Introduction
Oral cancer represents one of the most frequent
types of cancer worldwide with an incidence of about 9% of all
types or cancer (1). Oral malignant
tumors differ from benign tumors by their rate of high invasiveness
accompanied by local tissue destruction and a high lymphatic and
circulatory metastasis rate (2).
The most common sites for oral cancer are the lower
lip, the tongue and the mouth floor mucosa. Usually older patients
are most susceptible for developing oral cancers; individuals
between 50 and 55 years of age having the highest incidence
(3).
Varied clinical and histological forms of oral
malignant lesions have been described, oral squamous cell carcinoma
(OSCC) being the most frequently diagnosed form, comprising ~90% of
all oral cancers (4). This type of
cancer is characterized by a high mortality rate of over 50% due to
its high invasiveness, chemotherapy and radiotherapy resistance and
late stage diagnosis mostly in the inoperable state (5,6). One
key factor in lowering oral cancer morbidity is an early stage
diagnosis which greatly improves long term survivability (7).
Early stage diagnosis can be achieved by combining
clinical detection methods such as vital staining, brush biopsy,
auto-fluorescence spectroscopy, chemiluminescent illumination,
narrow band imaging and confocal microscopy (8). These methods are invasive and can
detect emergent oral cancer only after dysplastic changes have
occurred (9).
Combining clinical methods with laboratory testing
in which key biomarkers in early stage cancer development could be
analyzed and used to predict the existence of malignant
transformation or to evaluate the prognosis in late stage diagnose
of oral neoplasms is crucial. These key biomarkers could be
analyzed either in serum, saliva or tissue samples.
Cancer research has identified more than one hundred
biomarkers that show a potential for a laboratory diagnosis of oral
cancers. These biomarkers can be specific for each stage and
mechanism responsible for the initiation, proliferation and
metastasis of oral cancers (10,11).
From these potential biomarkers, in the present
study we include key inflammatory cytokines such as interleukin
(IL)-6 and IL-8 and tissue inhibitor of metalloproteinase-1
(TIMP-1), a protective factor against extracellular matrix
degradation.
Cytokines are a group of low-molecular-weight
glycoproteins produced by immune and non-immune cells and play a
crucial role as molecular messengers in inflammation, apoptosis,
and host resistance acting as signaling molecules in most cellular
interactions (12).
Key characteristics of cytokines are the ability to
interact only with cellular targets and not between themselves and
that one cell can respond to several cytokines (9).
IL-8 is a pro-inflammatory cytokine released by
various inflammatory cells such as neutrophils and macrophages
following the nuclear factor-κB (NF-κB) pathway activation by
various stimuli such as inflammatory signals and environmental
stresses (13). IL-8 acts on two
cellular receptors CRCX-1 and CRCX-2 which are structurally
similar, found on the surface of macrophages, neutrophils and most
important on the surface of cancer cells (14).
The traditional role of IL-8 is to promote direct
migration of inflammatory cells following a concentration gradient
leading to an accumulation at the site of IL-8 production and to
facilitate the degranulation of neutrophils (13). Cancer cells can obtain the ability
to release different cytokines such as IL-8 which can ultimately
lead to metastasis by promoting neutrophil recruitment,
angiogenesis, proliferation of endothelial cells and apoptosis
resistance (15). IL-8 binding to
CRCX-1 and CRCX-2 receptors triggers the activation of several
downstream signaling pathways such as the phosphatidylinositol-3
kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK) and
Janus kinase (JAK)/signal transducer and activator of transcription
(STAT) pathway (14,16).
IL-6 is a pro-inflammatory cytokine released by
various cells such as immune cells that infiltrate the tumor such
as macrophages, a wide range of stromal cells and also by the tumor
cells themselves, activating JAK/STAT3 and MAPK pathways leading to
encoding of proteins that induce tumor cell proliferation (cyclin
D1) and survival (BCL2-like protein) (17). STAT3 activation also leads to
increased expression of invasiveness and angiogenetic factors such
as vascular endothelial growth factor (VEGF) or matrix
metalloproteinases (MMPs) thus promoting metastasis (18). Another effect of JAK/STAT3
activation by IL-6 is the resulting immunosuppressive tumor
microenvironment due to the IL-6 inhibition of neutrophils, natural
killer cells and T cells leading to a decrease in antitumor
immunity (19).
Extracellular matrix (ECM) macromolecules create the
environment required for normal tissue function, including the
precise regulation of formation and degradation. Key enzymes in ECM
degradation include the matrix metalloproteinases (MMPs), a group
of proteolytic enzymes consisting of a prodomain, a catalytic
region, a hinge region and a hemopexin domain and are secreted by
both normal and cancer cells (20,21).
TIMPs are a group of four proteins that block the
action of various classes of MMPs by inhibiting the catalytic
domain of MMPs based on the interactions between the N-terminal
domain of TIMPs which is structurally similar with the substrate of
MMPs (22). Recent studies have
linked TIMPs with other inhibitory roles on non-MMP
metalloproteinase such as the metallopeptidases ADAMs and ADAMTS
(23). TIMPs also exhibit cell
growth-promoting roles; overexpression of some types of TIMPs
reduces tumor growth (20).
The aim of the present study was to analyze the
existence of various correlations between inflammation markers
(IL-6 and IL-8) and extracellular matrix degradation protection
markers such as TIMP-1 in OSCC tumors.
Patients and methods
Our study included 20 patients (12 females and 8
males) diagnosed with oral squamous cell carcinoma, recruited from
January to April 2020 from the patients treated at the
Oro-Maxillo-Facial Hospital ‘Prof. Dr. Dan Theodorescu’ Bucharest.
Patients age included in this study ranged from 36 to 75 years, the
mean age being 55.0±10.9 years (mean ± SD). Ten patients were
active smokers. Only one patient ceased smoking before inclusion in
this study and nine were nonsmokers.
Our prospective study was initiated following
approval of the Ethics Committee of UMF Carol Davila. Informed
written consent was obtained from all individual participants
included in the study. Oral cancer tissue samples were collected
from the included participants during surgery. Tumor cell lysates
were obtained according to the assay kit manufacturer's
recommendations.
IL-8, IL-6 and TIMP-1 levels were measured in the
tumor cell lysates by ELISA technique, using assay kits (cat. nos.
E-EL-H0048, E-EL-H0102 and E-EL-H0184) from Elabscience
numbers.
Statistical analysis
The results were statistically analyzed using IBM
SPSS Statistics 25 (IBM Corp.), Microsoft Office Excel/Word 2013
and the Shapiro-Wilk distribution test. For correlation analysis,
the Spearman's rho correlation coefficient was used.
Results
In the present study, possible correlations were
analyzed between inflammation markers IL-6 and IL-8 and TIMP-1, a
protective marker against degradation of the ECM.
Our results presented in Table I and Fig. 1 revealed a non-parametric
distribution, according to Shapiro-Wilk test (P<0.05) between
IL-8 and IL-6 inflammation markers. A positive and significant
correlation between IL-6 and IL-8 was also observed (P=0.005,
R=0.517) indicating that high IL-8 levels can be associated with a
significantly higher frequency of high IL-6 levels.
 | Table ICorrelation between IL-8 and IL-6
levels. |
Table I
Correlation between IL-8 and IL-6
levels.
| Correlation | P-value |
|---|
| IL-8 (P<0.001) x
IL-6 (P<0.001) | 0.005, R=0.517 |
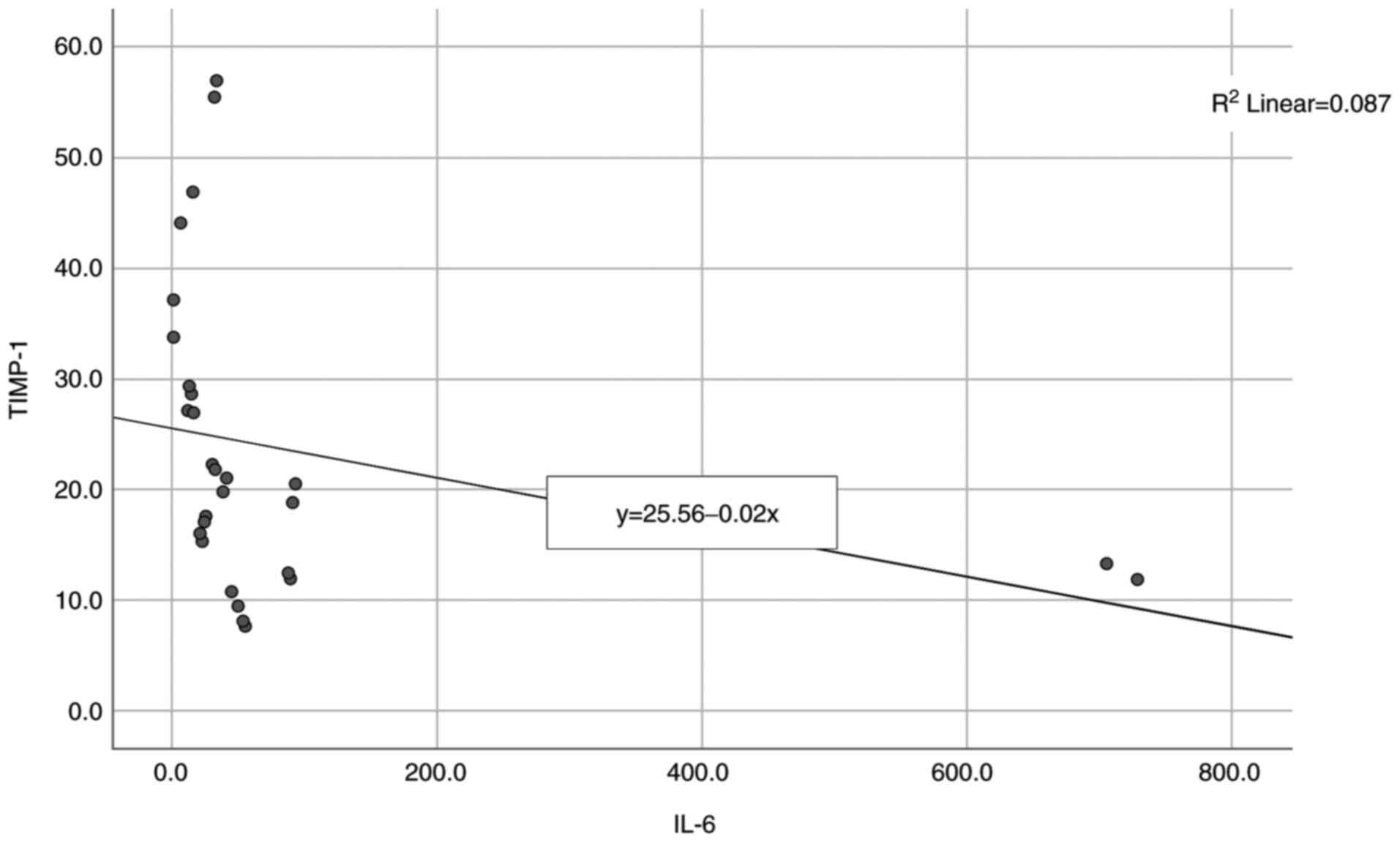
The next correlation analyzed was between IL-6 and
TIMP-1 and the results are presented in Table II and Fig. 2. A non-parametric distribution is
revealed, according to Shapiro-Wilk test (P<0.05) and the
correlation between the studied parameters showed a significant and
high degree negative correlation (P<0.001, R=-0.673) indicating
that high levels of IL-6 are significantly associated with lower
levels of TIMP-1.
 | Table IICorrelation between IL-6 and
TIMP-1. |
Table II
Correlation between IL-6 and
TIMP-1.
| Correlation | P-value |
|---|
| TIMP-1 (P=0.005) x
IL-6 (P<0.001) | <0.001,
R=-0.673 |
The final correlation to be analyzed was between
IL-8 and TIMP-1 and the results are presented in Table III and Fig. 3. A non-parametric distribution is
revealed, according to Shapiro-Wilk test (P<0.05) and the
correlation between the studied parameters showed significant and a
high negative correlation (P<0.001, R=-0.684) indicating that
high levels of IL-8 are significantly associated with lower levels
of TIMP-1.
 | Table IIICorrelation between IL-8 and
TIMP-1. |
Table III
Correlation between IL-8 and
TIMP-1.
| Correlation | P-value |
|---|
| TIMP-1 (P=0.005) x
IL-8 (P<0.001) | <0.001,
R=-0.684 |
Discussion
Inflammation represents the response to various
tissue aggressions caused by physical trauma, ischemic conditions,
infections or extended exposure to toxic agents resulting in an
inflammatory response aimed at repairing the damage consisting of
cellular transformation and proliferation (24). In the case of persistent tissue
damage or when control mechanisms are altered or incapacitated,
acute inflammation can result in a chronic state of inflammation
characterized by high mutation and cellular proliferation rates
creating conditions for the onset of cancer (25).
After initial malignant cell transformation, cancer
progression, according to Hanahan and Weinberg is based on 10 key
elements such as: Sustained proliferative signals, evasion of
cellular growth suppressors, metastasis through local invasion,
promotion of inflammation, immortality, induction of angiogenesis,
genetic instability and mutations, cellular death resistance and
metabolic imbalances (26).
It is widely accepted that chronic inflammation is
responsible for almost a quarter of all malignancies diagnosed
(27). Chronic inflammation can
play a crucial role in cancer progression acting on many of the key
inflammatory steps such as cellular proliferation, local
invasiveness, angiogenesis, metastasis, and cytokines such as IL-1,
IL-6, IL-8 and tumor necrosis factor (TNF)-α representing key
elements (28).
A wide number of both normal or cancer cells have
the ability to release IL-8 after the activation of nuclear factor
(NF)-κB as a response to various local and systemic stimuli
(9). In normal unstimulated cells,
IL-8 levels are virtually undetected. Apart from NF-κB activation
pathway, de-repression of the IL-8 gene promoter and IL-8 mRNA
stabilization by the p38 MAPK pathway play crucial roles in IL-8
release (29).
IL-8 has an autocrine and paracrine tumor-promoting
role, altering the local microenvironment, inducing cell growth
mainly in endothelial cells, stimulating leukocyte infiltration and
modification of immune responses (30).
IL-8 binds with high specificity with two membrane
receptors CXCR1 and CXCR2 located on tumor-associated macrophages,
neutrophils and cancer cells. This binding activates protein-G
mediated pathways leading to calcium release and activation of the
Ras/MAPK and PI3K signaling cascades (29). CXCR1 and not CXCR2 activates
phospholipase D leading to an increased oxidative burst through
increased reactive oxygen species production contributing to
altered metabolic conditions and leading to further mutations in
the tumor microenvironment (31,32).
The angiogenesis effect of IL-8 can be explained by the binding of
IL-8 to CXCR receptors that triggers the increase in Bcl-2
expression and matrix metalloproteinase (MMP) production, through
ERK phosphorylation, leading to endothelial cell proliferation and
a degradation of the extracellular matrix (ECM) which creates the
local conditions for proliferation (33,34).
Cellular proliferation and survival effects on other
types of cells of IL-8 can be explained through the activation of
Src-kinases and focal adhesion kinase (FAK) by increasing
phosphorylation (35,36).
The other pro-inflammatory cytokine included in this
study is IL-6. It plays important roles in cellular proliferation,
angiogenesis, local invasion, regenerative and metabolic processes.
It also regulates cellular metabolism protecting cancer cells from
the hypoxic conditions that characterize the tumor microenvironment
(9).
A host of cells located at the tumor microclimate
level secrete IL-6, including inflammatory cells, normal stromal
cells and also cancer cells. The effects of IL-6 are manifested at
both the local and systemic levels (37,38).
Cellular and metabolic actions of IL-6 can be
mediated through the JAK/STAT3 and Ras/Raf/MAPK pathways (9,18).
JAK/STAT3 pathway activation by IL-6 leads to the stimulation of
target genes, such as cyclin D1 responsible for essential roles in
the G1 phase of the cell cycle, the stimulation of Bcl-2 protein
affecting the regulatory mechanisms of apoptosis, the release of
angiogenesis-stimulating factors such as vascular endothelial
growth factor (VEGF) or increased levels of local invasiveness
markers such as MMPs (18,39,40).
Another effect of IL-6, coupled with high levels of oxidative
stress is the enhanced methylation of tumor-suppressor genes and
microRNAs through DNA methyltransferase 1 (DNMT1) transcription
alteration (41).
The release of IL-6 can be further increased by the
binding of STAT3 to the promotor sequence of IL-6 release
establishing a positive feedback loop increasing the biological
effects (42).
Local invasiveness is another key mechanism in oral
cancer progression. MMPs are a group of zinc-dependent
endoproteases with roles in degradation and remodeling of the ECM
and are secreted by both normal and tumor cells (43). They act on all types of collagen and
elastin in the ECM, hemopexin domain conferring substrate
specificity for different collagen types (44). High levels of MMPs, especially
MMP-9, were found in low to moderate differentiated tumors and were
found to be secreted by cancer and inflammatory cells such as
macrophages both in the primary tumor, mostly at the tumor invasion
margins, or in lymph node metastasis inducing angiogenesis thus
leading to invasiveness and cell growth (45). MMP expression is regulated at the
transcription level by cytokines, at pro-enzyme activation level by
oxidative stress and by the TIMP concentration (46).
The direct inhibitors and regulators of MMPs are
TIMPs. Any disruption in the balance between MMP activity and TIMP
inhibition may lead to invasion and metastasis and a worse
prognosis (47,48).
Lower levels of TIMP-1 can be explained by the
alteration in phosphorylation of key MAPK pathway molecules such as
JNK, Erk and p38 following miR-196 activation leading to suppressed
TIMP-1 levels and elevated MMP levels (49).
Many studies have analyzed either IL-8 or IL-6
levels separately or together with other biomarkers using either
saliva, serum or tumors as samples. Elevated levels of IL-6 and
IL-8 were found by St John et al both in the serum and
saliva of oral squamous cell carcinoma (OSCC) patients, IL-8 had
higher concentrations in saliva samples and IL-6 had higher
concentrations in serum (50).
These results were confirmed by SahebJamee et al using only
saliva as a diagnostic fluid (51).
Punyani and Sathawane found elevated levels of IL-8 in both
premalignant and OSCC cancer patients (52). Higher levels of IL-6 in OSCC
patients were found by Lotfi et al (53). A meta-analysis of 24 studies
conducted by Rezaei et al concluded that IL-8 and IL-6
levels were statistically higher in OSCC patients (54).
The diagnostic value of IL-8 levels could be
influenced by other diseases in which high levels of IL-8 are
found, such as asthma both allergic and non-allergic types, or
other common inflammatory diseases, such as viral infections
(55).
In the present study, both chronic inflammation
markers, IL-8 and IL-6, were present in high concentrations in OSCC
cell lysates and after statistical analysis we found a positive and
significant correlation between these two parameters (P=0.005,
R=0.517) indicating that high levels of IL-8 can be associated with
high levels of IL-6. A high concentration and correlation between
these biomarkers can suggest that both OSCC cells and normal cells
surrounding the tumor actively secrete cytokines. A high
concentration of IL-8 in the tumor microenvironment can be seen as
a defensive measure in order to attract more inflammatory cells.
IL-8 and IL-6 can also play a negative role in cancer progression
inducing angiogenesis, cellular proliferation and cancer cell
survival.
ECM degradation marker analyzed in this study was
TIMP-1. The level of this parameter was decreased in OSCC and after
statistical analysis, a negative and significant correlation
between IL-8 and TIMP-1 (P<0.001, R=-0.684) and between IL-6 and
TIMP 1 was found (P<0.001, R=-0.673) which indicates that high
levels of IL-6 or IL-8 can be statistically correlated with low
levels of TIMP-1 in OSCC patients. To the best of our knowledge,
this is the first study where the statistical correlation between
TIMP-1 and IL-6 and IL-8 was conducted.
The possible connection between IL-6 and IL-8 and
TIMP-1 can be explained by the inhibitory effect of these cytokines
on microRNA expression, which can control via MAPK pathways the
production of TIMPs (18). We can
speculate that another consequence of high cytokine expression in
cancers and in oral cancer in particular is the inhibition of ECM
degradation control mechanisms.
In conclusion, our study confirms the available
literature data on IL-6 and IL-8 as potential markers for oral
cancers such as OSCC. The negative correlations between IL-6 and
TIMP-1 and IL-8 and TIMP-1 suggest that pro-inflammatory cytokines
affect the tumor microenvironment by decreasing MMP control factors
such as TIMPs. All three biomarkers included in this study have the
potential to be used as detection or prognostic factors for oral
cancer.
Acknowledgements
Not applicable.
Funding
Funding: This paper was financially supported by ‘Carol Davila’
University of Medicine and Pharmacy (contract no. 23PFE/17.10.2018)
funded by the Ministry of Research and Innovation within PNCDI III,
Program 1-Development of the National R&D system, Subprogram
1.2-Institutional Performance-RDI excellence funding projects.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
RR, AD, MMI, ART, DM and MG made substantial
contributions to the conception and design of the research. ART,
RR, AD, DM and MMI made substantial contributions to the
acquisition, analysis, and interpretation of data for the research.
RR, ART and MMI drafted the work and revising it critically for
important intellectual content. AD and MG gave final approval of
the version to be published. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Our prospective study was initiated after the
approval of the Ethics Committee of ‘Carol Davila’ University of
Medicine and Pharmacy, no. 32698/11.12.2020. Informed written
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghantous Y and Abu Elnaaj I: Global
incidence and risk factors of oral cancer (In Hebrew). Harefuah.
156:645–649. 2017.PubMed/NCBI
|
|
2
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
3
|
Liu D, Yang F, Xiong F and Gu N: The smart
drug delivery system and its clinical potential. Theranostics.
6:1306–1323. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hema KN, Smitha T, Sheethal HS and
Mirnalini SA: Epigenetics in oral squamous cell carcinoma. J Oral
Maxillofac Pathol. 21:252–259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wong T and Wiesenfeld D: Oral cancer. Aust
Dent J. 63 (Suppl 1):S91–S99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bais MV: Impact of epigenetic regulation
on head and neck squamous cell carcinoma. J Dent Res. 98:268–276.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Scully C and Kirby J: Statement on mouth
cancer diagnosis and prevention. Br Dent J. 216:37–38.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kalavrezos N and Scully C: Mouth cancer
for clinicians part 7: Cancer diagnosis and pre-treatment
preparation. Dent Update. 43:50–54, 57-60, 63-65. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sahibzada HA, Khurshid Z, Khan RS, Naseem
M, Siddique KM, Mali M and Zafar MS: Salivary IL-8, IL-6 and TNF-α
as potential diagnostic biomarkers for oral cancer. Diagnostics
(Basel). 7(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cheng YS, Rees T and Wright J: A review of
research on salivary biomarkers for oral cancer detection. Clin
Transl Med. 3(3)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hussein AA, Forouzanfar T, Bloemena E, de
Visscher J, Brakenhoff RH, Leemans CR and Helder MN: A review of
the most promising biomarkers for early diagnosis and prognosis
prediction of tongue squamous cell carcinoma. Br J Cancer.
119:724–736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee S and Margolin K: Cytokines in cancer
immunotherapy. Cancers (Basel). 3:3856–3893. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
David JM, Dominguez C, Hamilton DH and
Palena C: The IL-8/IL-8R axis: A double agent in tumor immune
resistance. Vaccines (Basel). 4(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6341. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Larco JE, Wuertz BR and Furcht LT: The
potential role of neutrophils in promoting the metastatic phenotype
of tumors releasing interleukin-8. Clin Cancer Res. 10:4895–4900.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F,
Ren X and Yu J: IL-8, a novel messenger to cross-link inflammation
and tumor EMT via autocrine and paracrine pathways (Review). Int J
Oncol. 48:5–12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee H, Pal SK, Reckamp K, Figlin RA and Yu
H: STAT3: A target to enhance antitumor immune response. Curr Top
Microbiol Immunol. 344:41–59. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Arpino V, Brock M and Gill SE: The role of
TIMPs in regulation of extracellular matrix proteolysis. Matrix
Biol. 44-46:247–254. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Singh N, Baby D, Rajguru JP, Patil PB,
Thakkannavar SS and Pujari VB: Inflammation and cancer. Ann Afr
Med. 18:121–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Clevers H: At the crossroads of
inflammation and cancer. Cell. 118:671–674. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mantovani A: Cancer: Inflammation by
remote control. Nature. 435:752–753. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Ha H, Debnath B and Neamati N: Role of the
CXCL8-CXCR1/2 axis in cancer and inflammatory diseases.
Theranostics. 7:1543–1588. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brandolini L, Bertini R, Bizzarri C, Sergi
R, Caselli G, Zhou D, Locati M and Sozzani S: IL-1 beta primes
IL-8-activated human neutrophils for elastase release,
phospholipase D activity, and calcium flux. J Leukoc Biol.
59:427–434. 1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tappia PS, Dent MR and Dhalla NS:
Oxidative stress and redox regulation of phospholipase D in
myocardial disease. Free Radic Biol Med. 41:349–361.
2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li A, Dubey S, Varney ML, Dave BJ and
Singh RK: IL-8 directly enhanced endothelial cell survival,
proliferation, and matrix metalloproteinases production and
regulated angiogenesis. J Immunol. 170:3369–3376. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Khurram SA, Bingle L, McCabe BM, Farthing
PM and Whawell SA: The chemokine receptors CXCR1 and CXCR2 regulate
oral cancer cell behaviour. J Oral Pathol Med. 43:667–674.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Siesser PM and Hanks SK: The signaling and
biological implications of FAK overexpression in cancer. Clin
Cancer Res. 12:3233–3237. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kopetz S, Shah AN and Gallick GE: Src
continues aging: Current and future clinical directions. Clin
Cancer Res. 13:7232–7236. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bournazou E and Bromberg J: Targeting the
tumor microenvironment: JAK-STAT3 signaling. JAKSTAT.
2(e23828)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Walter M, Liang S, Ghosh S, Hornsby PJ and
Li R: Interleukin 6 secreted from adipose stromal cells promotes
migration and invasion of breast cancer cells. Oncogene.
28:2745–2755. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kurosaka M and Machida S:
Interleukin-6-induced satellite cell proliferation is regulated by
induction of the JAK2/STAT3 signalling pathway through cyclin D1
targeting. Cell Prolif. 46:365–373. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Adachi Y, Aoki C, Yoshio-Hoshino N,
Takayama K, Curiel DT and Nishimoto N: Interleukin-6 induces both
cell growth and VEGF production in malignant mesotheliomas. Int J
Cancer. 119:1303–1311. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rokavec M, Öner MG and Hermeking H:
Inflammation-induced epigenetic switches in cancer. Cell Mol Life
Sci. 73:23–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
O-Charoenrat P, Rhys-Evans PH and Eccles
SA: Expression of matrix metalloproteinases and their inhibitors
correlates with invasion and metastasis in squamous cell carcinoma
of the head and neck. Arch Otolaryngol Head Neck Surg. 127:813–820.
2001.PubMed/NCBI
|
|
44
|
Patterson ML, Atkinson SJ, Knäuper V and
Murphy G: Specific collagenolysis by gelatinase A, MMP-2, is
determined by the hemopexin domain and not the fibronectin-like
domain. FEBS Lett. 503:158–162. 2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Georgescu EF, Mogoantă SŞ, Costache A,
Pârvănescu V, Totolici BD, Pătraşcu Ş and Stănescu C: The
assessment of matrix metalloproteinase-9 expression and
angiogenesis in colorectal cancer. Rom J Morphol Embryol.
56:1137–1144. 2015.PubMed/NCBI
|
|
46
|
Maciejczyk M, Pietrzykowska A, Zalewska A,
Knaś M and Daniszewska I: The significance of matrix
metalloproteinases in oral diseases. Adv Clin Exp Med. 25:383–390.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Su CW, Lin CW, Yang WE and Yang SF: TIMP-3
as a therapeutic target for cancer. Ther Adv Med Oncol.
11(1758835919864247)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang Y, Goldberg ID and Shi YE: Complex
roles of tissue inhibitors of metalloproteinases in cancer.
Oncogene. 21:2245–2252. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lu YC, Chang JT, Liao CT, Kang CJ, Huang
SF, Chen IH, Huang CC, Huang YC, Chen WH, Tsai CY, et al:
OncomiR-196 promotes an invasive phenotype in oral cancer through
the NME4-JNK-TIMP1-MMP signaling pathway. Mol Cancer.
13(218)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
St John MA, Li Y, Zhou X, Denny P, Ho CM,
Montemagno C, Shi W, Qi F, Wu B, Sinha U, et al: Interleukin 6 and
interleukin 8 as potential biomarkers for oral cavity and
oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck
Surg. 130:929–935. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
SahebJamee M, Eslami M, AtarbashiMoghadam
F and Sarafnejad A: Salivary concentration of TNFalpha, IL1 alpha,
IL6, and IL8 in oral squamous cell carcinoma. Med Oral Patol Oral
Cir Bucal. 13:E292–E295. 2008.PubMed/NCBI
|
|
52
|
Punyani SR and Sathawane RS: Salivary
level of interleukin-8 in oral precancer and oral squamous cell
carcinoma. Clin Oral Investig. 17:517–524. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lotfi A, Shahidi N, Bayazian G,
AbdollahiFakhim S, Estakhri R, Esfahani A and Notash R: Serum level
of interleukin-6 in patients with oral tongue squamous cell
carcinoma. Iran J Otorhinolaryngol. 27:207–211. 2015.PubMed/NCBI
|
|
54
|
Rezaei F, Mozaffari HR, Tavasoli J,
Zavattaro E, Imani MM and Sadeghi M: Evaluation of serum and
salivary interleukin-6 and interleukin-8 levels in oral squamous
cell carcinoma patients: Systematic review and meta-analysis. J
Interferon Cytokine Res. 39:727–739. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Berghi NO, Dumitru M, Vrinceanu D,
Ciuluvica RC, Simioniuc-Petrescu A, Caragheorgheopol R, Tucureanu
C, Cornateanu RS and Giurcaneanu C: Relationship between chemokines
and T lymphocytes in the context of respiratory allergies (Review).
Exp Ther Med. 20:2352–2360. 2020.PubMed/NCBI View Article : Google Scholar
|