Introduction
Bone marrow mesenchymal stem cells (BMSCs),
nonhematopoietic stem cells that exist in bone marrow tissue, were
first reported by Friedenstein in the 1970s (1). BMSCs have the ability to differentiate
into adipocytes, osteoblasts, skeletal muscle cells, chondrocytes,
smooth muscle cells, tendon cells, hematopoietic support stroma
cells and other mesodermal cells under certain conditions. The
ability to differentiate into bone cells is important for bone
metabolism, and there is evidence that abnormal differentiation of
BMSCs is closely related to the occurrence of osteoporosis
(2). After BMSCs differentiate into
osteoblasts, their bone matrix synthesis, secretion and
mineralization abilities are enhanced, thereby resulting in bone
regeneration. Studies have demonstrated that transplanting cell
culture media containing BMSCs into the bone defect site can
promote bone defect repair (3-5).
This osteogenic differentiation characteristic of BMSCs provides
new treatment ideas for nonunion, bone defects, and osteolytic bone
disease.
Insulin growth factor-I (IGF-1) is a single-chain
basic polypeptide growth factor containing 70 amino acid residues
that plays an important role in regulating cell proliferation,
differentiation, and apoptosis (6).
At present, IGF-1 is considered to be widely involved in bone
growth and development. It has been reported that severe IGF-1
deficiency can cause short stature, and IGF-1 can significantly
improve this condition (7). A
previous study has confirmed that IGF-1 is positively correlated
with bone density and that IGF-1 in the blood of patients with
osteoporosis is significantly reduced (8). Studies have also confirmed that the
development of IGF-1 knockout in mice is related to a decrease in
the number of osteoblasts and a decrease in bone formation ability
(9,10). Moreover, under pathological
conditions, IGF-1 has been revealed to promote the mineralization
of mesenchymal stem cells, thereby promoting fracture healing
(11). However, the molecular
mechanism by which IGF-1 promotes the proliferation and
differentiation of BMSCs remains unclear.
The Wnt/β-catenin pathway is currently widely
investigated for its role in the pathogenesis of bone
system-related diseases and bone metabolism. It has an important
effect on bone remodeling and has the potential to control the
differentiation direction of BMSCs (12). The Wnt family is composed of 19
highly conserved cysteine-rich secreted glycoproteins. In the
Wnt/β-catenin pathway, the Wnt protein first binds to the
low-density lipoprotein receptor-related protein (LRP) 5/6 on the
cell surface and to the frizzled protein Fzd complex to promote
β-catenin polymerization and entry into the nucleus (13).
In the present study, the lentivirus carrying the
siRNA-Wnt3a gene was transfected into BMSCs to cause low expression
of the Wnt3a gene in BMSCs and then cells were treated with IGF-1.
The aim of the present study was to explore the role of the
Wnt/β-catenin pathway in IGF-1 in promoting the proliferation and
osteogenic differentiation of BMSCs.
Materials and methods
Cell culture
C57BL/6 mouse BMSCs were purchased from Cyagen
Biosciences, Inc. BMSCs were placed in a complete medium, which
consisted of basal medium (Cyagen Biosciences, Inc.), special 10%
fetal bovine serum (FBS; Cyagen Biosciences, Inc.), 1%
penicillin-streptomycin dual antibiotic solution and 1% glutamine.
Cells were cultured at 37˚C in an incubator containing 5%
CO2. When cell confluence reached 80-90%, the cells were
digested and passaged at a ratio of 1:2. The cells were maintained
in culture for no more than 10 passages.
Induction and identification of
osteogenic differentiation
When the cells reached 80% confluence, they were
incubated (37˚C, 5% CO2) with osteogenic induction
medium containing 80 ng/ml IGF-1 (PeproTech, Inc.), Dulbecco's
modified Eagle's medium [(DMEM)/F12 supplemented with 10% FBS, 0.25
mmol/l ascorbic acid, 10 mmol/l sodium β-glycerophosphate,
10-7 mol/l dexamethasone], and the medium was changed
once every 2 days on average. Osteogenesis was monitored
continuously, and 3 weeks after induction, the cells were fixed
with 4% paraformaldehyde for 15 min, stained with 0.1% Alizarin Red
(Cyagen Biosciences, Inc.), and observed under a fluorescence
microscope. Images were captured at 400 X magnification.
Drug toxicity test
BMSCs in the logarithmic growth phase were digested
and resuspended, and 100 µl of cell suspension
(1x104/ml) was added to a 96-well plate. The cells were
incubated at 5% CO2, 37˚C for 12 h, and then treated
with different concentrations of IGF-1 (0, 40, 60, 80 and 100
ng/ml) with 5 replicate wells for each concentration, after which
the cells were cultured for 5 days. Then, a total of 10 µl of Cell
Counting Kit-8 (CCK-8) reagent (Dojindo Molecular Technologies,
Inc.) was added to each well on days 1, 2, 3, 4, and 5. After
incubation for 2 h, a microplate reader was used to obtain the OD
value for each group of cells at a wavelength of 450 nm.
Lentiviral transfection of BMSCs and
cell grouping
BMSCs were transfected with a multiplicity of
infection (MOI) of 25, 50, 100, and 200 respectively, and 200 was
found to be the best MOI. BMSCs in good condition were selected and
inoculated on 24-well plates at a density of 1x104
cells/well and incubated overnight at 37˚C. After 24 h, recombinant
LV-small interfering (si)RNA-Wnt3a-mus (forward:
AGTGCCTCGGAGATGGTGGTAG; reverse: GGGTTAGGTTCGCAGAAGTTGGG)
lentivirus (GenePharma Co., Ltd.) or empty vector (GenePharma Co.,
Ltd.) lentivirus was added to the medium at a multiplicity of
infection of 200 (MOI=200). The normal group was cultured with
complete medium without adding lentivirus. After 96 h of infection,
green fluorescent protein (GFP) was observed at x400 under an
inverted fluorescence microscope (Olympus Corporation). Two groups
were used: IGF-1 + EV (empty vector) and IGF-1 + siRNA-Wnt3a.
CCK-8 assay for detection of cell
proliferation
A total of 1x104 cells/ml in good
condition were inoculated on a 96-well plate, after which 100 µl of
complete medium containing 80 ng/ml IGF-1 was added to 5 replicate
wells per group, and the cells were incubated in 5% CO2
at 37˚C. Then, a total of 10 µl of CCK-8 reagent (Dojindo Molecular
Technologies, Inc.) was added to each well on days 1, 2, 3, 4, and
5. After incubation for 2 h, a microplate reader was used to obtain
the OD value at a wavelength of 450 nm for each group of cells.
Cell cycle detection
Cells in logarithmic growth phase were digested and
centrifuged at 500 x g for 5 min at room temperature. Approximately
1x106 cells in each group were collected and washed
twice with PBS. Then, the supernatant was discarded, and the cells
were fixed with precooled 75% ethanol overnight at 4˚C. Next, the
cells were washed 2 times with PBS, after which the supernatant was
discarded, and the cells were incubated with 500 µl of PI/RNase
(1:9) Staining Buffer (Nanjing KeyGen Biotech Co., Ltd.) for 60 min
at room temperature. The proportions of cells in the cell cycle
(G1, S and G2 phases) were detected by flow cytometry (cat. no.
A00-1-1102; Beckman Coulter, Inc.).
Western blotting (WB)
Each group of cells was collected, total protein was
extracted with RIPA lysis buffer (KeyGen Biotech Co., Ltd.), and
the protein concentration was measured by the BCA method. The
proteins (50 µg/well) were used for 10% SDS-PAGE, then transferred
onto a PVDF membrane. Primary antibodies against runt-related
transcription factor 2 (RUNX2; 1:1,000; product code ab23981),
Wnt3a (1:1,000; product code ab219412), β-catenin (1:1,000; product
code ab32572; all from Abcam), GAPDH (1:2,000; cat. no. bsm-52262R;
BIOSS), cyclin D1 (1:10,000; product code ab134175) and osteopontin
(OPN; 1:1,000; product code ab63856; both from Abcam) were
incubated overnight at 4˚C. The membrane was washed 3 times with
TBST (1%), incubated at room temperature for 2 h with an
HRP-labeled secondary antibody (1:5,000; cat. no. bs-40295G-HRP;
BIOSS) and washed again with TBST. The ECL (KeyGen Biotech Co.,
Ltd.) signal was detected after exposure in a darkroom for
approximately 3 min. Imaging was performed using a Bio-Rad imaging
system (Bio-Rad Laboratories, Inc.), and the ratio of the target
protein level to GAPDH was calculated. The experiment was repeated
3 times.
RNA extraction and real-time
quantitative polymerase chain reaction (qPCR)
A total RNA extraction kit (Omega Bio-Tek, Inc.) was
used to extract total cell RNA according to the manufacturer's
instructions. RNA concentration was measured and reverse
transcribed, according to the Omega kit instructions. The detection
primers for Wnt3a, β-catenin, cyclin D1, RUNX2, OPN, and GAPDH were
designed and synthesized by Sangon Biotech, Co., Ltd. (Table I). The qPCR amplification process
was: Denaturation at 94˚C for 30 sec, annealing at 60˚C for 15 sec
and extension at 72˚C for 10 sec, for 45 cycles. qPCR was performed
using green PCR SuperMix (TransGen, Biotech Co., Ltd.) on an
Analytik Jena GmbH instrument (Qtower3G). GAPDH was used as the
internal reference gene, and the target gene expression level
obtained using the 2-ΔΔCT method (14).
 | Table IPrimers used for qPCR. |
Table I
Primers used for qPCR.
| Gene | Primer Sequence
(5'–3') |
|---|
| Wnt3a | F:
AGTGCCTCGGAGATGGTGGTAG |
| | R:
GGGTTAGGTTCGCAGAAGTTGGG |
| β-catenin | F:
GCTCTTCGTCATCTGACCAGC |
| | R:
GAGCAAGTTCACAGAGGACC |
| Cyclin D1 | F:
CGTATCTTACTTCAAGTGCGTG |
| | R:
ATGGTCTCCTTCATCTTAGAGG |
| RUNX2 | F:
TTTAGGGCGCATTCCTCATC |
| | R:
TGTCCTTGTGGATTAAAAGGACTTG |
| OPN | F:
GCAGACACTTTCACTCCAATCG |
| | R:
GGGACTCCTTAGACTCACCGC |
| GAPDH | F:
TGTGTCCGTCGTGGATCTGA |
| | R:
TTGCTGTTGAAGTCGCAGGAG |
Immunofluorescence (IF)
After BMSCs were cultured on a 24-well plate at 37˚C
for one week, Wnt3a, β-catenin, cyclin D1, RUNX2, and OPN were
detected by IF. The culture medium was discarded, and the cells
were fixed with 4% paraformaldehyde for 30 min at room temperature,
rinsed 3 times with PBS, permeabilized with 1% Triton X-100 for 15
min and blocked with 1% bovine serum albumin for 30 min at room
temperature. Then, the samples were washed and incubated overnight
at 4˚C with primary antibodies against Wnt3a (1:100), β-catenin
(1:250), cyclin D1 (1:50), RUNX2 (1:400) and OPN (1:500).
Subsequently, the cells were incubated with a
fluorescence-conjugated secondary antibody (1:800; cat. no.
SA00007-2; Proteintech, Inc.) for 1.5 h at room temperature. Nuclei
were stained with DAPI (OriGene Technologies, Inc.) for 3 min at
room temperature. The presence of the proteins was examined under a
fluorescence microscope (Olympus Corporation) at x400.
Alkaline phosphatase (ALP)
staining
Cells in good condition were selected, plated on
24-well plates, and cultured (37˚C, 5% CO2) in
osteogenic differentiation medium containing 80 ng/ml IGF-1 for 7
days. The solution was discarded, after which the cells were rinsed
twice with PBS, incubated with ALP fixative solution for 3 min at
room temperature, and rinsed again with PBS. Then, the cells were
incubated with the prepared 3% ALP incubation solution (Beijing
Solarbio Science & Technology Co., Ltd.) for 20 min in the dark
at 37˚C, rinsed with PBS, counterstained with nuclear fast red
staining solution (Beijing Solarbio Science & Technology Co.,
Ltd.) for 5 min at room temperature, rinsed with PBS again, and
observed under a fluorescence microscope (Olympus Corporation) at
x400.
Alizarin Red staining (ARS)
Cells in good condition were selected, plated on
6-well plates, and cultured (37˚C, 5% CO2) in osteogenic
differentiation medium containing 80 ng/ml IGF-1 for 3 weeks. After
the culture medium was discarded, the cells were rinsed twice with
PBS, fixed with 4% paraformaldehyde for 30 min, rinsed twice with
PBS, incubated with a 0.2% Alizarin Red (Cyagen Biosciences, Inc.)
solution for 5 min at room temperature and rinsed again with PBS.
The cells were placed under an inverted fluorescence microscope
(Olympus Corporation) for observation at x400.
Statistical analysis
Quantitative data are expressed as the means ±
standard deviation. The experiment was repeated 3 times, and
GraphPad Prism 8 software (GraphPad Software Inc.) was used for
data analysis. Comparisons of the mean differences between groups
were performed by one-way analysis of variance (ANOVA) with Tukey's
post hoc test, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Optimal drug concentration of
IGF-1
Drug toxicity was detected by CCK-8 assay. It was
revealed that cell proliferation was increased with increasing
IGF-1 concentration. When the concentration was 80 ng/ml, cell
proliferation was the highest, and the difference was statistically
significant (P<0.001); a concentration of 100 ng/ml resulted in
decreased cell proliferation (Fig.
1). Therefore, 80 ng/ml was the optimal drug concentration of
IGF-1 used in subsequent experiments.
Lentiviral transfection
BMSCs were transfected with an MOI of 25, 50, 100,
and 200 for 96 h. When the MOI was 200, the cells with green
fluorescence reached 90%. (Fig. 2).
In the subsequent experiment, transfection was performed with an
MOI of 200.
BMSCs are transfected with lentivirus
carrying siRNA-Wnt3a
BMSCs were transfected with lentivirus carrying
siRNA-Wnt3a or empty vector. WB revealed that in the siRNA-Wnt3a
group, the Wnt3a protein level was significantly lower than that in
the empty vector group and the normal group (P<0.001 vs. both
groups), but the difference between the empty vector group and the
normal group was not significant (Fig.
3A). It was revealed by qPCR that the RNA level of Wnt3a in the
siRNA-Wnt3a group was significantly lower than that in the empty
vector group and the normal group (P<0.001 vs. both groups),
with no significant difference between the empty vector group and
the normal group (Fig. 3B). In
subsequent experiments, the empty vector group served as the
control group. IF also revealed that the Wnt3a protein expression
level in the siRNA-Wnt3a group was significantly lower than that in
the empty vector group (Fig.
3C).
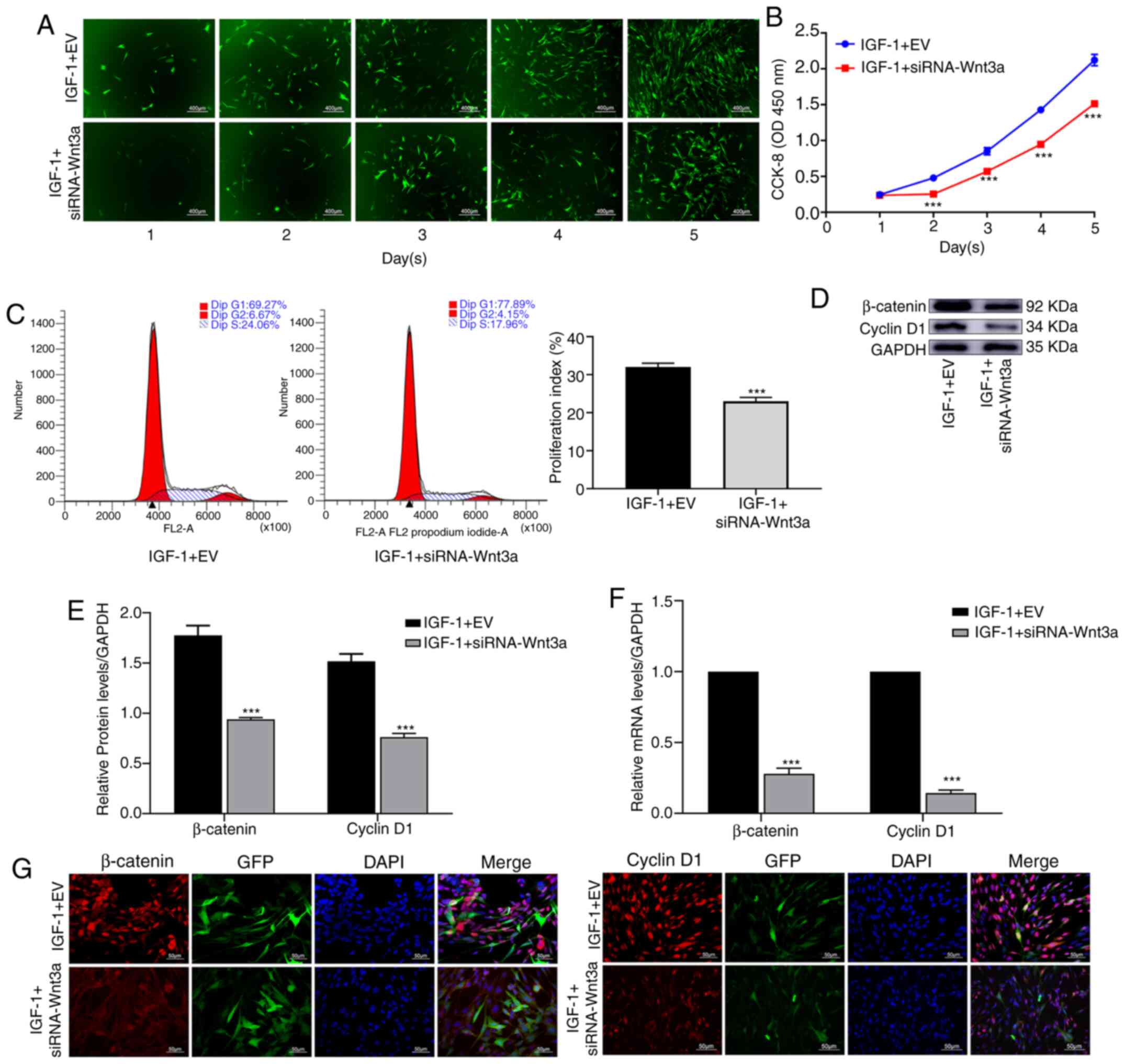
After blocking Wnt3a, IGF-1 decreases
the proliferation of BMSCs
Cells in each group were treated with IGF-1. It was
revealed that in the IGF-1 + siRNA-Wnt3a group, cell proliferation
was significantly slower than that in the IGF-1 + EV group
(Fig. 4A). The CCK-8 assay verified
that cell proliferation in the IGF-1 + siRNA-Wnt3a group was
significantly decreased (P<0.001) (Fig. 4B). Flow cytometry was used to
investigate the cell cycle, and the proliferation index was
revealed to be significantly different between the two groups
(P<0.001) (Fig. 4C). WB revealed
that the protein levels of β-catenin and cyclin D1 in the IGF-1 +
siRNA-Wnt3a group were significantly lower than those in the
control group (P<0.001) (Fig. 4D
and E). Based on qPCR, RNA levels
of β-catenin and cyclin D1 were significantly lower in the IGF-1 +
siRNA-Wnt3a group than in the control group (P<0.001) (Fig. 4F). IF also confirmed that the
fluorescence intensity of β-catenin and cyclin D1 in the IGF-1 +
siRNA-Wnt3a group was significantly lower than that in the control
group (Fig. 4G).
After blocking Wnt3a, IGF-1 decreases
the osteogenic differentiation of BMSCs
Cells of each group were stained with ALP for 7 days
after osteogenic differentiation and staining in the IGF-1 +
siRNA-Wnt3a group was weaker than that in the control group
(Fig. 5A). Cells were also stained
with ARS for 21 days after osteogenic differentiation, which
revealed that the number and area of reddish-brown mineralized
nodules in the IGF-1 + siRNA-Wnt3a group were markedly lower than
those in the control group (Fig.
5B). WB and qPCR revealed that the protein and RNA levels of
β-catenin, RUNX2 and OPN were significantly different between the
two groups (P<0.001) (Fig. 5C
and D). IF indicated that the
fluorescence intensity of β-catenin, RUNX2 and OPN was markedly
weaker in the IGF-1 + siRNA-Wnt3a group than in the IGF-1 + EV
group (Fig. 5E).
 | Figure 5By inhibiting the Wnt/β-catenin
pathway, IGF-1 reduces the osteogenic differentiation of BMSCs.
BMSCs were transfected with empty vector or the siRNA-Wnt3a gene.
Then, the cells were treated with IGF-1 (80 ng/ml). (A) Alkaline
phosphatase staining of BMSCs for 1 week. (B) Alizarin Red staining
of BMSCs for 3 weeks. (C) β-catenin, RUNX2 and OPN protein
expression was determined by western blotting for 1 week. (D) The
relative mRNA expression of β-catenin, RUNX2, and OPN was evaluated
by quantitative polymerase chain reaction for 1 week. (E)
Immunofluorescence was used to detect the expression of β-catenin,
RUNX2 and OPN in BMSCs for 1 week. ***P<0.001 vs.
IGF-1 + EV. IGF, insulin growth factor; BMSCs, bone marrow
mesenchymal stem cells; RUNX2, runt-related transcription factor 2;
OPN, osteopontin; si, small interfering; EV, empty vector. |
Discussion
In the present study, it was revealed that IGF-1
enhanced the proliferation and osteogenic differentiation of BMSCs.
When Wnt3a gene expression in BMSCs was inhibited, the ability of
IGF-1 to promote the proliferation and osteogenic differentiation
of BMSCs was reduced. A study has confirmed that the Wnt/β-catenin
pathway plays an important role in the process by which IGF-1
promotes the proliferation and osteogenic differentiation of BMSCs
(15).
BMSCs are nonhematopoietic stem cells with
multilineage differentiation potential that exist in a variety of
tissues (16). In particular, their
ability to differentiate into bone cells has become a research
focus in recent years. IGF-1 is a type of growth factor that plays
an important role in regulating the proliferation, differentiation
and apoptosis of tissue cells (17). Previous studies have also reported
that IGF-1 could regulate the proliferation, differentiation and
apoptosis of BMSCs through the STAT3/Akt, ERK1/2, BMP2, PI3K/AKT
signaling pathways (18,19). However, there are few reports on the
role of Wnt/β-catenin pathway in these processes. In the present
study, it was mainly investigated whether IGF-1 regulates the
proliferation and differentiation of BMSCs through the
Wnt/β-catenin pathway.
A lentivirus carrying siRNA-Wnt3a was transfected
into BMSCs to silence Wnt3a gene expression. The lentiviral vector
is a gene therapy vector, which is developed based on human
immunodeficiency type I virus (HIV-I). It can infect nondividing
cells, integrate large fragments of exogenous genes into
chromosomes and support long-term stable expression (20). In the present study, WB, qPCR and IF
confirmed that Wnt3a protein and RNA levels were significantly
lower in the siRNA-Wnt3a group than in the control group. This
model provides a strong background to study the role of the
Wnt/β-catenin pathway in IGF-1-mediated promotion of BMSC
proliferation and differentiation.
CCK-8 and cell cycle assays were used to assess cell
proliferation. The CCK-8 assay is a fast, highly sensitive,
nonradioactive colorimetric method widely used in cell
proliferation and toxicity (21).
Cell cycle detection includes G1, G2, and S phases, and the
proliferation index (G2 + S/G1 + G2 + S) is used to assess cell
proliferation (22). In our
experiments, CCK-8 binding and the proliferation index were
significantly reduced after the Wnt/β-catenin pathway was
inhibited. WB, qPCR, and IF confirmed that β-catenin and cyclin D1
were significantly downregulated at the protein and mRNA levels.
β-catenin is a key regulator in the Wnt/β-catenin pathway. When
Wnt3a is inhibited, β-catenin level in the cytoplasm is decreased,
inhibiting downstream pathways (23). Cyclin D1 plays an important role in
cell proliferation and is a downstream target gene in the
Wnt/β-catenin pathway (24). The
present study revealed that IGF-1 can promote the proliferation of
BMSCs, which is consistent with previously reported results
(18). However, when the
Wnt/β-catenin pathway was inhibited, the proliferation of BMSCs
induced by IGF-1 was decreased. Therefore, it was concluded that
IGF-1 promoted the proliferation of BMSCs at least partially
through the Wnt/β-catenin pathway.
In addition, ALP staining and ARS were used to
detect osteogenic differentiation. ALP is a phosphatase that is
widely present in mammals and is often used as an indicator of
early osteogenic differentiation (25). Alizarin Red reacts with calcium to
produce a deep red color and is often used as an indicator of late
osteogenic differentiation (26).
In the present study, the intensity and area of ALP staining and
ARS were significantly reduced after the Wnt/β-catenin pathway was
inhibited. WB, qPCR and IF confirmed that β-catenin, RUNX2 and OPN
were significantly downregulated at the protein and mRNA levels.
RUNX2 is a member of the RUNX2 family of transcription factors,
which plays an important role in the formation and reconstruction
of bone tissue (27). OPN is a
glycosylated protein that is widely present in the extracellular
matrix. It is closely related to bone formation and development
(28). In the present study, IGF-1
promoted the osteogenic differentiation of BMSCs, which is
consistent with the results of recent related study (29). However, after the Wnt/β-catenin
pathway was inhibited, the ability of IGF-1 to promote osteogenic
differentiation was decreased. Therefore, it was inferred that
IGF-1 promoted the differentiation of BMSCs at least partially
through the Wnt/β-catenin pathway.
In the present study, the role of the Wnt/β-catenin
pathway in the mechanism by which IGF-1 promotes the proliferation
and differentiation of BMSCs was verified through in vitro
experiments. However, further in vivo experiments are
required to support our conclusions and provide new strategies and
directions for the treatment of nonunion, bone defects and other
diseases. Upon inhibition of the Wnt/β-catenin pathway, IGF-1
promoted the proliferation of BMSCs and decreased their
differentiation ability. In summary, IGF-1 at least partially
promoted the proliferation and osteogenic differentiation of BMSCs
through the Wnt/β-catenin pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the General Hospital
of Ningxia Medical University (Clinical Medicine Research Center of
Autonomous Region) Open Project (grant no. 020007004127).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JF performed the experiments, collected the results
and wrote the manuscript. ZM designed the experiments, analyzed the
data and revised the manuscript. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedenstein AJ, Latzinik NV, Gorskaya
YuF, Luria EA and Moskvina IL: Bone marrow stromal colony formation
requires stimulation by haemopoietic cells. Bone Miner. 18:199–213.
1992.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang A, Yu C, You F, He C and Li Z:
Mechanisms of zuogui pill in treating osteoporosis: Perspective
from bone marrow mesenchymal stem cells. Evid Based Complement
Alternat Med. 2018(3717391)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yin N, Wang Y, Ding L, Yuan J, Du L, Zhu
Z, Pan M, Xue F and Xiao H: Platelet-rich plasma enhances the
repair capacity of muscle-derived mesenchymal stem cells to large
humeral bone defect in rabbits. Scie Rep. 10(6771)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yanagihara K, Uchida S, Ohba S, Kataoka K
and Itaka K: Treatment of bone defects by transplantation of
genetically modified mesenchymal stem cell spheroids. Mol Ther
Methods Clin Dev. 9:358–366. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang XR, Yang HY, Zhang XX, Lin GD, Meng
YC, Zhang PX, Jiang S, Zhang CL, Huang F and Xu L: Repair of bone
defects with prefabricated vascularized bone grafts and
double-labeled bone marrow-derived mesenchymal stem cells in a rat
model. Sci Rep. 7(39431)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Křížková K, Chrudinová M, Povalová A,
Selicharová I, Collinsová M, Vaněk V, Brzozowski AM, Jiráček J and
Žáková L: Insulin-insulin-like growth factors hybrids as molecular
probes of hormone: Receptor binding specificity. Biochemistry.
55:2903–2913. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Backeljauw P: Therapy with recombinant
human IGF-1 for children with primary insulin-like growth factor-I
deficiency. Growth Horm IGF Res. 51:22–26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gillberg P, Olofsson H, Mallmin H, Blum
WF, Ljunghall S and Nilsson AG: Bone mineral density in femoral
neck is positively correlated to circulating insulin-like growth
factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men.
Calcif Tissue Int. 70:22–29. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Halmos T and Suba I: The physiological
role of growth hormone and insulin-like growth factors. Orv Hetil.
160:1774–1783. 2019.PubMed/NCBI View Article : Google Scholar : (In Hu).
|
|
10
|
Salih DA, Mohan S, Kasukawa Y, Tripathi G,
Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ and Pell
JM: Insulin-like growth factor-binding protein-5 induces a
gender-related decrease in bone mineral density in transgenic mice.
Endocrinology. 146:931–940. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aboalola D and Han VKM: Insulin-like
growth factor binding protein-6 promotes the differentiation of
placental mesenchymal stem cells into skeletal muscle independent
of insulin-like growth factor receptor-1 and insulin receptor. Stem
Cells Int. 2019(9245938)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Du JH, Lin SX, Wu XL, Yang SM, Cao LY,
Zheng A, Wu JN and Jiang XQ: The function of Wnt ligands on
osteocyte and bone remodeling. J Dent Res. 98:930–938.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saad FA: Novel insights into the complex
architecture of osteoporosis molecular genetics. Ann N Y Acad Sci.
1462:37–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gugjoo MB, Amarpal Abdelbaset-Ismail A,
Aithal HP, Kinjavdekar P, Pawde AM, Kumar GS and Sharma GT:
Mesenchymal stem cells with IGF-1 and TGF-β1 in laminin gel for
osteochondral defects in rabbits. Biomed Pharmacother.
93:1165–1174. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8(784)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ahmad S, Ahmad K, Lee EJ, Lee YH and Choi
I: Implications of insulin-like growth factor-1 in skeletal muscle
and various diseases. Cells. 9(1773)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang XH, Wu HY, Gao J, Wang XH, Gao TH and
Zhang SF: IGF1R facilitates epithelial-mesenchymal transition and
cancer stem cell properties in neuroblastoma via the STAT3/AKT
axis. Cancer Manag Res. 11:5459–5472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang C, Hong FF, Wang CC, Li L, Chen JL,
Liu F, Quan RF and Wang JF: TRIB3 inhibits proliferation and
promotes osteogenesis in hBMSCs by regulating the ERK1/2 signaling
pathway. Sci Rep. 7(10342)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song L, He J, Gao Y, Fang Y, Zhang L, Wang
J, Sun F, Zhang F, Zeng Y, Zeng F and Zhang J: Improved biosafety
of a lentiviral vector by reducing cellular gene activation. J Gene
Med. 21(e3087)2019.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Cai L, Qin X, Xu Z, Song Y, Jiang H, Wu Y,
Ruan H and Chen J: Comparison of cytotoxicity evaluation of
anticancer drugs between real-time cell analysis and CCK-8 method.
ACS Omega. 4:12036–12042. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sosnowska M, Kutwin M, Jaworski S, Strojny
B, Wierzbicki M, Szczepaniak J, Łojkowski M, Święszkowski W,
Bałaban J, Chwalibog A and Sawosz E: Mechano-signalling, induced by
fullerene C60 nanofilms, arrests the cell cycle in the
G2/M phase and decreases proliferation of liver cancer cells. Int J
Nanomedicine. 14:6197–6215. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Annavarapu SR, Cialfi S, Dominici C, Kokai
GK, Uccini S, Ceccarelli S, McDowell HP and Helliwell TR:
Characterization of Wnt/β-catenin signaling in rhabdomyosarcoma.
Lab Invest. 93:1090–1099. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng W, Lin P, Ma Y, Shao X, Chen H, Chen
D, Liu X, Li X and Ye H: Psoralen promotes the expression of cyclin
D1 in chondrocytes via the Wnt/β-catenin signaling pathway. Int J
Mol Med. 40:1377–1384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Posa F, Di Benedetto A, Cavalcanti-Adam
EA, Colaianni G, Porro C, Trotta T, Brunetti G, Lo Muzio L, Grano M
and Mori G: Vitamin D promotes MSC osteogenic differentiation
stimulating cell adhesion and αvβ3 expression. Stem Cells Int.
2018(6958713)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schürmann M, Wolff A, Widera D, Hauser S,
Heimann P, Hütten A, Kaltschmidt C and Kaltschmidt B: Interaction
of adult human neural crest-derived stem cells with a nanoporous
titanium surface is sufficient to induce their osteogenic
differentiation. Stem Cell Res. 13:98–110. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang J, Xie J, Chen W, Tang C, Wu J, Wang
Y, Zhou XD, Zhou HD and Li YP: Runt-related transcription factor 1
is required for murine osteoblast differentiation and bone
formation. J Biol Chem. 295:11669–11681. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luukkonen J, Hilli M, Nakamura M, Ritamo
I, Valmu L, Kauppinen K, Tuukkanen J and Lehenkari P: Osteoclasts
secrete osteopontin into resorption lacunae during bone resorption.
Histochem Cell Biol. 151:475–487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Reible B, Schmidmaier G, Moghaddam A and
Westhauser F: Insulin-like growth factor-1 as a possible
alternative to bone morphogenetic protein-7 to induce osteogenic
differentiation of human mesenchymal stem cells in vitro. Int J Mol
Sci. 19(1674)2018.PubMed/NCBI View Article : Google Scholar
|