Jpn J Cancer Res. 78:748–755. 1987.PubMed/NCBI
|
|
39
|
Oguri T, Achiwa H, Bessho Y, Muramatsu H,
Maeda H, Niimi T, Sato S and Ueda R: The role of thymidylate
synthase and dihydropyrimidine dehydrogenase in resistance to
5-fluorouracil in human lung cancer cells. Lung Cancer. 49:345–351.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shirasaka T, Shimamoto Y and Fukushima M:
Inhibition by oxonic acid of gastrointestinal toxicity of
5-fluorouracil without loss of its antitumor activity in rats.
Cancer Res. 53:4004–4009. 1993.PubMed/NCBI
|
|
41
|
Kaira K, Sunaga N, Yanagitani N, Imai H,
Utsugi M, Iwasaki Y, Shimizu K, Iijima H, Tsurumaki H, Tomizawa Y,
et al: Phase 2 study of S-1 plus carboplatin in patients with
advanced non-small cell lung cancer. Lung Cancer. 68:253–257.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hattori K, Heissig B, Wu Y, Dias S, Tejada
R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, et al: Placental
growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+)
stem cells from bone-marrow microenvironment. Nat Med. 8:841–849.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Hood JD, Meininger CJ, Ziche M and Granger
HJ: VEGF upregulates ecNOS message, protein, and NO production in
human endothelial cells. Am J Physiol. 274:H1054–H1058.
1998.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Alexandrescu D, Vaillant J and Dasanu C:
Effect of treatment with a colloidal oatmeal lotion on the acneform
eruption induced by epidermal growth factor receptor and multiple
tyrosine-kinase inhibitors. Clin Exp Dermatol. 32:71–74.
2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Socinski MA, Novello S, Sanchez JM,
Brahmer JA, Govindan R, Belani CP, Atkins JN, Gillenwater HH,
Palleres C and Chao RC: Efficacy and safety of sunitinib in
previously treated, advanced non-small cell lung cancer (NSCLC):
Preliminary results of a multicenter phase II trial. J Clin Oncol.
24 (18 Suppl)(S7001)2006.
|