Introduction
The global burden of gastric cancer has drastically
decreased over the last few decades (1). However, the disease remains a leading
cause of cancer-associated mortality with an overall poor prognosis
(2,3). One of the major factors increasing the
mortality of gastric cancer is late diagnosis. It is estimated that
~80% of cases are diagnosed in the late stages of malignancy
(1,3). Thus, early and accurate diagnosis
along with appropriate TNM staging of all the gastric cancers is
essential (4-7).
Early detection enables the clinician to appropriately select the
treatment strategy and correctly predict overall prognosis
(8).
Several imaging modalities, including endoscopic
ultrasound (EUS), contrast-enhanced computed tomography (CECT),
magnetic resonance imaging (MRI) and
18F-fluorodeoxyglucose positron emission tomography
(18F-FDG PET)/CT may be used for the diagnosis and TNM
staging of gastric cancers (9).
However, no specific guidelines exist regarding the most
appropriate diagnostic modality for the staging of gastric cancer
(10). In addition, there are
limitations to each diagnostic tool for assessing gastric cancer.
EUS cannot be used to evaluate the greater curvature wall, the
fundus or the lymphatic spread (11,12)
and it is highly dependent on the body habitus of the patient
(13). CECT scans have limitations
detecting flat lesions and feature poor contrast resolution for
soft tissues (14,15). This may result in inaccurate
assessments of lymph nodes, as CECT cannot detect microscopic nodal
invasion and cannot exclude malignancy from normal large reactive
nodes (14). MRI also has
limitations including respiratory motion artifacts, high costs,
long examination times and lack of standard gastric cancer
protocols (16,17). Furthermore, nodal assessments via
MRI are also limited by size criteria and the body coverage of a
single examination is not suitable for metastasis staging (18). 18F-FDG PET/CT is a
semi-quantitative method that assesses the FDG uptake in gastric
tumors (19). However, standardized
uptake values depend on numerous factors, including the time
interval post-FDG injection, tumor size, technical parameters and
normoglycemia (20,21). In addition, uptake values vary with
pathological cancer types and mucinous cancers may provide
false-negative results (22).
Such limitations associated with each imaging
modality preclude the accurate preoperative staging of gastric
cancer. Furthermore, ~50% of patients with advanced gastric cancers
develop recurrences after treatment (23,24).
Early detection of recurrence is also essential to reduce mortality
associated with the disease. Out of the several imaging modalities,
CECT and 18F-FDG PET/CT have been commonly used for the
diagnosis and staging of gastric cancer. Studies have assessed the
accuracy of each imaging tool in different settings with variable
results. There is a requirement for high-quality evidence to
determine the accuracy of these imaging modalities to guide
clinical practice. Hence, the present systematic review and
meta-analysis was performed to assess the accuracy of the
diagnostic performance of 18F-FDG PET/CT and CECT for
TNM staging of primary tumors and diagnosis of recurrences in
patients with gastric cancer.
Materials and methods
Inclusion criteria
All types of studies examining the accuracy of CECT
or 18F-FDG PET/CT for diagnosing and staging primary and
recurrent gastric cancer were included. Studies were to compare the
diagnostic accuracy of 18F-FDG PET/CT or CECT (screening
tests) with the histopathological examination result, which was
considered the ‘reference standard’. Full-text articles that
reported on the sensitivity and specificity or provided information
to calculate these values were included. Studies with sample sizes
of <10 patients were excluded.
Search strategy
A systematic electronic search was performed in the
databases PubMed Central, Medline, Scopus, Cochrane Library and
Embase. The following medical subject headings and free-text terms
were used for the search: ‘Validation studies’, ‘gastric
carcinoma’, ‘staging’, ‘prognosis’, ‘gastric cancer’, ‘recurrence’,
‘sensitivity’, ‘specificity’, ‘diagnosis’, ‘computed tomography’,
‘positron emission tomography’, ‘fluorodeoxyglucose’ and
‘diagnostic accuracy studies’. The search included entries from the
inception of the databases up to 1st January 2020 without any
language restrictions. The reference lists of primary trials were
also examined to further identify any relevant articles for
inclusion in the present review.
Selection of studies
A total of two authors (ZZ and BZ) independently
performed the primary screening of titles, key words and abstracts.
Full texts of relevant studies were then retrieved. Secondary
screening of the retrieved articles was then performed to select
studies meeting the inclusion criteria. All disagreements were
resolved in discussion with a third investigator (WC).
Data extraction and management
The primary investigators (ZZ and BZ) extracted the
relevant data from the studies, which included the following: Study
setting, design, inclusion and exclusion criteria, sample size,
comorbidities, the mean age of participants, index test, and
sensitivity and specificity values of the imaging modality. The
data extracted were double-checked during the review and the study
reports to ensure correctness. The study outcomes were as follows:
Sensitivity, specificity, diagnostic odds ratio (DOR), positive
likelihood ratio (LR+), negative likelihood ratio (LR-).
Risk of bias assessment
The Quality Assessment of Diagnostic Accuracy
Studies-2 tool was used to assess the risk of bias for each study
(25). The tool comprises the
following domains: Patient selection bias, conduct and
interpretation of index tests and reference standards, as well as
time interval of outcome assessments. The studies in each domain
were graded as having unclear, high or low risk of bias.
Statistical analysis
The present meta-analysis was performed using the
STATA 14.2 software (StataCorp). The pooled values for sensitivity,
specificity, LR-, LR+ and DOR for each the 18F-FDG
PET/CT and the CECT imaging techniques were obtained using the
bivariate meta-analysis method. A summary receiver operating
characteristic (SROC) curve was generated and the area under the
curve (AUC) was obtained. An AUC value closer to 1 indicated better
diagnostic accuracy. Study-specific and pooled values of
sensitivity and specificity were graphically represented using
forest plots. The clinical values for both 18F-FDG
PET/CT and CECT were determined by generating LR scattergrams. In
addition, the probability that a patient with gastric cancer had
nodal or distant metastases or recurrences was tested using Fagan
plots. Bivariate boxplots were generated and heterogeneity was
tested using the χ2 and I2 statistics
(I2<25%, mild; I2=25-75%, moderate; and
I2>75%, substantial heterogeneity). Publication bias
was assessed graphically by funnel plots and also by Deek's test.
The ‘Midas’ command package in STATA 14.2 software (StataCorp, LP)
was used for all analyses.
Results
Selection of studies
In the database search, a total of 2,934 records
were identified, of which, 1,388 studies were from Medline, 880
from Scopus, 557 from Embase and 109 from the Cochrane library.
After the first stage of screening, 247 studies were retrieved
based on relevance. The full texts of these articles were extracted
and it was assessed whether they fulfilled the inclusion criteria.
Finally, a total of 58 studies met the inclusion criteria and were
included in the review (Fig.
1).
Characteristics of the included
studies
Table I lists the
characteristics of the included studies (14,23,26-81).
The majority of them (37/58) were retrospective in nature. Data
from a total of 9,997 participants were analyzed in the included
studies. The sample sizes of individual studies varied from 18 to
1,964 patients. All of the included studies used histopathology as
the reference standard. Among the studies using 18F-FDG
PET/CT as the index test, 11 reported data on lymph node metastases
and 8 reported on distant metastases, while 16 reported on the
accuracy of the imaging modality for detecting recurrent gastric
cancer tumors. Among the studies using CECT as the index test, 37
studies reported data on lymph node metastases, 7 on distant
metastasis and 4 on recurrent gastric cancer tumors.
 | Table ICharacteristics of the included
studies (n=58). |
Table I
Characteristics of the included
studies (n=58).
| Study number | First author and
year | Country | Study design | Sample size | Type of diagnostic
modality | Gold standard
comparator | Outcomes
reported | Sensitivity and
specificity | (Refs) |
|---|
| 1 | Ahn et al,
2009 | South Korea | Retrospective | 434 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=17.0%
Specificity=91.6% | (26) |
| 2 | Bilici et
al, 2011 | Turkey | Retrospective | 34 | 18F-FDG PET/CT and
CECT | Histopathology | Recurrent gastric
cancer | Sensitivity
(FDG-PET)=95.8% Specificity (FDG-PET)=100.0% Sensitivity
(CECT)=62.5% Specificity (CECT)=100.0% | (27) |
| 3 | Blackshaw et
al, 2003 | United Kingdom | Prospective | 100 | CECT | Histopathology | Distant
metastasis | Sensitivity
(CECT)=46.2% Specificity (CECT)=100.0% | (28) |
| 4 | Bosch et al,
2020 | United Kingdom | Retrospective | 105 | CECT | Histopathology | Distant
metastasis | Sensitivity
(CECT)=40.0% Specificity (CECT)=73.3% | (29) |
| 5 | Cayvarlı et
al, 2014 | Turkey | Retrospective | 130 | 18F-FDG PET/CT and
CECT | Histopathology | Recurrent gastric
cancer | Sensitivity=91.2%
Specificity=61.5% | (30) |
| 6 | Chen et al,
2005 | South Korea | Prospective | 68 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node and
distant metastasis | FDG PET (LN):
Sensitivity=56.0% Specificity=92.0% FDG PET (Distant):
Sensitivity=30.0% Specificity=98.0% CECT (Distant):
Sensitivity=80.0% Specificity=91.0% CECT (LN): Sensitivity=78.0%
Specificity=61.0% | (31) |
| 7 | Chen et al,
2007 | Taiwan | Retrospective | 64 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=88.0%
Specificity=80.0% | (32) |
| 8 | Chen et al,
2006 | Taiwan | Prospective
study | 55 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=86.0%
Specificity=77.0% | (14) |
| 9 | De Potter et
al, 2002 | Belgium | Retrospective
study | 33 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=70.0%
Specificity=69.0% | (33) |
| 10 | D'Elia F et
al, 2000 | Italy | Prospective | 107 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=97.0%
Specificity=65.0% | (34) |
| 11 | Feng et al,
2013 | China | Prospective | 610 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=84.9%
Specificity=61.0% | (35) |
| 12 | Filik et al,
2015 | Turkey | Retrospective | 25 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node
metastasis | FDG PET:
Sensitivity=82.0% Specificity=75.0% CECT: Sensitivity=64.0%
Specificity=100.0% | (36) |
| 13 | Fujikawa et
al, 2014 | Japan | Prospective | 525 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=4.0%
Specificity=98.0% | (37) |
| 14 | Giganti et
al, 2016 | Italy | Prospective | 55 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=90.0%
Specificity=91.0% | (38) |
| 15 | Graziosi et
al, 2011 | Italy | Retrospective | 50 | 18F-FDG PET/CT and
CECT | Histopathology | Recurrent gastric
cancer | Sensitivity=89.0%
Specificity=85.0% | (39) |
| 16 | Ha et al,
2011 | South Korea | Retrospective | 78 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node
metastasis | FDG PET:
Sensitivity=89.0% Specificity=85.0% CECT: Sensitivity=69.0%
Specificity=86.0% | (40) |
| 17 | Hasegawa et
al, 2013 | Japan | Prospective | 315 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=46.4%
Specificity=96.0% | (41) |
| 18 | Hwang et al,
2010 Korea | South | Prospective | 247 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=44.5%
Specificity=85.3% | (42) |
| 19 | Jadvar et
al, 2003 | United States of
America | Retrospective | 18 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=77.7%
Specificity=77.7% | (43) |
| 20 | Joo et al,
2015 | South Korea | Prospective | 47 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=43.3%
Specificity=100.0% | (44) |
| 21 | Karakoyun et
al, 2014 | Turkey | Prospective | 55 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=97.5%
Specificity=73.3% | (45) |
| 22 | Kawanaka et
al, 2016 | Japan | Retrospective
study | 101 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node and
distant metastasis | FDG PET (LN):
Sensitivity=80.0% Specificity=70.0% CECT (Distant):
Sensitivity=75.0% Specificity=97.0% FDG PET (Distant):
Sensitivity=81.0% Specificity=100.0% CECT (LN): Sensitivity=84.0%
Specificity=70.0% | (46) |
| 23 | Kim et al,
2005 | South Korea | Prospective | 106 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=71.7%
Specificity=63.3% | (47) |
| 24 | Kim et al,
2009 | South Korea | Retrospective | 102 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=50.0%
Specificity=91.0% | (48) |
| 25 | Kim et al,
2011 | South Korea | Retrospective | 71 | 18F-FDG PET/CT | Histopathology | Lymph node
metastasis and recurrent gastric cancer | Lymph node
metastasis: Sensitivity=40.0% Specificity=100.0% Recurrent gastric
cancer: Sensitivity=51.0% Specificity=84.0% | (49) |
| 26 | Kim et al,
2013 | South Korea | Retrospective | 171 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=60.0%
Specificity=89.0% | (50) |
| 27 | Kim et al,
2017 | South Korea | Retrospective | 600 | CECT | Histopathology | Recurrent gastric
cancer | Sensitivity=75.9%
Specificity=98.4% | (51) |
| 28 | Kudou et al,
2018 | Japan | Retrospective | 117 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node and
distant metastasis | FDG PET (LN):
Sensitivity=22.6% Specificity=90.0% CECT (Distant):
Sensitivity=60.8% Specificity=67.6% FDG PET (Distant):
Sensitivity=80.0% Specificity=64.0% CECT (LN): Sensitivity=52.0%
Specificity=71.0% | (52) |
| 29 | Lee et al,
2010 | South Korea | Retrospective | 148 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=26.3%
Specificity=98.8% | (53) |
| 30 | Lee et al,
2011 | South Korea | Retrospective | 93 | 18F-FDG PET/CT and
CECT | Histopathology | Recurrent gastric
cancer | FDG PET:
Sensitivity=42.0% Specificity=57.0% CECT: Sensitivity=85.0%
Specificity=87.0% | (54) |
| 31 | Lee et al,
2014 | South Korea | Retrospective | 46 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=100.0%
Specificity=88.0% | (55) |
| 32 | Lim et al,
2006 | South Korea | Retrospective | 112 | CECT | Histopathology | Lymph node and
distant metastasis | Sensitivity=35.0%
Specificity=98.9% | (56) |
| 33 | Marrelli et
al, 2011 | Italy | Prospective | 92 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=84.6%
Specificity=95% | (57) |
| 34 | Mochiki et
al, 2004 | Japan | Prospective | 85 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node
metastasis | FDG PET:
Sensitivity=35.0% Specificity=100.0% CECT: Sensitivity=65.0%
Specificity=77.0% | (23) |
| 35 | Nakamoto et
al, 2009 | Japan | Retrospective | 92 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=77.2%
Specificity=91.7% | (58) |
| 36 | Namikawa et
al, 2014 | Japan | Retrospective | 90 | 18F-FDG PET/CT | Histopathology | Lymph node
metastasis | Sensitivity=64.0%
Specificity=85.0% | (59) |
| 37 | Pan et al,
2013 | China | Prospective | 96 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=91.0%
Specificity=60.0% | (60) |
| 38 | Park et al,
2009 | South Korea | Retrospective | 105 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=74.0%
Specificity=76.0% | (61) |
| 39 | Park et al,
2010 | South Korea | Retrospective | 1964 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=57.0%
Specificity=80.0% | (62) |
| 40 | Park et al,
2014 | South Korea | Retrospective | 74 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=51.0%
Specificity=81.0% | (63) |
| 41 | Perlaza et
al, 2018 | Spain | Prospective | 50 | 18F-FDG PET/CT and
CECT | Histopathology | Distant
metastasis | FDG PET:
Sensitivity=63.0% Specificity=92.0% CECT: Sensitivity=65.0%
Specificity=100.0% | (64) |
| 42 | Ren et al,
2007 | China | Retrospective | 77 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=83.0%
Specificity=75.0% | (65) |
| 43 | Saito et al,
2015 | Japan | Retrospective | 90 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=55.0%
Specificity=86.0% | (66) |
| 44 | Sharma et
al, 2012 | India | Retrospective | 93 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=95.0%
Specificity=79.0% | (67) |
| 45 | Shinohara et
al, 2005 | Japan | Prospective | 451 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=67.0%
Specificity=90.0% | (68) |
| 46 | Sim et al,
2009 | South | Retrospective
Korea | 52 | 18F-FDG PET/CT | Histopathology and
CECT | Recurrent gastric
cancer | FDG PET:
Sensitivity=68.0% Specificity=71.0% CECT: Sensitivity=89.0%
Specificity=64.0% | (69) |
| 47 | Smyth et al,
2012 | United States of
America | Prospective | 113 | 18F-FDG PET/CT | Histopathology | Distant
metastasis | Sensitivity=35.0%
Specificity=98.7% | (70) |
| 48 | Stell et al,
1996 | United Kingdom | Prospective | 65 | CECT | Histopathology | Lymph node and
distant metastasis | LN:
Sensitivity=26.0% Specificity=100.0% Distant: Sensitivity=7.6%
Specificity=100.0% | (71) |
| 49 | Sun et al,
2008 | China | Retrospective | 23 | 18F-FDG PET/CT | Histopathology | Distant
metastasis | Sensitivity=85.0%
Specificity=77.7% | (72) |
| 50 | Tsujimoto et
al, 2010 | Japan | Prospective | 205 | 18F-FDG PET/CT | Histopathology | LN metastasis | Sensitivity=21.0%
Specificity=89.0% | (73) |
| 51 | Turlakow A et
al, 2003 | United States of
America | Retrospective | 37 | 18F-FDG PET/CT | Histopathology | Distant
metastasis | Sensitivity=56.0%
Specificity=93.0% | (74) |
| 52 | Yan et al,
2009 | China | Prospective | 670 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=86.0%
Specificity=76.0% | (75) |
| 53 | Yan et al,
2010 | China | Prospective | 61 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=77.0%
Specificity=73.0% | (76) |
| 54 | Yang et al,
2008 | Japan | Retrospective | 44 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=84.0%
Specificity=84.0% | (77) |
| 55 | Yoon et al,
2012 | South Korea | Retrospective | 372 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node
metastasis | FDG PET:
Sensitivity=59.0% Specificity=88.0% CECT: Sensitivity=70.0%
Specificity=82.0% | (78) |
| 56 | Yun et al,
2005 | South Korea | Retrospective | 30 | 18F-FDG PET/CT | Histopathology | Recurrent gastric
cancer | Sensitivity=94.0%
Specificity=69.0% | (79) |
| 57 | Yun et al,
2005 | South Korea | Retrospective | 81 | 18F-FDG PET/CT and
CECT | Histopathology | Lymph node
metastasis | FDG PET:
Sensitivity=50.0% Specificity=98.0% CECT: Sensitivity=50.0%
Specificity=98.0% | (80) |
| 58 | Zhong et al,
2012 | China | Retrospective | 115 | CECT | Histopathology | Lymph node
metastasis | Sensitivity=87.0%
Specificity=75.0% | (81) |
Methodological quality
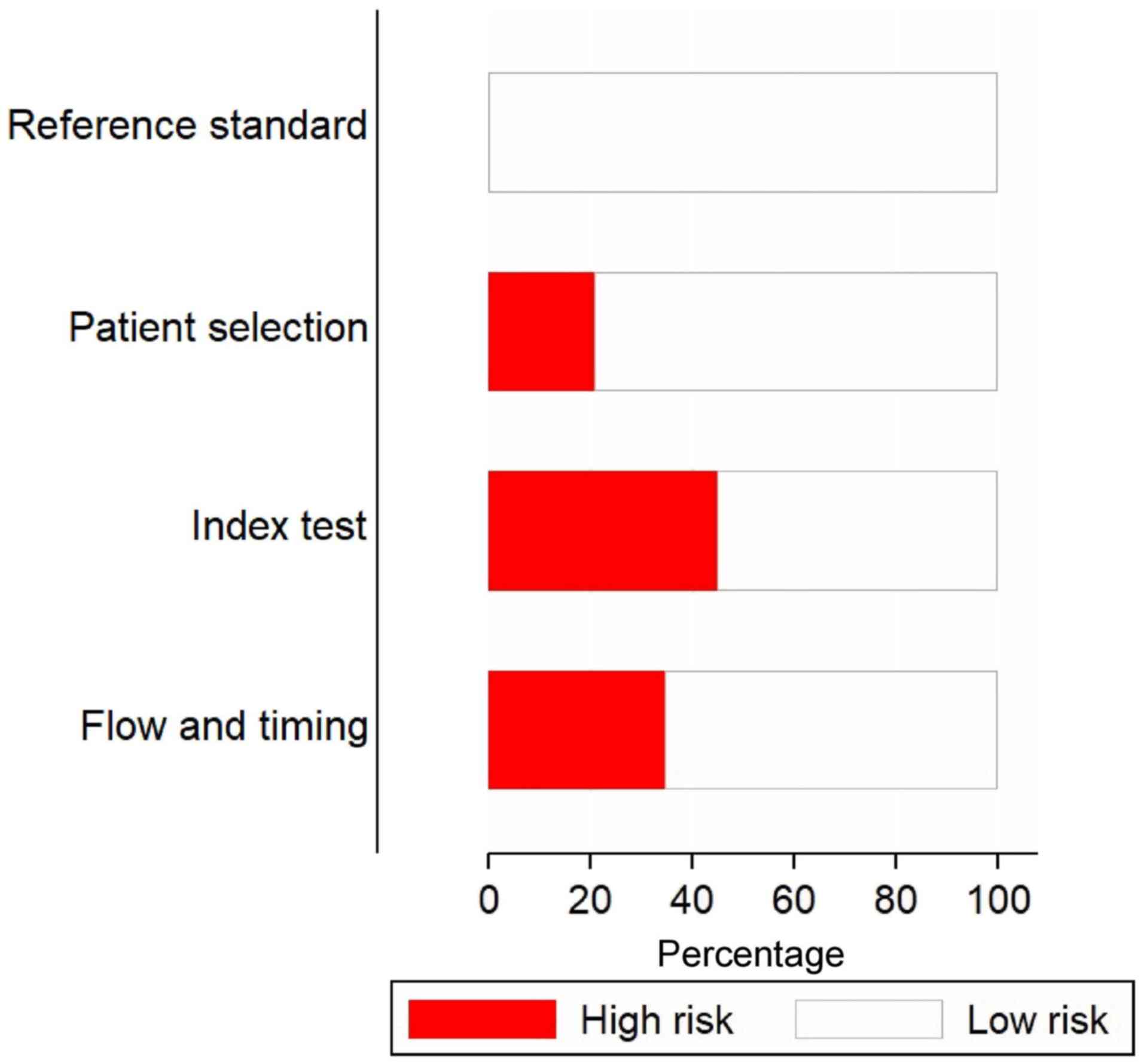
Fig. 2 depicts the
risk of bias assessments for the included studies. A high risk of
patient selection bias was present in almost 20% of the studies.
Furthermore, >40% of the studies had a high risk of bias for
conduct and interpretation of the index test. All of the studies
had a low risk of bias for conduct and interpretation of reference
standards. In addition, ~70% of the studies had low risks of bias
for patient flow and interval between index tests and reference
standards.
Diagnostic performance of
18F-FDG PET/CT Lymph node metastasis
Overall, 11 studies evaluated the accuracy of
18F-FDG PET/CT for diagnosing lymph node metastases (N
staging) among patients with gastric cancer. The pooled sensitivity
and specificity were 49% (95% CI, 37-61%) and 92% (95% CI, 86-96%),
respectively (Fig. 3). The DOR was
11 (95% CI, 6-21). The LR+ was 6.1 (95% CI, 3.5-10.6) and the LR-
was 0.56 (0.44-0.70). The LR+ and LR- values were in the right
lower quadrant of the LR scattergram, indicating that the
18F-FDG PET/CT cannot be used for confirmation or
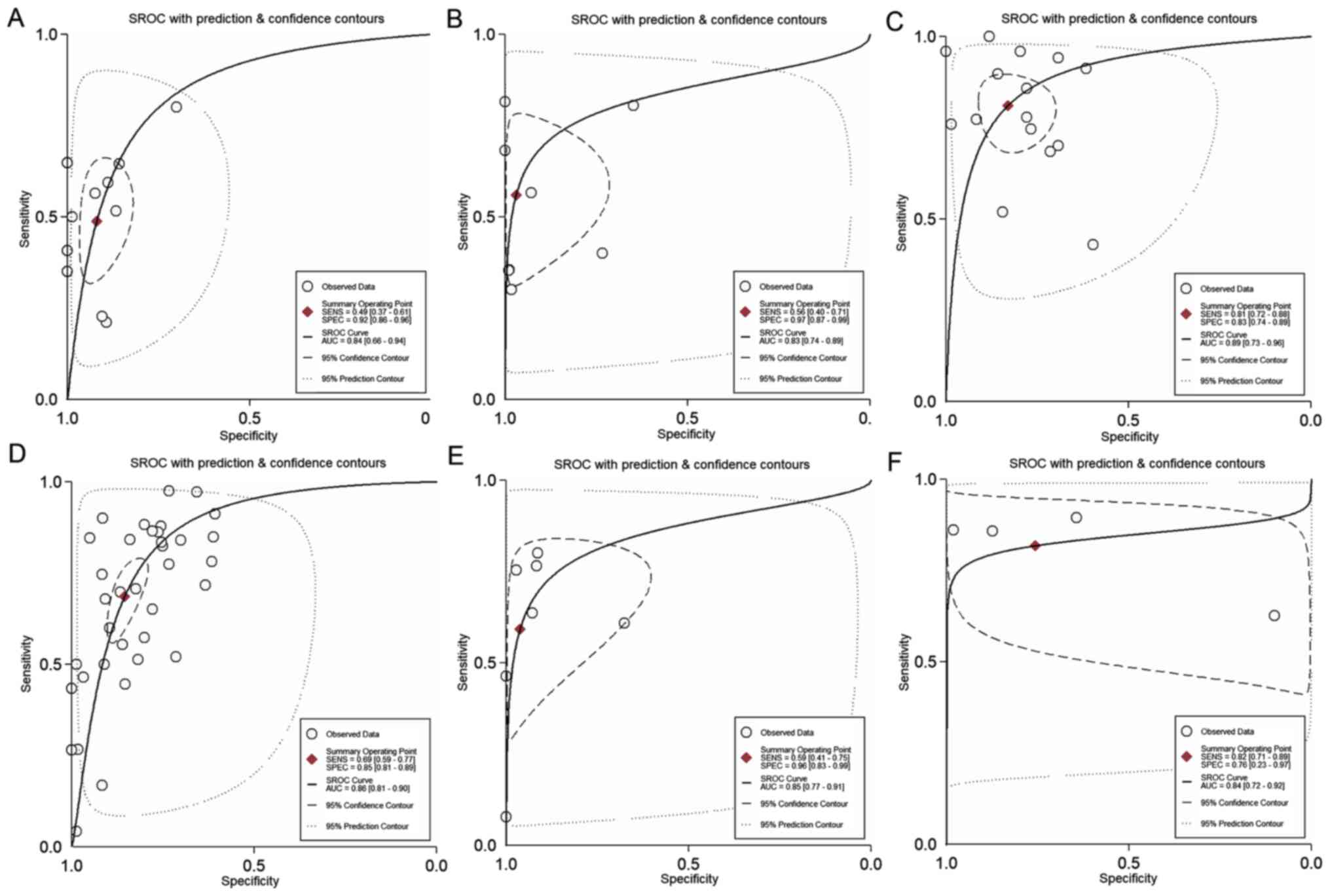
exclusion (Fig. 4). Fig. 5 presents the SROC curve for
diagnosing nodal metastases using 18F-FDG PET/CT. The
AUC was 0.84 (95% CI, 0.66-0.94), indicating a high diagnostic
performance for 18F-FDG PET/CT. Fagan's nomogram
indicated an average clinical utility of 18F-FDG PET/CT
for diagnosing nodal metastasis, as the post-test probability
(positive, 85%; negative, 35%) differed slightly from the pre-test
probability (49%; Fig. 6).
Considerable heterogeneity with a significant
χ2 test (P<0.001) and an I2 value of 87.6%
for pooling the sensitivity and 64.2% for specificity was
determined, indicating substantial heterogeneity (Fig. 3). Of note, two studies were outside
the circle of the bivariate box plot, indicating the possibility of
between-study heterogeneity (Fig.
7). The funnel plot was symmetrical, indicating the absence of
publication bias (Fig. S1), which
was confirmed with a non-significant Deek's test (P=0.44).
Distant metastasis
In total, 8 studies evaluated the accuracy of
18F-FDG PET/CT for diagnosing distant metastases (M
staging) among patients with gastric cancer. The pooled sensitivity
and specificity were 56% (95% CI, 40-71%) and 97% (95% CI, 87-99%),
respectively (Fig. 3). The DOR was
41 (95% CI, 8-206). The LR+ was 18.5 (95% CI, 4.1-83.6) and the LR-
was 0.45 (0.32-0.65). LR+ and LR- values were in the right upper
quadrant of the LR scattergram, indicating that the
18F-FDG PET/CT may be used for confirmation only
(Fig. 4). Fig. 5 presents the SROC curve for
diagnosing distant metastases using 18F-FDG PET/CT. The
AUC of 0.83 (95% CI, 0.74-0.89) suggested a high diagnostic
performance of 18F-FDG PET/CT. Fagan's nomogram
indicated a good clinical utility for 18F-FDG PET/CT for
diagnosing distant metastasis, as the post-test probability
(positive, 91%; negative, 20%) was significantly different from the
pre-test probability (35%) (Fig.
6).
Considerable heterogeneity with a significant
Chi-square test (P<0.001) and an I2 value of 83.5%
for pooling the sensitivity and 94.1% for specificity was
determined, indicating substantial heterogeneity (Fig. 3). Of note, 1 study was outside of
the bivariate box plot circle, indicating the possibility of
between-study heterogeneity (Fig.
7). Publication bias was not assessed, as <10 studies
reported on this outcome.
Recurrent gastric cancer
In total, 16 studies evaluated the accuracy of
18F-FDG PET/CT for diagnosing recurrent gastric cancer.
The pooled sensitivity and specificity were 81% (95% CI, 72-88%)
and 83% (95% CI, 74-89%), respectively (Fig. 3). The DOR was 21 (95% CI, 10-45).
The LR+ was 4.8 (95% CI, 3-7.5) and the LR- was 0.23 (0.15-0.35).
The LR+ and LR- values were in the right lower quadrant of the LR
scattergram, indicating that the 18F-FDG PET/CT should
not be used for confirmation or exclusion (Fig. 4). Fig.
5 presents the SROC curve for diagnosing recurrent gastric
cancer tumors using 18F-FDG PET/CT. The AUC was 0.89
(95% CI, 0.73-0.96), indicating a high diagnostic performance of
18F-FDG PET/CT. Fagan's nomogram suggested a good
clinical utility of 18F-FDG PET/CT for recurrent gastric
cancer diagnosis, as the post-test probability (positive, 73%;
negative, 11%) differed from the pre-test probability (36%;
Fig. 6).
Considerable heterogeneity was determined with a
significant Chi-square test (P<0.001) and an I2 value
of 75.7% for pooling the sensitivity and 89.7% for specificity,
indicating substantial heterogeneity (Fig. 3). A total of 4 studies were outside
of the bivariate box plot circle, implying the possibility of
between-study heterogeneity (Fig.
7). The funnel plot was symmetrical, indicating the absence of
publication bias (Fig. S2). This
was confirmed with a non-significant Deek's test (P=0.10).
Diagnostic performance of CECT. Lymph
node metastasis
In total, 37 studies evaluated the accuracy of CECT
for diagnosing lymph node metastases (N staging) among patients
with gastric cancer. The pooled sensitivity and specificity were
69% (95% CI, 59-77%) and 85% (95% CI, 81-89%), respectively
(Fig. 3). The DOR was 12 (95% CI,
9-17). The LR+ was 4.7 (95% CI, 3.8-5.8) and the LR- was 0.38
(0.30-0.50). The LR+ and LR- values were in the right lower
quadrant of the LR scattergram, indicating that the CECT cannot be
used for confirmation or exclusion (Fig. 4). Fig.
5 presents the SROC curve for diagnosing nodal metastases using
CECT. The AUC was 0.86 (95% CI, 0.81-0.90), indicating a high
diagnostic performance for CECT. Fagan's nomogram suggested an
average clinical utility of CECT for nodal metastasis diagnosis, as
the post-test probability (positive, 77%; negative, 14%) differed
slightly from the pre-test probability (42%; Fig. 6).
Considerable heterogeneity with a significant
Chi-square test (P<0.001) and an I2 value of 94.6%
for pooling the sensitivity and 91.7% for specificity was
determined, indicating substantial heterogeneity (Fig. 3). A total of six studies were
outside the bivariate box plot circle, implying the possibility of
between-study heterogeneity (Fig.
7). The funnel plot was found to be asymmetrical according to
Deeks' test (P=0.02), indicating the presence of publication bias
(Fig. S3).
Distant metastasis
A total of 7 studies evaluated the accuracy of CECT
for diagnosing distant metastasis (M staging) among patients with
gastric cancer. The pooled sensitivity and specificity were 59%
(95% CI, 41-75%) and 96% (95% CI, 83-99%), respectively (Fig. 3). The DOR was 36 (95% CI, 9-147).
The LR+ was 15.4 (95% CI, 3.7-64.3) and the LR- was 0.42
(0.28-0.64). The LR+ and LR- values were in the right upper
quadrant of the LR scattergram, indicating that the CECT may be
used for confirmation only (Fig.
4). Fig. 5 presents the SROC
curve for diagnosing distant metastases using CECT. The AUC was
0.85 (95% CI, 0.77-0.91), indicating a high diagnostic performance
of CECT. Fagan's nomogram suggested a good clinical utility of CECT
for distant metastasis diagnosis, as the post-test probability
(positive, 90%; negative, 20%) differed significantly from the
pre-test probability (37%) (Fig.
6).
Considerable heterogeneity with a significant
Chi-square test (P<0.001) and an I2 value of 79.7%
for pooling the sensitivity and 89.7% for specificity was
determined, indicating substantial heterogeneity (Fig. 3). A total of 2 studies were outside
the bivariate box plot circle, suggesting between-study
heterogeneity (Fig. 7). Publication
bias was not assessed, as <10 studies reported on this
outcome.
Recurrent gastric cancer
In total, 4 studies evaluated the accuracy of CECT
for diagnosing patients with recurrent gastric cancer. The pooled
sensitivity and specificity were 82% (95% CI, 71-89%) and 76% (95%
CI, 23-97%), respectively (Fig. 3).
The DOR was 14 (95% CI, 0.89-217). The LR+ was 3.4 (95% CI,
0.54-21) and the LR- was 0.24 (0.09-0.63). The LR+ and LR- values
were in the right lower quadrant of the LR scattergram, indicating
that the CECT cannot be used for confirmation or exclusion
(Fig. 4). Fig. 5 presents the SROC curve for
diagnosing recurrent gastric cancer using CECT. The AUC was 0.84
(95% CI, 0.72-0.92), indicating a high diagnostic performance of
CECT. Fagan's nomogram suggested a good clinical utility of CECT
for diagnosing recurrent gastric cancer, as the post-test
probability (positive, 66%; negative, 12%) differed from the
pre-test probability (37%) (Fig.
6).
Considerable heterogeneity was determined with a
significant Chi-square test (P<0.001) and an I2 value
of 65.5% for pooling the sensitivity and 95.4% for specificity,
indicating substantial heterogeneity (Fig. 3). Of note, one study was outside the
bivariate box plot circle, indicating the possibility of
between-study heterogeneity (Fig.
7). Publication bias was not assessed, as <10 studies
reported on this outcome.
Discussion
Various imaging modalities are available for the
staging of primary gastric cancers and diagnosing recurrent
lesions. For several years, CECT scans have been routinely used for
preoperative staging of gastric cancer around the world. However,
18F-FDG PET/CT is a relatively new technique that is
being incorporated for the pre-operative staging of several
malignant lesions (19,20). An important advantage offered by
18F-FDG PET/CT is that it combines functional images
from PET and anatomical details of the CT scan, thereby overcoming
the limitations of the individual imaging modalities (21). Both PET and CT are acquired in the
same session for 18F-FDG PET/CT and the modality allows
for the accurate anatomical localization of malignant lesions.
Evidence suggests that 18F-FDG PET/CT may also
facilitate early diagnosis, particularly for recurrent lesions with
negative findings on conventional imaging (19-21).
In order to present high-level evidence to guide clinical practice,
the current literature was reviewed to analyze the diagnostic
accuracies of both 18F-FDG PET/CT and CECT for patients
with primary and recurrent gastric cancers.
The present study provided a pooled analysis of data
from a large number of studies comprising a total of 9,997
participants. Initially, the diagnostic accuracy of both imaging
modalities for lymph node metastases was assessed and it was
revealed that 18F-FDG PET/CT had a pooled sensitivity of
49% and specificity of 92% with a high diagnostic performance
(AUC=0.84). On the other hand, CECT had a better pooled sensitivity
(69%) but lower specificity (85%) and higher diagnostic accuracy
(AUC=0.86) for the same. For distant metastasis, the diagnostic
accuracies of both techniques (sensitivity and specificity) were
similar. Furthermore, for recurrent gastric cancer, the pooled
sensitivities were similar for both techniques, but the pooled
specificity was higher for 18F-FDG PET/CT than for CECT.
The results of the present study concur with previous reviews
conducted by Zhong et al (81) in 2012 and Li et al (82) in 2016, which demonstrated that
18F-FDG PET/CT had a higher diagnostic performance than
CECT for recurrent gastric cancer but CECT is better for
preoperative staging of nodal metastasis. These studies also
suggested that both techniques are equally accurate in detecting
distant metastases among patients with gastric cancer.
The LR scattergrams of both techniques had the LR+
and LR- in the right lower quadrant, indicating that these
techniques cannot be used to exclude or confirm the presence of
lymph node metastases or recurrent gastric cancer tumors. However,
both 18F-FDG PET/CT and CECT had LR scattergrams
occupying the right upper quadrant for distant metastases,
indicating that both techniques may be used for confirming the M
staging of gastric cancer. The clinical values of both
18F-FDG PET/CT and CECT for all the outcomes were high,
as Fagan's nomogram exhibited a significant increase in the
post-test probabilities compared to the pre-test probabilities.
However, while inferring these results, the quality and methodology
differences between the included studies should be considered, as
these may potentially influence the conclusions. There was
significant inter-study heterogeneity among the included studies as
indicated by a significant Chi-square test and I2
statistic results. Furthermore, Deek's test and the funnel plots
indicated the possibility of publication bias among the studies
reporting on the diagnostic accuracy of CECT for lymph node
metastasis. Publication bias for other outcomes for CECT was not
assessed due to an insufficient number of studies in the analysis.
However, there was no evidence of publication bias among the
studies reporting on the outcomes for 18F-FDG
PET/CT.
The present study has the following strengths: As
compared with previous reviews on the subject (81,82),
the present study provided comprehensive and updated evidence on
the accuracy of 18F-FDG PET/CT and CECT for primary
gastric cancer TNM staging and detection of recurrence. The lack of
publication bias for the 18F-FDG PET/CT analysis in the
present review adds credibility to the overall results. However,
the present study also has certain limitations. First, there was a
high risk of bias in certain studies assessing the accuracy of
CECT, which may have influenced the final estimates. In addition,
significant inter-study heterogeneity was identified between the
studies included in the present review. This may have influenced
the accuracy of the pooled results. Finally, no meta-regression was
performed to explore the sources of heterogeneity among the
included studies.
Despite these limitations, the present study
provided valuable insight regarding the diagnostic performance of
two important non-invasive imaging modalities for screening
patients with gastric cancer for preoperative TNM staging and
postoperative recurrence. 18F-FDG PET/CT has a
sensitivity well below the acceptable threshold for N staging for
gastric cancer, indicating that it cannot be used for diagnosing
nodal metastasis in patients with gastric cancer. Although CECT had
a satisfactory sensitivity and specificity for all the outcomes, it
did not meet the SnNout triage test criteria for sensitivity and
the SpPin criteria for the specificity of a diagnostic test for N
staging of gastric cancer and recurrent gastric cancer (83). This means that CECT cannot be used
to confirm or rule out nodal metastases or recurrent gastric cancer
tumors in patients. However, both 18F-FDG PET/CT and
CECT meet the SpPin criteria for the specificity of a diagnostic
test for gastric cancer M staging, which indicates that both
techniques may be used to confirm distant metastasis with a high
level of confidence in patients with gastric cancer. The present
results may prompt a change in clinical practices for the diagnosis
and staging of gastric cancer. Both 18F-FDG PET/CT and
CECT may be used as first-line imaging modalities for M staging of
the disease. However, further studies from different geographical
regions of the world are also required, as current evidence from
low- and middle-income regions is limited. With more generalizable
data, new global guidelines and practices may be generated for
patients with gastric cancer irrespective of the setting.
Affordability of the tests should also be considered by
cost-effectiveness analyses to choose the best and the most
cost-effective technique for gastric cancer diagnosis and
staging.
In conclusion, the present study indicated that both
FDG PET/CT and CECT are highly useful imaging modalities for
diagnosing recurrent gastric cancer due to their high sensitivities
and specificities. These techniques cannot be used to exclude or
confirm the presence of lymph node metastases or recurrent gastric
cancer tumors, but can be used for the confirmation of distal
metastasis.
Supplementary Material
Deeks' Funnel plot for publication
bias. Diagnostic performance of 18F-fluorodeoxyglucose
positron emission tomography/CT for lymph node metastasis. Number
inside each of the circles indicate the study number. EES-Estimated
Effect Size.
Deeks' Funnel plot for publication
bias: Diagnostic performance of 18F-fluorodeoxyglucose
positron emission tomography/CT for recurrent gastric cancer.
Number inside each of the circles indicate the study number. EES.
Estimated Effect Size.
Deeks' Funnel plot for publication
bias: Diagnostic performance of contrast-enhanced CT for lymph node
metastasis. Number inside each of the circles indicate the study
number. EES-Estimated Effect Size.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ, BZ and CJ designed the project; WC and HX were
involved in data collection and data analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balakrishnan M, George R, Sharma A and
Graham DY: Changing trends in stomach cancer throughout the world.
Curr Gastroenterol Rep. 19(36)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Martel C, Forman D and Plummer M:
Gastric cancer: Epidemiology and risk factors. Gastroenterol Clin
North Am. 42:219–240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ono H, Yao K, Fujishiro M, Oda I, Nimura
S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M and Matsui T:
Guidelines for endoscopic submucosal dissection and endoscopic
mucosal resection for early gastric cancer. Dig Endosc. 28:3–15.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Eom BW, Yu JS, Ryu KW, Kook MC, Kim YI,
Cho SJ, Lee JY, Kim CG, Choi IJ, Yoon HM and Kim YW: Optimal
submucosal invasion of early gastric cancer for endoscopic
resection. Ann Surg Oncol. 22:1806–1812. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ajani JA, Bentrem DJ, Besh S, D'Amico TA,
Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et
al: Gastric cancer, version 2.2013: Featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 11:531–546. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
de Steur WO, Hartgrink HH, Dikken JL,
Putter H and van de Velde CJ: Quality control of lymph node
dissection in the Dutch gastric cancer trial. Br J Surg.
102:1388–1393. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Hwang SW and Lee DH: Is endoscopic
ultrasonography still the modality of choice in preoperative
staging of gastric cancer? World J Gastroenterol. 20:13775–13782.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
National Comprehensive Cancer Network:
NCCN clinical practice guidelines in oncology. Gastric Cancer,
V.2.2010, 2010.
|
|
11
|
Lim JH, Ko YT and Lee DH: Transabdominal
US staging of gastric cancer. Abdom Imaging. 19:527–531.
1994.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Suk KT, Lim DW, Kim MY, Park DH, Kim KH,
Kim JM, Kim JW, Kim HS, Kwon SO, Baik SK and Park SJ: Thickening of
the gastric wall on transabdominal sonography: A sign of gastric
cancer. J Clin Ultrasound. 36:462–466. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Solbiati L, Tonolini M, Cova L and
Goldberg SN: The role of contrast-enhanced ultrasound in the
detection of focal liver leasions. Eur Radiol. 11 (Suppl
3):E15–E26. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen CY, Wu DC, Kang WY and Hsu JS:
Staging of gastric cancer with 16-channel MDCT. Abdom Imaging.
31:514–520. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim AY, Kim HJ and Ha HK: Gastric cancer
by multidetector row CT: Preoperative staging. Abdom Imaging.
30:465–472. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tunaci M: Carcinoma of stomach and
duodenum: Radiologic diagnosis and staging. Eur J Radiol.
42:181–192. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kwee RM and Kwee TC: Imaging in local
staging of gastric cancer: A systematic review. J Clin Oncol.
25:2107–2116. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koh DM and Collins DJ: Diffusion-weighted
MRI in the body: Applications and challenges in oncology. AJR Am J
Roentgenol. 188:1622–1635. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JF
and Bosscha K: FDG-PET has no definite role in preoperative imaging
in gastric cancer. Eur J Surg Oncol. 35:449–455. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Thie JA: Understanding the standardized
uptake value, its methods, and implications for usage. J Nucl Med.
45:1431–1434. 2004.PubMed/NCBI
|
|
21
|
Boellaard R, Krak NC, Hoekstra OS and
Lammertsma AA: Effects of noise, image resolution, and ROI
definition on the accuracy of standard uptake values: A simulation
study. J Nucl Med. 45:1519–1527. 2004.PubMed/NCBI
|
|
22
|
Long NM and Smith CS: Causes and imaging
features of false positives and false negatives on F-PET/CT in
oncologic imaging. Insights Imaging. 2:679–698. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mochiki E, Kuwano H, Katoh H, Asao T,
Oriuchi N and Endo K: Evaluation of 18F-2-deoxy-2-fluoro-D-glucose
positron emission tomography for gastric cancer. World J Surg.
28:247–253. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2 Group. QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Internal Med.
155:529–536. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim
SH, Kim WH, Lee KU and Yang HK: Diagnostic accuracy of T and N
stages with endoscopy, stomach protocol CT, and endoscopic
ultrasonography in early gastric cancer. J Surg Oncol. 99:20–27.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bilici A, Ustaalioglu BB, Seker M, Kefeli
U, Canpolat N, Tekinsoy B, Ozugur S and Gumus M: The role of
18F-FDG PET/CT in the assessment of suspected recurrent
gastric cancer after initial surgical resection: Can the results of
FDG PET/CT influence patients' treatment decision making? Eur J
Nucl Med Mol Imaging. 38:64–73. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Blackshaw GR, Barry JD, Edwards P, Allison
MC, Thomas GV and Lewis WG: Laparoscopy significantly improves the
perceived preoperative stage of gastric cancer. Gastric Cancer.
6:225–229. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bosch KD, Chicklore S, Cook GJ, Davies AR,
Kelly M, Gossage JA and Baker CR: Staging FDG PET-CT changes
management in patients with gastric adenocarcinoma who are eligible
for radical treatment. Eur J Nucl Med Mol Imaging. 47:759–767.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cayvarlı H, Bekiş R, Akman T and Altun D:
The role of 18F-FDG PET/CT in the evaluation of gastric cancer
recurrence. Mol Imaging Radionucl Ther. 23:76–83. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen J, Cheong JH, Yun MJ, Kim J, Lim JS,
Hyung WJ and Noh SH: Improvement in preoperative staging of gastric
adenocarcinoma with positron emission tomography. Cancer.
103:2383–2390. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen BB, Liang PC, Liu KL, Hsiao JK, Huang
JC, Wong JM, Lee PH, Shun CT and Ming-Tsang Y: Preoperative
diagnosis of gastric tumors by three-dimensional multidetector row
ct and double contrast barium meal study: Correlation with surgical
and histologic results. J Formos Med Assoc. 106:943–952.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
De Potter T, Flamen P, Van Cutsem E,
Penninckx F, Filez L, Bormans G, Maes A and Mortelmans L:
Whole-body PET with FDG for the diagnosis of recurrent gastric
cancer. Eur J Nucl Med Mol Imaging. 29:525–529. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
D'Elia F, Zingarelli A, Palli D and Grani
M: Hydro-dynamic CT preoperative staging of gastric cancer:
Correlation with pathological findings. A prospective study of 107
cases. Eur Radiol. 10:1877–1885. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, Li
W, Sun XW, Li YF, Xu DZ, Guan YX, et al: Comparison of endoscopic
ultrasonography and multislice spiral computed tomography for the
preoperative staging of gastric cancer-results of a single
institution study of 610 Chinese patients. PLoS One.
8(e78846)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Filik M, Kir KM, Aksel B, Soyda Ç, Özkan
E, Küçük ÖN, İbiş E and Akgül H: The role of 18F-FDG PET/CT in the
primary staging of gastric cancer. Mol Imaging Radionucl Ther.
24:15–20. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fujikawa H, Yoshikawa T, Hasegawa S,
Hayashi T, Aoyama T, Ogata T, Cho H, Oshima T, Rino Y, Morita S and
Masuda M: Diagnostic value of computed tomography for staging of
clinical T1 gastric cancer. Ann Surg Oncol. 21:3002–3007.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Giganti F, Orsenigo E, Arcidiacono PG,
Nicoletti R, Albarello L, Ambrosi A, Salerno A, Esposito A, Petrone
MC, Chiari D, et al: Preoperative locoregional staging of gastric
cancer: Is there a place for magnetic resonance imaging?
Prospective comparison with EUS and multidetector computed
tomography. Gastric Cancer. 19:216–225. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Graziosi L, Bugiantella W, Cavazzoni E,
Cantarella F, Porcari M, Baffa N and Donini A: Role of FDG-PET/CT
in follow-up of patients treated with resective gastric surgery for
tumour. Ann Ital Chir. 82:125–129. 2011.PubMed/NCBI
|
|
40
|
Ha TK, Choi YY, Song SY and Kwon SJ:
F18-fluorodeoxyglucose-positron emission tomography and computed
tomography is not accurate in preoperative staging of gastric
cancer. J Korean Surg Soc. 81:104–110. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hasegawa S, Yoshikawa T, Shirai J,
Fujikawa H, Cho H, Doiuchi T, Yoshida T, Sato T, Oshima T, Yukawa
N, et al: A prospective validation study to diagnose serosal
invasion and nodal metastases of gastric cancer by
multidetector-row CT. Ann Surg Oncol. 20:2016–2022. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hwang SW, Lee DH, Lee SH, Park YS, Hwang
JH, Kim JW, Jung SH, Kim NY, Kim YH, Lee KH, et al: Preoperative
staging of gastric cancer by endoscopic ultrasonography and
multidetector-row computed tomography. J Gastroenterol Hepatol.
25:512–518. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jadvar H, Tatlidil R, Garcia AA and Conti
PS: Evaluation of recurrent gastric malignancy with [F-18]-FDG
positron emission tomography. Clin Radiol. 58:215–221.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Joo I, Lee JM, Kim JH, Shin CI, Han JK and
Choi BI: Prospective comparison of 3T MRI with diffusion-weighted
imaging and MDCT for the preoperative TNM staging of gastric
cancer. J Magn Reson Imaging. 41:814–821. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Karakoyun R, Demirci E, Karakoyun M,
Karakaş B, Gündüz U, Sener Z, Gülenay S, Erol B and Sağtaş E:
Reliability of MDCT, with MPR and hydro-CT technique, in
resectability and lymphnode staging of gastric cancer. Minerva
Chir. 69:129–140. 2014.PubMed/NCBI
|
|
46
|
Kawanaka Y, Kitajima K, Fukushima K, Mouri
M, Doi H, Oshima T, Niwa H, Kaibe N, Sasako M, Tomita T, et al:
Added value of pretreatment (18)F-FDG PET/CT for staging of
advanced gastric cancer: Comparison with contrast-enhanced MDCT.
Eur J Radiol. 85:989–995. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim
PN, Lee MG and Ha HK: Gastric cancer staging at multi-detector row
CT gastrography: Comparison of transverse and volumetric CT
scanning. Radiology. 236:879–885. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim DS, Hong SH, Choi JY, Paeng JC, Kim
NR, Jun WS and Kang HS: Magnetic resonance imaging diagnoses of
bone scan abnormalities in breast cancer patients. Nucl Med Commun.
30:736–741. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY,
Kim BT and Kim HS: The value of PET/CT for preoperative staging of
advanced gastric cancer: Comparison with contrast-enhanced CT. Eur
J Radiol. 79:183–188. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kim SH, Kim JJ, Lee JS, Kim SH, Kim BS,
Maeng YH, Hyun CL, Kim MJ and Jeong IH: Preoperative N staging of
gastric cancer by stomach protocol computed tomography. J Gastric
Cancer. 13:149–156. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim JH, Heo SH, Kim JW, Shin SS, Min JJ,
Kwon SY, Jeong YY and Kang HK: Evaluation of recurrence in gastric
carcinoma: Comparison of contrast-enhanced computed tomography and
positron emission tomography/computed tomography. World J
Gastroenterol. 23:6448–6456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kudou M, Kosuga T, Kubota T, Okamoto K,
Komatsu S, Shoda K, Konishi H, Shiozaki A, Fujiwara H, Arita T, et
al: Value of preoperative PET-CT in the prediction of pathological
stage of gastric cancer. Ann Surg Oncol. 25:1633–1639.
2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lee IJ, Lee JM, Kim SH, Shin CI, Lee JY,
Kim SH, Han JK and Choi BI: Diagnostic performance of 64-channel
multidetector CT in the evaluation of gastric cancer:
Differentiation of mucosal cancer (T1a) from submucosal involvement
(T1b and T2). Radiology. 255:805–814. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lee MR, Unger JG and Rohrich RJ:
Management of the nasal dorsum in rhinoplasty: A systematic review
of the literature regarding technique, outcomes, and complications.
Plast Reconstr Surg. 128:538e–550e. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee DY, Lee CH, Seo MJ, Lee SH, Ryu JS and
Lee JJ: Performance of (18)F-FDG PET/CT as a postoperative
surveillance imaging modality for asymptomatic advanced gastric
cancer patients. Ann Nucl Med. 28:789–795. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH,
Hwang HS, Park MS, Cha SW, Lee JD, Noh SH, et al: Comparison of CT
and 18F-FDG pet for detecting peritoneal metastasis on the
preoperative evaluation for gastric carcinoma. Korean J Radiol.
7:249–256. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Marrelli D, Mazzei MA, Pedrazzani C, Di
Martino M, Vindigni C, Corso G, Morelli E, Volterrani L and
Roviello F: High accuracy of multislices computed tomography (MSCT)
for para-aortic lymph node metastases from gastric cancer: A
prospective single-center study. Ann Surg Oncol. 18:2265–2272.
2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nakamoto Y, Togashi K, Kaneta T, Fukuda H,
Nakajima K, Kitajima K, Murakami K, Fujii H, Satake M, Tateishi U,
et al: Clinical value of whole-body FDG-PET for recurrent gastric
cancer: A multicenter study. Jpn J Clin Oncol. 39:297–302.
2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Namikawa T, Okabayshi T, Nogami M, Ogawa
Y, Kobayashi M and Hanazaki K: Assessment of
(18)F-fluorodeoxyglucose positron emission tomography combined with
computed tomography in the preoperative management of patients with
gastric cancer. Int J Clin Oncol. 19:649–655. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pan Z, Pang L, Ding B, Yan C, Zhang H, Du
L, Wang B, Song Q, Chen K and Yan F: Gastric cancer staging with
dual energy spectral CT imaging. PLoS One. 8(e53651)2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Park MJ, Lee WJ, Lim HK, Park KW, Choi JY
and Kim BT: Detecting recurrence of gastric cancer: The value of
FDG PET/CT. Abdom Imaging. 34:441–447. 2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Park SR, Kim MJ, Ryu KW, Lee JH, Lee JS,
Nam BH, Choi IJ and Kim YW: Prognostic value of preoperative
clinical staging assessed by computed tomography in resectable
gastric cancer patients: A viewpoint in the era of preoperative
treatment. Ann Surg. 251:428–435. 2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Park K, Jang G, Baek S and Song H:
Usefulness of combined PET/CT to assess regional lymph node
involvement in gastric cancer. Tumori. 100:201–206. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Perlaza P, Ortín J, Pagès M, Buxó E,
Fernández-Esparrach G, Colletti PM, Rubello D, Mayoral M, Sánchez
N, Ruiz C, et al: Should 18F-FDG PET/CT be routinely performed in
the clinical staging of locally advanced gastric adenocarcinoma?
Clin Nucl Med. 43:402–410. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ren G, Cai R and Chen KM: Clinical value
of multidetector computed tomography in detecting lymph node
metastasis of early gastric cancer. Zhonghua Zhong Liu Za Zhi.
29:852–855. 2007.PubMed/NCBI(In Chinese).
|
|
66
|
Saito T, Kurokawa Y, Takiguchi S, Miyazaki
Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Mori M and Doki
Y: Accuracy of multidetector-row CT in diagnosing lymph node
metastasis in patients with gastric cancer. Eur Radiol. 25:368–374.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sharma P, Singh H, Suman SK, Sharma A,
Reddy RM, Thulkar S, Bal C, Malhotra A and Kumar R: 18F-FDG PET-CT
for detecting recurrent gastric adenocarcinoma: Results from a
non-oriental Asian population. Nucl Med Commun. 33:960–966.
2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Shinohara T, Ohyama S, Yamaguchi T, Muto
T, Kohno A, Ogura T, Kato Y and Urashima M: Preoperative TNM
staging of advanced gastric cancer with multi-detector row computed
tomography. JMAJ. 48:175–182. 2005.
|
|
69
|
Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW,
Kang WJ, Im SA, Kim TY, Kim WH, Heo DS and Bang YJ: The role of
PET/CT in detection of gastric cancer recurrence. BMC Cancer.
9(73)2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Smyth E, Schöder H, Strong VE, Capanu M,
Kelsen DP, Coit DG and Shah MA: A prospective evaluation of the
utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission
tomography and computed tomography in staging locally advanced
gastric cancer. Cancer. 118:5481–5488. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Stell DA, Carter CR, Stewart I and
Anderson JR: Prospective comparison of laparoscopy, ultrasonography
and computed tomography in the staging of gastric cancer. Br J
Surg. 83:1260–1262. 1996.PubMed/NCBI
|
|
72
|
Sun L, Su XH, Guan YS, Pan WM, Luo ZM, Wei
JH and Wu H: Clinical role of 18F-fluorodeoxyglucose positron
emission tomography/computed tomography in post-operative follow up
of gastric cancer: Initial results. World J Gastroenterol.
14:4627–4632. 2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Tsujimoto H, Sugasawa H, Ono S, Ichikura
T, Yamamoto J and Hase K: Has the accuracy of preoperative
diagnosis improved in cases of early-stage gastric cancer? World J
Surg. 34:1840–1846. 2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Turlakow A, Yeung HW, Salmon AS,
Macapinlac HA and Larson SM: Peritoneal carcinomatosis: Role of
(18)F-FDG PET. J Nucl Med. 44:1407–1412. 2003.PubMed/NCBI
|
|
75
|
Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL,
Chen J, Xiang M, Chen MM, Liu BY, Yin HR and Lin YZ: Value of
multidetector-row computed tomography in the preoperative T and N
staging of gastric carcinoma: A large-scale Chinese study. J Surg
Oncol. 100:205–214. 2009.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL,
Chen J, Xiang M, Chen MM, Liu BY, Yin HR and Lin YZ: Size of the
largest lymph node visualized on multi-detector-row computed
tomography (MDCT) is useful in predicting metastatic lymph node
status of gastric cancer. J Int Med Res. 38:22–33. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yang QM, Kawamura T, Itoh H, Bando E,
Nemoto M, Akamoto S, Furukawa H and Yonemura Y: Is PET-CT suitable
for predicting lymph node status for gastric cancer?
Hepatogastroenterology. 55:782–785. 2008.PubMed/NCBI
|
|
78
|
Yoon NR, Park JM, Jung HS, Cho YK, Lee IS,
Choi MG, Chung IS, Song KY and Park CH: Usefulness of
18F-fluoro-2-deoxyglucose positron emission tomography in
evaluation of gastric cancer stage. Korean J Gastroenterol.
59:347–353. 2012.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yun M, Choi HS, Yoo E, Bong JK, Ryu YH and
Lee JD: The role of gastric distention in differentiating recurrent
tumor from physiologic uptake in the remnant stomach on 18F-FDG
PET. J Nucl Med. 46:953–957. 2005.PubMed/NCBI
|
|
80
|
Yun M, Lim JS, Noh SH, Hyung WJ, Cheong
JH, Bong JK, Cho A and Lee JD: Lymph node staging of gastric cancer
using (18)F-FDG PET: A comparison study with CT. J Nucl Med.
46:1582–1588. 2005.PubMed/NCBI
|
|
81
|
Zhong BY, Liu YX, Huang WF, Liu QQ, Liu SQ
and Liu Y: Clinical value of 64-slice spiral 3-phase CT enhanced
scanning for preoperative TNM staging assessment of gastric
carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi. 15:706–709.
2012.PubMed/NCBI(In Chinese).
|
|
82
|
Li P, Liu Q, Wang C, Wang T, Liu J, Huang
G and Song S: Fluorine-18-fluorodeoxyglucose positron emission
tomography to evaluate recurrent gastric cancer after surgical
resection: A systematic review and meta-analysis. Ann Nucl Med.
30:179–187. 2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Seevaratnam R, Cardoso R, McGregor C,
Lourenco L, Mahar A, Sutradhar R, Law C, Paszat L and Coburn N: How
useful is preoperative imaging for tumor, node, metastasis (TNM)
staging of gastric cancer? A meta-analysis. Gastric Cancer. 15
(Suppl 1):S3–S18. 2012.PubMed/NCBI View Article : Google Scholar
|