Introduction
Increasing evidence has indicated that the existence
of donor-specific human leukocyte antigen (HLA) antibodies (DSAs)
may adversely affect the long-term survival of grafts (1). DSAs in kidney and heart transplant
recipients have been associated with acute T-cell-mediated
rejection, antibody-mediated rejection (AMR), progression to
chronic rejection, late graft dysfunction, vasculopathy and
allograft loss (2-6).
In adult liver transplantation, the effect of DSAs
on long-term survival is controversial, but DSAs may be a risk
factor for poor survival (7-9).
Patients undergoing liver transplantation with preformed DSAs have
been indicated to be at increased risk of hyperacute rejection
(10) and AMR within the first
weeks after transplantation (11-13).
In addition, DSAs have been associated with chronic rejection
(14,15), accelerated fibrosis (16,17)
and anastomotic biliary strictures (18). Compared with adult recipients,
pediatric liver transplant recipients exhibit a higher incidence of
DSAs after liver transplantation. It has been reported that the
positive rate of DSAs in pediatric liver transplant recipients can
be as high as 54% (19). However,
the relationship between the presence of DSAs and AMR after
pediatric liver transplantation and how it affects the survival of
allogeneic liver transplant recipients is not clear.
The purpose of the present study was to analyze the
effect of DSAs on the liver function and survival of 48 pediatric
patients undergoing liver transplantation at the Tianjin First
Central Hospital (Tianjin, China).
Materials and methods
Patients and samples
A retrospective analysis of 48 pediatric patients
undergoing liver transplantation (age, 0-11 years) at the Tianjin
First Central Hospital, enrolled between January 2015 and December
2018, was conducted. The following criteria were used to select
patients for the present study: i) All children with complete
follow-up data after liver transplantation; and ii) all pediatric
liver transplant recipients who received immunosuppressive therapy
consisting of tacrolimus combined with corticosteroids.
Immunosuppression
All recipients were treated with tacrolimus and
corticosteroids. Methylprednisolone and basiliximab were also used
to treat patients following pediatric liver transplantations;
basiliximab was given on the fourth day post-operatively.
Sequential treatment with methylprednisolone was administered
post-operatively and stopped at 6 months. Tacrolimus was
administered on the second day after liver transplantation, and the
trough concentration (7-10 ng/ml) was maintained up to 3 months
after pediatric liver transplantation. Following detection of DSAs,
the recipients were treated with mycophenolate mofetil (MMF; 600
mg/m2 per dose twice daily).
Diagnostic criteria for AMR following
pediatric liver transplantation
The diagnostic criteria for AMR after pediatric
liver transplantation included the following: i) early liver
transplantation insufficiency; ii) diffuse vascular endothelial
injury and small perivascular inflammation; iii) high DSA
positivity; iv) strong diffuse C4d linear staining of liver tissue;
and v) improvement in liver function (measured using standard
biochemical tests), decreased DSA level, improvement in liver
histology (such as fibrosis and inflammation) and disappearance of
C4d deposition after anti-AMR treatment.
HLA typing and HLA antibody
determination
All patients and donors were typed for HLA-A, -B,
-DRB1 and -DQB1. HLA class I and II typing was performed by
molecular methods (PCR-sequence-specific oligonucleotide
techniques; One Lambda Inc.) (20).
The detection of HLA antibodies was performed using Lifecodes
single-antigen beads class I and II (Immucor Inc.), according to
the manufacturer's protocol (21).
Antibody results of liver transplantation recipients were compared
with the HLA of the corresponding donor to determine whether they
were donor-specific. The interpretation criteria for positive
samples were as follows: Mean fluorescence intensity (MFI)
>10,000 was strongly positive, 4,000< MFI <10,000 was
moderately positive, 750< MFI <4,000 was weakly positive and
<750 was negative.
Liver histology
Liver tissue specimens obtained by ultrasound-guided
biopsy were fixed for 12 h in 4% formaldehyde solution at room
temperature, and 3-µm paraffin-embedded sections were cut according
to the conventional procedure. For H&E staining, hematoxylin
staining was performed for 5 min, followed by immersion in 1%
hydrochloric acid alcohol for 30 sec, light ammonia water for 30
sec, eosin staining for 1 min and gradient alcohol dehydration at
room temperature (all from Wexis Group Limited). For Masson's
trichrome, the slides were stained with hematoxylin for 3-5 min,
followed by Ponceau acid red for 5-8 min at room temperature. After
washing with water, the slides were sequentially immersed in
phosphomolybdic acid for 3 min, acetic acid for 20 sec,
water-soluble aniline blue for 30 sec, 95% ethanol and xylene for
dehydration at room temperature (all from Beijing Yili Fine
Chemicals co., Ltd.). For C4d staining, after antigen retrieval
(high pressure for 3 min at 120˚C), the anti-C4d antibody (cat no.
ab187931; Abcam) was incubated at 4˚C overnight, followed by
HRP-labeled anti-mouse IgG antibody (provided in the ultraView
Universal DAB Detection Kit; cat. no. 760-500; Roche Diagnostics)
at 37˚C for 30 min, incubation with 3,3-diaminobenzidine at room
temperature for 5 min for color development and hematoxylin
counterstain for 30 sec (both from Roche Applied Science).
Statistical analysis
Statistical analysis was performed using SPSS
(version 20.0; IBM Corp.) and GraphPad Prism (version 5.0; GraphPad
Software, Inc.) software. Continuous variables are presented as the
mean ± standard deviation for normally distributed data and as
median (range) when the values were not normally distributed. The
differences between the groups were tested by independent samples
t-test or χ2 test, as appropriate. The Mann-Whitney U
test was used when parameters exhibited a non-normal distribution.
Kaplan-Meier survival analysis was performed to analyze clinical
events following liver transplantation, and statistical
significance was determined by log rank testing. Multivariate
analyses were performed using the Cox proportional hazards
regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
All patients were children who underwent a primary
liver transplant. Patient characteristics are outlined in Table I. The mean recipient age was 3.7±2.6
years. The primary disease of 38 (79.2%) patients was biliary
atresia. A total of 35 livers were from parental donors and 13
livers were from deceased organ donors.
 | Table ICharacteristics of pediatric liver
transplant recipients (n=48). |
Table I
Characteristics of pediatric liver
transplant recipients (n=48).
| Characteristics | Data at
transplantation |
|---|
| Age, mean ± SD
(range) | 3.7±2.6 (1-11) |
| Sex
(male/female) | 27/21 |
| Clinical diagnosis
(n) | |
|
Biliary
atresia | 38 |
|
Autoimmune
liver cirrhosis | 1 |
|
Congenital
bile duct dilatation | 1 |
|
Bile
cirrhosis | 1 |
|
Alagille
syndrome | 1 |
|
Cholestasis | 6 |
| Liver transplant type
(n) | |
|
Parental
transplantation | 35 |
|
Liver
transplantation after donor death | 13 |
| Blood type
combination (n) | |
|
Identical | 31 |
|
Compatible | 17 |
Prevalence of DSAs in pediatric liver
transplantation
It was observed that 10 of 48 (20.8%) pediatric
patients developed DSAs post-liver transplantation (Table II). One of the 10 patients (10%)
developed DSAs against HLA class I and II antigens, and nine (90%)
developed DSAs against HLA class II antigens. Six of the 10
patients (60%) developed one DSA, 2 patients (20%) developed two
DSAs and 2 patients (20%) developed three DSAs. The
locus-specificity of DSAs was as follows: One (10%) against the A
locus, 4 (40%) against the DR locus and 9 (90%) against the DQ
locus. AMR occurred after pediatric liver transplant in in 4 of the
10 patients (40%). In patients with AMR, the mean MFI of HLA-DQ was
16,807 (range, 13,707-19,356) and the mean MFI of HLA-DR was 12,291
(range, 6,351-18,231). In patients with no AMR, the mean MFI of
HLA-DQ was 5,461 (range, 1,297-18,045) and the mean MFI of HLA-DR
was 1,079 (range, 761-1,397).
 | Table IIDemographic and clinical data and
specifications of patients with DSA (n=10). |
Table II
Demographic and clinical data and
specifications of patients with DSA (n=10).
| |
Patients |
|---|
| Patient
characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|
| Sex (M/F) | F | M | M | F | F | M | M | M | F | F |
| Age at LT
(years) | 2 | 3 | 11 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| Donor age
(years) | 1 | 34 | 34 | 32 | 29 | 34 | 32 | 31 | 1 | 28 |
| Donor type
(DCD/P) | DCD | DCD | P | P | P | P | P | P | DCD | P |
| Disease type | BA | AS | ALC | BA | BA | BA | BA | BA | BA | BA |
| Amount of blood
transfusions during surgery |
|
|
Erythrocytes
(U) | 2 | 4 | 4 | 4 | 2 | 2 | 4 | 4 | 4 | 2 |
|
Fresh-frozen
plasma (ml) | 400 | 400 | 600 | 400 | 200 | 200 | 400 | 400 | 400 | 200 |
| Time post-LT,
months | 7 | 4 | 1 | 1 | 1 | 2 | 4 | 24 | 12 | 8 |
| AMR (Y/N) | N | N | N | Y | Y | Y | Y | N | N | N |
| Type of DSA
(MFI) | DQ6 (18,045) | DQ5(807) DQ6
(2,573) DR15(761) | DQ7 (3,496) | DQ7 (15,527) | DR7 (6,351) DQ8
(13,707) | DQ4 (18,640) | A2 (2,463) DR7
(18,231) DQ8 (19,356) | DR7 (1,397) DR9
(1,365) | DQ7 (1,894) | DQ6 (1,297) |
|
Immunosuppression | Tac | Tac | Tac | Tac | Tac | Tac | Tac | Tac | Tac | Tac |
| Tacrolimus level in
the presence of DSAs (ng/ml) | 3.5 | 6.9 | 12.6 | 4.3 | 6.5 | 8.1 | 7.6 | 5.0 | 3.4 | 7.6 |
| Biochemical liver
test in the presence of DSAs |
|
|
AST
(U/l) | 288.6 | 418 | 280.2 | 331.6 | 273.9 | 208.9 | 277.1 | 40.4 | 21.7 | 40.8 |
|
ALT
(U/l) | 381 | 317.4 | 232.4 | 307.8 | 237.5 | 160.2 | 301.1 | 50.9 | 35.9 | 48.8 |
|
ALP
(U/l) | 279 | 382 | 181 | 463.7 | 231 | 360.3 | 287.5 | 240 | 331 | 294 |
|
Bilirubin
(mg/dl) | 5.4 | 223.7 | 13.38 | 207.2 | 234.8 | 174.9 | 156.4 | 14.9 | 3.5 | 3.58 |
Differences in various indexes in
different pediatric liver transplantation groups
According to the HLA classification of donors and
recipients, and the DSA detection results, the recipients were
divided into two groups: A DSA-positive group and a DSA-negative
group. There was no significant difference in age, sex, donor age,
donor type and intraoperative blood transfusion between the two
groups (P>0.05). There were significant differences in the
tacrolimus level in the presence of DSA, postoperative alanine
aminotransferase (ALT), aspartate aminotransferase (AST), total
bilirubin and follow-up time (time post-LT) between the two groups
(P<0.05; Table III).
 | Table IIIComparison of demographic and
clinical parameters between pediatric transplant patient
groups. |
Table III
Comparison of demographic and
clinical parameters between pediatric transplant patient
groups.
|
Characteristics | DSA group | Non-DSA group | P-value |
|---|
| Number of
patients | 10 | 38 | |
| Sex
(male/female) | 5/5 | 22/16 | 0.6543 |
| Age at LT, mean ±
SD (years) | 2.6±0.9 | 2.6±0.4 | 0.9961 |
| Donor age, mean ±
SD (years) | 25±4.2 | 26±1.9 | 0.9321 |
| Donor type
(DCD/Parental) | 3/7 | 10/28 | 0.8156 |
| Amount of blood
transfusions during surgery | | | |
|
Erythrocytes
(U) | 3.2 (2-4) | 2.9 (2-4) | 0.4745 |
|
Fresh-frozen
plasma (ml) | 360 (200-400) | 340 (200-600) | 0.8329 |
| Number of
transfusions, mean ± SD (n) | 0.9±0.32 | 0.74±0.45 | 0.0110 |
| Time post-LT, mean
± SD (months) | 7.4±4.5 | 17.5±7.8 | 0.0091 |
| Acute rejection
(Y/N) | 6/4 | 3/35 | 0.0010 |
| Antibody-mediated
rejection (Y/N) | 4/6 | 0/38 | 0.0010 |
| Tacrolimus level in
the presence of DSA (ng/ml) | 6.6±0.9 | 3.9±0.3 | 0.0005 |
| Biochemical liver
test at biopsy | | | |
|
ALT
(U/l) | 227.4±46.0 | 43.0±7.5 | <0.0001 |
|
AST
(U/l) | 198.1±36.4 | 43.6±3.8 | <0.0001 |
|
ALP
(U/l) | 305.0±26.0 | 261.8±17.9 | 0.2684 |
|
Bilirubin
(mg/dl) | 103.8±32.7 | 7.6±1.0 | <0.0001 |
Impact of DSAs on patient and
allograft survival
The role of DSAs in predicting patient survival was
then analyzed using Cox proportional hazard analysis (Table IV). The multivariate model
identified DSAs and recipient age <2-years-old as independent
predictors of patient death (P<0.05).
 | Table IVCox regression analysis of patient
survival predictors. |
Table IV
Cox regression analysis of patient
survival predictors.
| | Multivariate
analysis |
|---|
| Variables | 95% CI | P-value |
|---|
| DSAs | 0.182-0.952 | 0.038 |
| Patient age
<2-years-old | 0.678-0.996 | 0.045 |
| Donor age
<2-years-old | 0.967-1.042 | 0.837 |
| Donor type | 0.124-1.071 | 0.066 |
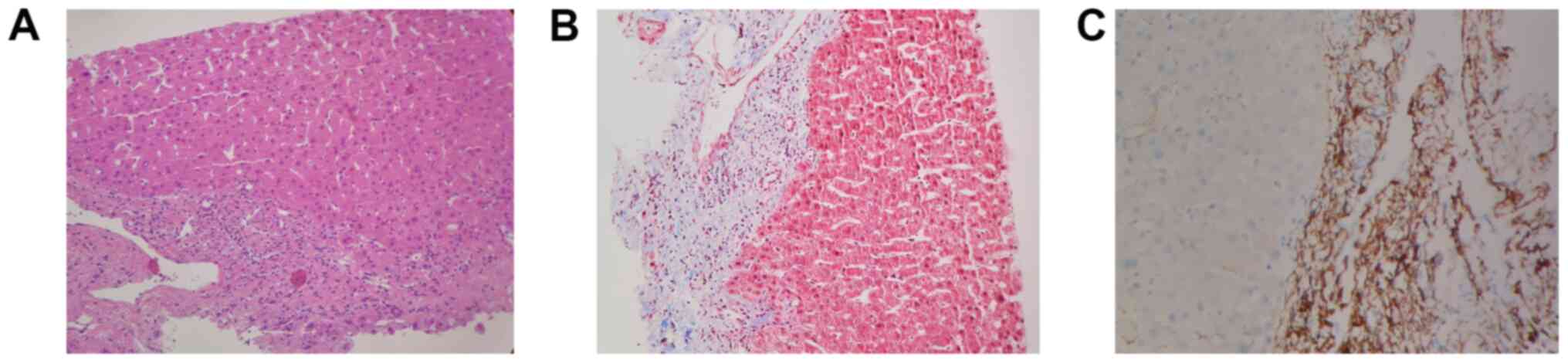
Liver biopsies were performed in 10 patients with
abnormal liver function in the DSA-positive group (7 cases) and
DSA-negative group (3 cases). Among 7 cases in the DSA-positive
group, the pathological results of 4 cases indicated an
antibody-mediated rejection, and 3 of the 4 cases were positive for
C4d (Fig. 1). Two cases
demonstrated T-cell-mediated rejection. The 3 cases in the
DSA-negative group indicated a T-cell-mediated rejection.
Kaplan-Meier survival analysis revealed that there was a
significant difference in the survival of graft recipients between
the DSA-positive and the DSA-negative group (Fig. 2; P<0.05).
Treatment and outcome of AMR in
DSA-positive recipients
Of the 10 DSA-positive recipients, four had AMR.
Liver function returned to normal after hormone therapy. All four
patients with AMR were treated with MMF and 3 of the patients were
treated with plasma exchange 1-3 times. During and after plasma
exchange therapy, human immunoglobulin (intravenous immunoglobulin,
2.5 g/day) was administered intravenously. One patient was treated
with bortezomib five times, and the other 3 patients were treated
with rituximab once each. During treatment, except for the routine
daily use of MMF, other treatment methods were carried out
alternately. Among the patients with AMR, only one case was cured
after treatment, and the other three cases finally underwent
re-transplantation.
Discussion
The liver is recognized as an immune-privileged
organ (22); therefore, AMR is less
common after liver transplantation (23). However, the humoral immunological
mechanism of acute rejection after liver transplantation is
consistent with that of other organ transplantations (24). A re-evaluation of the importance of
DSAs in liver transplants was required, and the present study
demonstrated a higher risk of rejection and decreased allograft
survival in DSA-positive patients.
In the present study, it was observed that DSAs
against HLA class II antigens were more common than those against
class I antigens and represented 90% of all DSAs. The HLA-II
antigen is dominated by the HLA-DQ site. It was observed that in
patients with AMR, the mean value of HLA-DQ MFI was >15,000 and
the mean value of HLA-DR MFI was >10,000. Therefore, a high MFI
value was closely related to the occurrence of AMR. Previous
research has demonstrated that the occurrence of AMR, bile duct
complications and hepatic fibrosis in DSA-positive liver
transplantation recipients is closely related to the
characteristics, intensity and IgG classification of antibodies
(25). In a retrospective cohort
study, such as that by Kaneku et al (26), DSA analysis of serum samples before
and after transplantation was performed in 749 adult liver
transplant recipients. It was found that 8.1% of patients developed
DSAs 1 year after transplant. Almost all DSAs were targeted at HLA
II antigens, most of which were targeted at HLA-DQ antigens.
Miyagawa-Hayashino et al (18) retrospectively analyzed 79 children
with good graft function >5 years after transplantation. The
donor specificity of antibodies was identified in 67 patients;
specifically, DSAs were detected in 32 cases the patients were
treated with plasma exchange 1-3 times. and after plasma exchange
therapy, human immunoglobulin (intravenous immunoglobulin, 2.5
g/day) was administered intravenously. One patient was treated with
bortezomib five times, and the other three patients were treated
with rituximab once each. During treatment, except for the routine
daily use of MMF, other treatment methods were carried out
alternately. Among the patients with AMR, only one case was cured
after treatment, and the other three cases finally underwent
re-transplantation.
Discussion
The liver is recognized as an immune-privileged
organ (22); therefore, AMR is less
common after liver transplantation (23). However, the humoral immunological
mechanism of acute rejection after liver transplantation is
consistent with that of other organ transplantations (24). A re-evaluation of the importance of
DSAs in liver transplants was required, and the present study
demonstrated a higher risk of rejection and decreased allograft
survival in DSA-positive patients.
In the present study, it was observed that DSAs
against HLA class II antigens were more common than those against
class I antigens and represented 90% of all DSAs. The HLA-II
antigen is dominated by the HLA-DQ site. It was observed that in
patients with AMR, the mean value of HLA-DQ MFI was >15,000 and
the mean value of HLA-DR MFI was >10,000. Therefore, a high MFI
value was closely related to the occurrence of AMR. Previous
research has demonstrated that the occurrence of AMR, bile duct
complications and hepatic fibrosis in DSA-positive liver
transplantation recipients is closely related to the
characteristics, intensity and IgG classification of antibodies
(25). In a retrospective cohort
study, such as that by Kaneku et al (26), DSA analysis of serum samples before
and after transplantation was performed in 749 adult liver
transplant recipients. It was found that 8.1% of patients developed
DSAs 1 year after transplant. Almost all DSAs were targeted at HLA
II antigens, most of which were targeted at HLA-DQ antigens.
O’Leary and Klintmalm (27)
retrospectively analyzed 79 children with good graft function >5
years after transplantation. The donor specificity of antibodies
was identified in 67 patients; specifically, DSAs were detected in
32 cases (48%). The DSAs were usually targeted at HLA class II (30
cases) and rarely against class I (2 cases). Although the liver has
the ability to absorb pre-stored HLA class I antibodies, the
persistence of HLA class II antibodies increases the incidence of
acute and chronic rejection (27).
Another finding of the present study was that
DSA-positive recipients exhibited abnormal liver function before
AMR and exhibited abnormal elevation of serum transaminase,
followed by hypercholanemia. O'Leary et al (28) retrospectively evaluated 1,270 cases
of liver transplantation and demonstrated that immunosuppressant
concentration and hormone dosage were closely related to rejection.
Another study reported that the detection rate of DSAs in the
non-tolerant group was 54%, mainly targeting HLA class II antigens
(DR, 41%; DQ, 53%). The average levels of AST, ALT, total bilirubin
and γ-glutamyltransferase were also higher in DSA-positive patients
(19).
The formation of DSAs in the early stages after
organ transplantation suggests a poor prognosis, but the
pathogenesis between DSAs and rejection has not been determined. In
other organ transplants, such as renal, complement C4d has been
recognized as a sensitive index to predict rejection; however, the
role of C4d in liver transplantation has been controversial
(29). Although it has been
reported that C4d serves an important role in the prediction of
rejection in DSA-positive cases, it is highly specific but not
highly sensitive. Some research has shown that acute rejection
after liver transplantation is closely related to the accumulation
of vascular endothelial cells in the portal area. However, Ali
et al (30) revealed that
positive staining of C4d can be found in different liver lesions,
i.e., acute cell rejection (52%), chronic catheterization rejection
(50%), recurrent liver disease (48%), preservation injury (18%) and
liver necrosis (54%). Furthermore, C4d positivity was rarely found
in ABO blood group-compatible liver transplantation, but it was
closely related to liver fibrosis after transplant. Musat et
al (15) performed liver
biopsies in 43 patients with acute or chronic rejection after liver
transplantation. There was a significant positive association
between C4d deposition and DSA intensity (MFI). The incidence of
chronic rejection among DQ-DSA-positive patients was higher than
that among DQ-DSA-negative patients. The higher the MFI value of
DSAs, the higher the risk of chronic rejection. Del Bello et
al (31) performed a
retrospective analysis of 152 patients with liver transplantation
and demonstrated that 21 patients (14%) developed DSAs, including 5
patients with C1q-DSA (24%) and 9 patients (43%) with AMR. The
positive rate of C4d staining was higher in liver biopsies from
patients with AMR (P<0.0001). In the present study, liver
biopsies were performed in 10 patients with abnormal liver function
in the DSA-positive group. The pathological results of four cases
revealed that the cellular rejection was grade 0, and three cases
were positive for C4d, suggesting that systemic fluid rejection
should be further assessed. C1q-DSA should also be further
assessed. In recent years, additional knowledge on complement C1q
has been obtained (32). It has
been revealed that the damage caused by DSA was related to its own
ability to bind C1q. C1q-DSA is considered to exhibit potential
cytotoxicity and has been associated with acute rejection and graft
loss (33).
In the present study, all the patients with AMR were
treated with MMF, and 3 of them were treated with plasma exchange.
During and after plasma exchange therapy, human immunoglobulin was
administered intravenously. One patient was treated with
bortezomib, and the other 3 patients were treated with rituximab.
Among the patients with AMR, only one case was cured after
treatment, and the other three cases finally underwent
re-transplantation. DSAs are closely related to rejection after
liver transplantation. Common treatments for abnormal liver
function caused by DSAs include plasma exchange, immunoglobulin,
rituximab and bortezomib. Bortezomib is used to treat AMR; however,
considering the increased risk of viral hepatitis and other
infections, the pros and cons should be assessed prior to treatment
(34-36).
In a study by Paterno et al (13), bortezomib was used to treat three
ABO-compatible liver transplant recipients with AMR. These patients
developed a severe, acute rejection of steroids and antithymocyte
globulin resistance; they also had histological evidence of plasma
cell infiltration. After treatment with bortezomib, the liver
function of all patients with C4d positivity and high DSA levels
improved or was normal, the deposition of C4d disappeared and the
level of DSAs decreased significantly.
The present study had some limitations. Firstly, HLA
antibody analysis in pediatric liver transplants is not a routine
test for all patients before transplant. Thus, the authors were
unable to determine whether the detected antibodies in patients
were preformed or de novo. Secondly, the sample size in the
present study was small; more samples need to be collected to
further analyze the relationship between DSAs and graft loss in
patients. Thirdly, the follow-up time of pediatric liver
transplants needs to be extended to study the 5-year graft survival
rate of children. Finally, more clinical data and practical
experience should be accumulated in the diagnosis and treatment of
AMR after liver transplantation in children. When the diagnosis of
AMR is unknown or highly suspected, liver biopsies and DSA tests
should be performed in a timely manner, and C4d, IgG subclass, C1q
and C3d should be assessed at the same time (37). In conclusion, regular monitoring of
DSA levels may have an important role in predicting graft survival
and DSA treatment choice.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Tianjin Natural
Science Foundation (grant no. 17JCYBJC27500) and the China Organ
Transplant Development Foundation ‘Elite Project’ (grant no.
2019JYJH03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DHL conceived the study. WL performed the research,
analyzed the data and wrote the first draft of the manuscript. KW
collected the clinical data and revised the draft. YLX and CL
performed the measurement of DSA and HLA typing. WG screened the
clinical data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patients' parents/guardians. The present study was approved by the
Medical Ethics Committee of Tianjin First Central Hospital
(approval no. 2015017S).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vionnet J, Sempoux C, Pascual M,
Sánchez-Fueyo A and Colmenero J: Donor-specific antibodies in liver
transplantation. Gastroenterol Hepatol. 43:34–45. 2020.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
2
|
Kannabhiran D, Lee J, Schwartz JE,
Friedlander R, Aull M, Muthukumar T, Campbell S, Epstein D, Seshan
SV, Kapur S, et al: Characteristics of circulating donor human
leukocyte antigen-specific immunoglobulin G antibodies predictive
of acute antibody-mediated rejection and kidney allograft failure.
Transplantation. 99:1156–1164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mohan S, Palanisamy A, Tsapepas D,
Tanriover B, Crew RJ, Dube G, Ratner LE, Cohen DJ and Radhakrishnan
J: Donor-specific antibodies adversely affect kidney allograft
outcomes. J Am Soc Nephrol. 23:2061–2071. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Einecke G, Sis B, Reeve J, Mengel M,
Campbell PM, Hidalgo LG, Kaplan B and Halloran PF:
Antibody-mediated microcirculation injury is the major cause of
late kidney transplant failure. Am J Transplant. 9:2520–2531.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Venick RS, Farmer DG, McDiarmid SV, Duffy
JP, Gordon SA, Yersiz H, Hong JC, Vargas JH, Amment ME and Busuttil
RW: Predictors of survival following liver transplantation in
infants: A single-center analysis of more than 200 cases.
Transplantation. 89:600–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bishara A, Brautbar C, Eid A, Scherman L,
Ilan Y and Safadi R: Is presensitization relevant to liver
transplantation outcome? Hum Immunol. 63:742–750. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Muro M, López-Álvarez MR, Campillo JA,
Marin L, Moya-Quiles MR, Bolarin JM, Botella C, Salgado G, Martinez
P, Sánchez-Bueno F, et al: Influence of human leukocyte antigen
mismatching on rejection development and allograft survival in
liver transplantation: Is the relevance of HLA-A locus matching
being underestimated? Transpl Immunol. 26:88–93. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kaczmarek I, Deutsch MA, Kauke T,
Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B,
Spannagl M and Ueberfuhr P: Donor-specific HLA alloantibodies:
Long-term impact on cardiac allograft vasculopathy and mortality
after heart transplant. Exp Clin Transplant. 63:229–235.
2008.PubMed/NCBI
|
|
9
|
Smith JD, Banner NR, Hamour IM, Ozawa M,
Goh A, Robinson D, Terasaki PI and Rose ML: De novo donor
HLA-specific antibodies after heart transplantation are an
independent predictor of poor patient survival. Am J Transplant.
11:312–319. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Taner T, Gandhi MJ, Sanderson SO,
Poterucha CR, De Goey SR, Stegall MD and Heimbach JK: Prevalence,
course and impact of HLA donor-specific antibodies in liver
transplantation in the first year. Am J Transplant. 12:1504–1510.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Starzl TE, Demetris AJ, Todo S, Kang Y,
Tzakis A, Duquesnoy R, Makowka L, Banner B, Concepcion W and
Kendrick A: Evidence for hyperacute rejection of human liver
grafts: The case of the canary kidneys. Clin Transplant. 3:37–45.
1989.PubMed/NCBI
|
|
12
|
Kozlowski T, Rubinas T, Nickeleit V,
Woosley J, Schmitz J, Collins D, Hayashi P, Passannante A and
Andreoni K: Liver allograft antibody-mediated rejection with
demonstration of sinusoidal C4d staining and circulating
donor-specific antibodies. Liver Transpl. 17:357–368.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Paterno F, Shiller M, Tillery G, O'Leary
JG, Susskind B, Trotter J and Klintmalm GB: Bortezomib for acute
antibody-mediated rejection in liver transplantation. Am J
Transplant. 12:2526–2531. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Musat AI, Pigott CM, Ellis TM, Agni RM,
Leverson GE, Powell AJ, Richards KR, D'Alessandro AM and Lucey MR:
Pretransplant donor-specific anti-HLA antibodies as predictors of
early allograft rejection in ABO-compatible liver transplantation.
Liver Transpl. 19:1132–1141. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Musat AI, Agni RM, Wai PY, Pirsch JD,
Lorentzen DF, Powell A, Leverson GE, Bellingham JM, Fernandez LA,
Foley DP, et al: The significance of donor-specific HLA antibodies
in rejection and ductopenia development in ABO compatible liver
transplantation. Am J Transplant. 11:500–510. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Demetris AJ, Markus BH, Burnham J,
Nalesnik M, Gordon RD, Makowka L and Starzl TE: Antibody deposition
in liver allografts with chronic rejection. Transplant Proc. 19 (4
Suppl 5):S121–S125. 1987.PubMed/NCBI
|
|
17
|
Del Bello A, Congy-Jolivet N, Muscari F,
Lavayssière L, Esposito L, Cardeau-Desangles I, Guitard J, Dörr G,
Suc B, Duffas JP, et al: Prevalence, incidence and risk factors for
donor-specific anti-HLA antibodies in maintenance liver transplant
patients. Am J Transplant. 14:867–875. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Miyagawa-Hayashino A, Yoshizawa A, Uchida
Y, Egawa H, Yurugi K, Masuda S, Minamiguchi S, Maekawa T, Uemoto S
and Haga H: Progressive graft fibrosis and donor-specific human
leukocyte antigen antibodies in pediatric late liver allografts.
Liver Transpl. 18:1333–3342. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wozniak LJ, Hickey MJ, Venick RS, Vargas
JH, Farmer DG, Busuttil RW, McDiarmid SV and Reed EF:
Donor-specific HLA antibodies are associated with late allograft
dysfunction after pediatric liver transplantation. Transplantation.
99:1416–1422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dalva K and Beksac M: HLA typing with
sequence-specific oligonucleotide primed PCR (PCR-SSO) and use of
the Luminex technology. Methods Mol Med. 134:61–69. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshizawa A, Egawa H, Yurugi K, Hishida R,
Tsuji H, Ashihara E, Miyagawa-Hayashino A, Teramukai S, Maekawa T,
Haga H and Uemoto S: Significance of semiquantitative assessment of
preformed donor-specific antibody using luminex single bead assay
in living related liver transplantation. Clin Dev Immunol.
2013(972705)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen ZH: Problems and significance of
liver allograft as an immunologically privileged organ. Zhonghua
Gan Zang Bing Za Zhi. 13(221)2005.PubMed/NCBI(In Chinese).
|
|
23
|
Guo H: Brief summary of recent findings on
pathology of antibody-mediated rejection in organ transplantation.
Chin J Transplant (Electronic Edition). 6:138–143. 2012.
|
|
24
|
Zhou J and Shao CK: Advances of cellular
and molecular immunology of acute rejection in liver
transplantation. Chin J Organ Transplant. 6:381–383. 2007.
|
|
25
|
Valcnzucla NM, Hickey MJ and Reed EF:
Antibody subclass repertoire and graft outcome following solid
organ transplantation. Front Immunol. 7(433)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kaneku H, O'Leary JG, Bannuelos N, Jenning
LW, Susskind BM, Klintmalm GB and Terasaki PI: De novo
donor-specific HLA antibodies decrease patient and graft survival
in liver transplant recipients. Am J Transplant. 13:1541–1548.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
O'Leary JG and Klintmalm GB: Impact of
donor-specific antibodies on results of liver transplantation. Curr
Opin Organ Transplant. 18:279–284. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
O'Leary JG, Kaneku H, Jennings LW,
Banuelos N, Susskind BM, Terasaki PI and Klintmalm GB: Preformed
class II donor-specific antibodies are associated with an increased
risk of early rejection after liver transplantation. Liver Transpl.
19:973–980. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Böhming GA, Exner M, Habicht A,
Schillinger M, Lang U, Kletzmayr J, Säemann MD, Hörl WH,
Watschinger B and Regele H: Capillary C4d deposition in kidney
allografts: A specific marker of alloantibody-dependent graft
injury. J Am Soc Nephrol. 13:1091–1099. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ali S, Ormsby A, Shah V, Segovia MC, Kantz
KL, Skorupski S, Eisenbrey AB, Mahan M and Huang MA: Significance
of complement split product C4d in ABO-compatible liver allograft:
Diagnosing utility in acute antibody mediated rejection. Transpl
Immunol. 26:62–69. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Del Bello A, Congy-Jolivet N, Danjoux M,
Muscari F, Lavayssière L, Esposito L, Cardeau-Desangles I, Guitard
J, Dörr G, Milongo D, et al: De novo donor-specific anti-HLA
antibodies mediated rejection in liver-transplant patients. Transpl
Int. 28:1371–1382. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kovandova B, Slavcev A, Sekerkova Z,
Honsova E and Trunecka P: Antibody-mediated rejection after liver
transplantation-relevance of C1q and C3d-binding antibodies. HLA.
92 (Suppl 2):S34–S37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sutherland SM, Chen G, Sequeira FA, Lou
CD, Alexander SR and Tyan DB: Complement-fixing donor-specific
antibodies identified by a novel C1q assay are associated with
allograft loss. Pediatr Transplant. 16:12–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wilson CH, Agarwal K, Carter V, Burt AD,
Hübscher S, Talbot D, Jaques BC and Manas DM: Late humoral
rejection in a compliant ABO-compatible liver transplant recipient.
Transplantation. 82:988–989. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Watson R, Kozlowski T, Nickeleit V,
Woosley JT, Schmitz JL, Zacks SL, Fair JH, Gerber DA and Andreoni
KA: Isolated donor specific alloantibody-mediated rejection after
ABO compatible liver transplantation. Am J Transplant. 6:3022–3029.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kamar N, Lavayssière L, Muscari F, Selves
J, Guilbeau-Frugier C, Cardeau I, Esposito L, Cointault O, Nogier
MB, Peron JM, et al: Early plasmapheresis and rituximab for acute
humoral rejection after ABO-compatible liver transplantation. World
J Gastroenterol. 15:3426–3430. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Couchonnal E, Rivet C, Ducreux S,
Dumortier J, Bosch A, Boillot O, Collardeau-Frachon S, Dubois R,
Hervieu V, André P, et al: Deleterious impact of C3d-binding
donor-specific anti-HLA antibodies after pediatric liver
transplantation. Transpl Immunol. 45:8–14. 2017.PubMed/NCBI View Article : Google Scholar
|