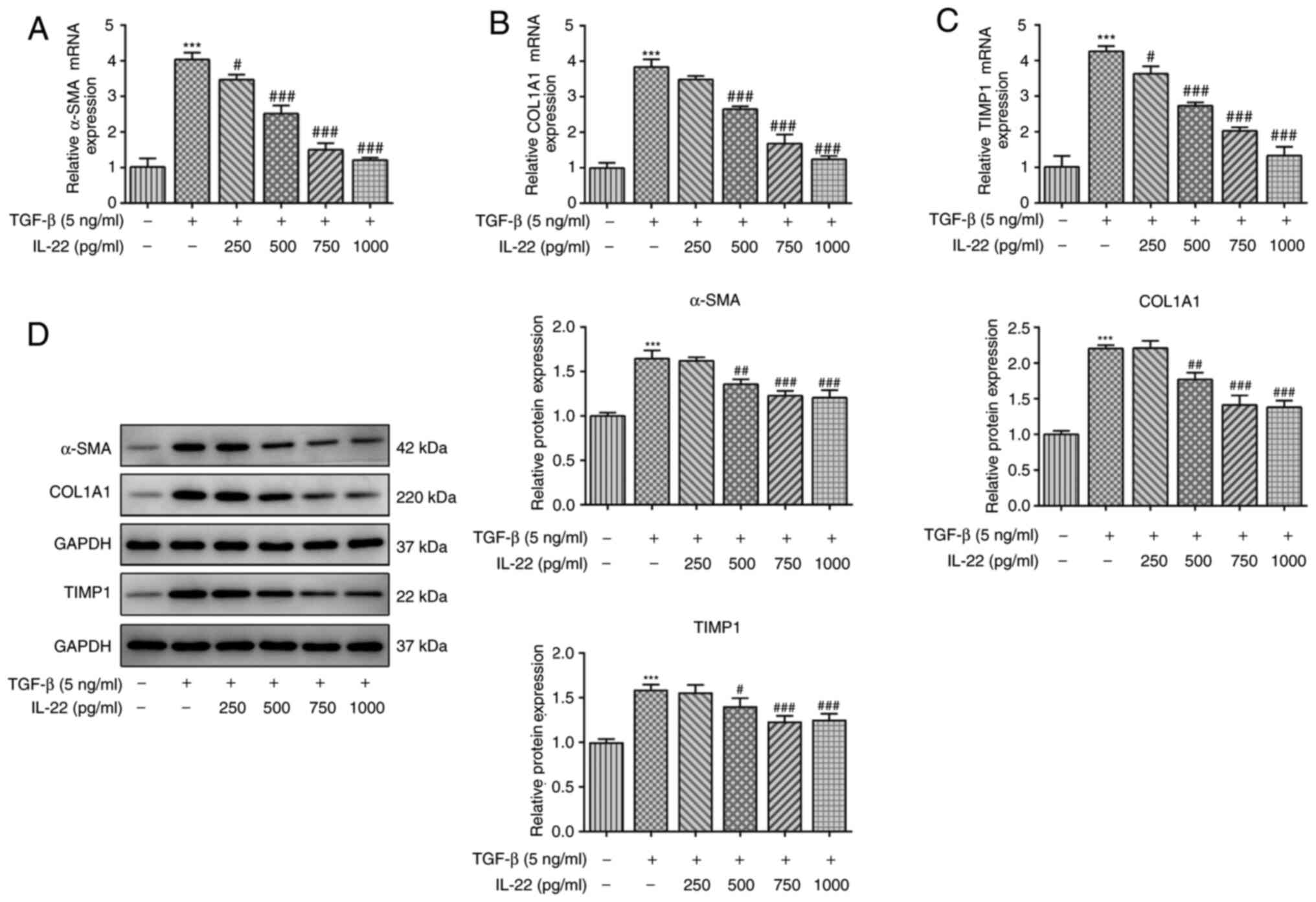
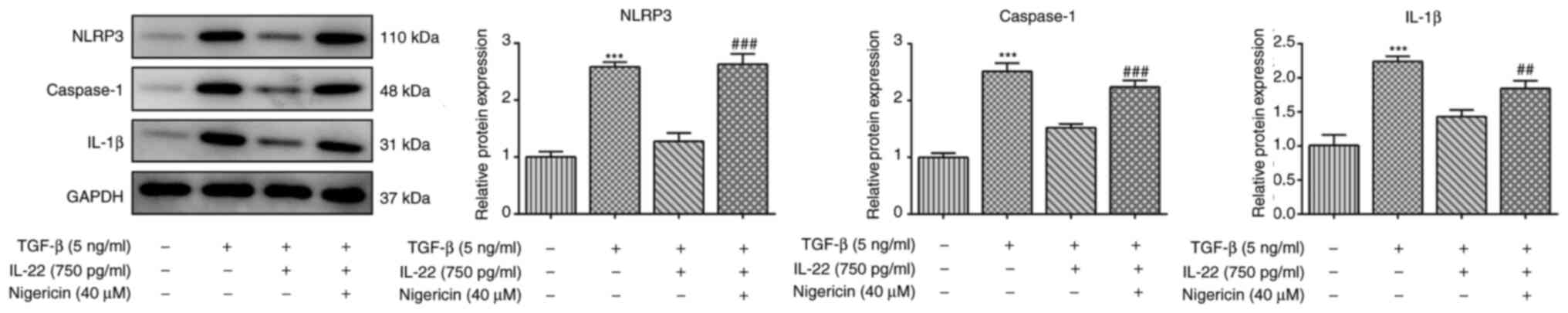
|
1
|
Seki E and Brenner DA: Recent advancement
of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat
Sci. 22:512–518. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Matsuda M and Seki E: The liver fibrosis
niche: Novel insights into the interplay between fibrosis-composing
mesenchymal cells, immune cells, endothelial cells, and
extracellular matrix. Food Chem Toxicol. 143(111556)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Friedman SL: Mechanisms of disease:
Mechanisms of hepatic fibrosis and therapeutic implications. Nat
Clin Pract Gastroenterol Hepatol. 1:98–105. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu S, Liu L, Yang S, Kuang G, Yin X, Wang
Y, Xu F, Xiong L, Zhang M, Wan J, et al: Paeonol alleviates
CCl4-induced liver fibrosis through suppression of hepatic stellate
cells activation via inhibiting the TGF-β/Smad3 signaling.
Immunopharmacol Immunotoxicol. 41:438–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumar P, Smith T, Rahman K, Thorn NE and
Anania FA: Adiponectin agonist ADP355 attenuates CCl4-induced liver
fibrosis in mice. PLoS One. 9(e110405)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schuppan D: Liver fibrosis: Common
mechanisms and antifibrotic therapies. Clin Res Hepatol
Gastroenterol. 39 (Suppl 1):S51–S59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kunkl M, Amormino C, Frascolla S, Sambucci
M, De Bardi M, Caristi S, Arcieri S, Battistini L and Tuosto L:
CD28 autonomous signaling orchestrates IL-22 expression and
IL-22-regulated epithelial barrier functions in human T
lymphocytes. Front Immunol. 11(590964)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang
Z, Stinson J, Wood WI, Goddard AD and Gurney AL: Interleukin
(IL)-22, a novel human cytokine that signals through the interferon
receptor-related proteins CRF2-4 and IL-22R. J Biol Chem.
275:31335–31339. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nagem RA, Colau D, Dumoutier L, Renauld
JC, Ogata C and Polikarpov I: Crystal structure of recombinant
human interleukin-22. Structure. 10:1051–1062. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang S, Li Y, Fan J, Zhang X, Luan J, Bian
Q, Ding T, Wang Y, Wang Z, Song P, et al: Interleukin-22
ameliorated renal injury and fibrosis in diabetic nephropathy
through inhibition of NLRP3 inflammasome activation. Cell Death
Dis. 8(e2937)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xing WW, Zou MJ, Liu S, Xu T, Gao J, Wang
JX and Xu DG: Hepatoprotective effects of IL-22 on fulminant
hepatic failure induced by d-galactosamine and lipopolysaccharide
in mice. Cytokine. 56:174–179. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim DK, Jo A, Lim HS, Kim JY, Eun KM, Oh
J, Kim JK, Cho SH and Kim DW: Enhanced type 2 immune reactions by
increased IL-22/IL-22Ra1 signaling in chronic rhinosinusitis with
nasal polyps. Allergy Asthma Immunol Res. 12:980–993.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y
and Zhou D: Interleukin-22 in liver injury, inflammation and
cancer. Int J Biol Sci. 16:2405–2413. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Que R, Shen Y, Ren J, Tao Z, Zhu X and Li
Y: Estrogen receptor β dependent effects of saikosaponin d on the
suppression of oxidative stress induced rat hepatic stellate cell
activation. Int J Mol Med. 41:1357–1364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pan X, Shao Y, Wang F, Cai Z, Liu S, Xi J,
He R, Zhao Y and Zhuang R: Protective effect of apigenin magnesium
complex on H2O2-induced oxidative stress and
inflammatory responses in rat hepatic stellate cells. Pharm Biol.
58:553–560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Novo E, Busletta C, Bonzo LV, Povero D,
Paternostro C, Mareschi K, Ferrero I, David E, Bertolani C,
Caligiuri A, et al: Intracellular reactive oxygen species are
required for directional migration of resident and bone
marrow-derived hepatic pro-fibrogenic cells. J Hepatol. 54:964–974.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li C, Meng M, Guo MZ, Wang MY, Ju AN and
Wang CL: The polysaccharides from Grifola frondosa attenuate
CCl4-induced hepatic fibrosis in rats via the TGF-beta/Smad
signaling pathway. RSC Advances. 9:33684–33692. 2019.
|
|
20
|
Ting JP, Lovering RC, Alnemri ES, Bertin
J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA,
et al: The NLR gene family: A standard nomenclature. Immunity.
28:285–287. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu X, Dong L, Lin X and Li J: Relevance of
the NLRP3 inflammasome in the pathogenesis of chronic liver
disease. Front Immunol. 8(1728)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Feng D, Mukhopadhyay P, Qiu J and Wang H:
Inflammation in liver diseases. Mediators Inflamm.
2018(3927134)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang S, Li Y, Fan J, Zhang X, Luan J, Bian
Q, Ding T, Wang Y, Wang Z, Song P, et al: Interleukin-22
ameliorated renal injury and fibrosis in diabetic nephropathy
through inhibition of NLRP3 inflammasome activation. Cell Death
Dis. 8(e2937)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang S, Fan J, Mei X, Luan J, Li Y, Zhang
X, Chen W, Wang Y, Meng G and Ju D: Interleukin-22 attenuated renal
tubular injury in aristolochic acid nephropathy via suppressing
activation of NLRP3 inflammasome. Front Immunol.
10(2277)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen E, Cen Y, Lu D, Luo W and Jiang H:
IL-22 inactivates hepatic stellate cells via downregulation of the
TGF-β1/Notch signaling pathway. Mol Med Rep. 17:5449–5453.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mu M, Zuo S, Wu RM, Deng KS, Lu S, Zhu JJ,
Zou GL, Yang J, Cheng ML and Zhao XK: Ferulic acid attenuates liver
fibrosis and hepatic stellate cell activation via inhibition of
TGF-β/Smad signaling pathway. Drug Des Devel Ther. 12:4107–4115.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Martine P, Chevriaux A, Derangère V,
Apetoh L, Garrido C, Ghiringhelli F and Rébé C: HSP70 is a negative
regulator of NLRP3 inflammasome activation. Cell Death Dis.
10(256)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aslamazova EB, Stroganova EV and
Dzhinanian VL: Pathogenesis of liver cirrhosis. Ter Arkh. 47:30–34.
1975.PubMed/NCBI(In Russian).
|
|
30
|
Lakner AM, Steuerwald NM, Walling TL,
Ghosh S, Li T, McKillop IH, Russo MW, Bonkovsky HL and Schrum LW:
Inhibitory effects of microRNA 19b in hepatic stellate
cell-mediated fibrogenesis. Hepatology. 56:300–310. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hwang S, He Y, Xiang X, Seo W, Kim SJ, Ma
J, Ren T, Park SH, Zhou Z, Feng D, et al: Interleukin-22
ameliorates neutrophil-driven nonalcoholic steatohepatitis through
multiple targets. Hepatology. 72:412–429. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zenewicz LA, Yancopoulos GD, Valenzuela
DM, Murphy AJ, Karow M and Flavell RA: Interleukin-22 but not
interleukin-17 provides protection to hepatocytes during acute
liver inflammation. Immunity. 27:647–659. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu DH, Guo XY, Qin SY, Luo W, Huang XL,
Chen M, Wang JX, Ma SJ, Yang XW and Jiang HX: Interleukin-22
ameliorates liver fibrogenesis by attenuating hepatic stellate cell
activation and downregulating the levels of inflammatory cytokines.
World J Gastroenterol. 21:1531–1545. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu K, Sun J, Chen S and Li Y, Peng X, Li M
and Li Y: Hydrodynamic delivery of IL-38 gene alleviates
obesity-induced inflammation and insulin resistance. Biochem
Biophys Res Commun. 508:198–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Vandanmagsar B, Youm YH, Ravussin A,
Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM and Dixit
VD: The NLRP3 inflammasome instigates obesity-induced inflammation
and insulin resistance. Nat Med. 17:179–188. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Swanson KV, Deng M and Ting JPY: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Duewell P, Kono H, Rayner KJ, Sirois CM,
Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr
M, et al: NLRP3 inflammasomes are required for atherogenesis and
activated by cholesterol crystals. Nature. 464:1357–1361.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y,
Fan D and Tan H: Activation of NLRP3 inflammasomes contributes to
hyperhomocysteinemia-aggravated inflammation and atherosclerosis in
apoE-deficient mice. Lab Invest. 97:922–934. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Abderrazak A, Couchie D, Mahmood DF,
Elhage R, Vindis C, Laffargue M, Matéo V, Büchele B, Ayala MR, El
Gaafary M, et al: Anti-inflammatory and antiatherogenic effects of
the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a
high-fat diet. Circulation. 131:1061–1070. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Qu J, Yuan Z, Wang G, Wang X and Li K: The
selective NLRP3 inflammasome inhibitor MCC950 alleviates
cholestatic liver injury and fibrosis in mice. Int Immunopharmacol.
70:147–155. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wree A, Eguchi A, McGeough MD, Pena CA,
Johnson CD, Canbay A, Hoffman HM and Feldstein AE: NLRP3
inflammasome activation results in hepatocyte pyroptosis, liver
inflammation, and fibrosis in mice. Hepatology. 59:898–910.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu X, Zhang F, Xiong X, Lu C, Lian N, Lu Y
and Zheng S: Tetramethylpyrazine reduces inflammation in liver
fibrosis and inhibits inflammatory cytokine expression in hepatic
stellate cells by modulating NLRP3 inflammasome pathway. IUBMB
Life. 67:312–321. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Mridha AR, Wree A, Robertson AAB, Yeh MM,
Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou
GN, et al: NLRP3 inflammasome blockade reduces liver inflammation
and fibrosis in experimental NASH in mice. J Hepatol. 66:1037–1046.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Watanabe A, Sohail MA, Gomes DA, Hashmi A,
Nagata J, Sutterwala FS, Mahmood S, Jhandier MN, Shi Y, Flavell RA,
et al: Inflammasome-mediated regulation of hepatic stellate cells.
Am J Physiol Gastrointest Liver Physiol. 296:G1248–G1257.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Inzaugarat ME, Johnson CD, Holtmann TM,
McGeough MD, Trautwein C, Papouchado BG, Schwabe R, Hoffman HM,
Wree A and Feldstein AE: NLR family pyrin domain-containing 3
inflammasome activation in hepatic stellate cells induces liver
fibrosis in mice. Hepatology. 69:845–859. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu D, Qin H, Yang B, Du B and Yun X:
Oridonin ameliorates carbon tetrachloride-induced liver fibrosis in
mice through inhibition of the NLRP3 inflammasome. Drug Dev Res.
81:526–533. 2020.PubMed/NCBI View Article : Google Scholar
|