Introduction
Lung cancer is one of the most invasive malignancies
worldwide. Non-small cell lung cancer (NSCLC) is the major type of
lung cancer, with an estimated 5-year survival rate of only 16% in
2014 in the United States (1).
While great effort has been made to improve the prognosis of NSCLC,
metastasis is still a serious hurdle for its treatment. Metastasis
is a complex process; its foundation depends on the activity of
cellular movement machinery (2).
Therefore, the migration and invasion abilities may be potential
targets for preventing metastasis.
Focal adhesions (FAs) act as important connectors
between cells and the extracellular matrix (ECM). FA kinase (FAK)
is a major kinase with a crucial role in cell motility (3). FAKs are recruited to FAs once
integrins bind to ECM proteins in the tumor microenvironment
(4). FAK forms a complex with Src
when recognized by its SH2 domain. The FAK/Src complex has been
demonstrated to promote cell migration and invasion in many types
of cancer, including NSCLC (5-7).
Inhibition of FAK/Src signaling decreases the migration and
metastasis of ovarian and lung cancer cells (8-10).
However, current drugs targeting FAK/Src (such as defactinib and
dasatinib) showed limited success in the treatment of solid tumors
(11).
β-elemene, a major component of Curcuma Rhizoma, has
been demonstrated to have anti-tumor effects on different types of
cancer, including gastric cancer and breast cancer (12,13).
Studies performed on cells and animals have shown that β-elemene
induced apoptosis and decreased proliferation in esophageal
squamous carcinoma cell, gastric cancer cell and rheumatoid
arthritis fibroblast-like synoviocytes (12,14,15).
Several experiments have demonstrated the chemotherapeutic effects
of β-elemene by decreasing cytotoxicity (16,17).
β-elemene has been shown to decrease the migration and invasion of
lung cancer cells by suppressing the epithelial-to-mesenchymal
transition (EMT) (18). β-elemene
was also shown to exert an anti-metastatic effect on breast cancer
cells by blocking aerobic glycolysis (13). However, the role of β-elemene in
cell motility in lung cancer and its possible underlying mechanism
remain poorly defined.
In the present study, it was hypothesized that
β-elemene inhibits cell motility in lung cancer cells through the
FAK-Src signaling pathway. The effects of β-elemene on cell
migration and invasiveness were investigated in two NSCLC cell
lines (A549 and H1299) using wound-healing and Transwell assays.
The impact of β-elemene on the expression levels of mRNA and
protein associated with FAK-Src signaling was determined. The
results suggest that β-elemene could suppress NSCLC cell motility
via the inhibition of FAK-Src signaling.
Materials and methods
Reagents
β-elemene was obtained from CSCP Pharmaceutical
Group Ltd. Fetal bovine serum (FBS) and Dulbecco's modified Eagle's
medium (DMEM/H) were purchased from Cytvia. Dimethyl sulfoxide
(DMSO) was purchased from BioFROXX and
3-(4,5-dimethylthiazol-2-yl)-2, 5-dephenyltetrazolium bromide (MTT)
was obtained from (Shanghai Ica Biotechnology Co., Ltd.; cat. no.
MO105-1G). A Matrigel-coated Transwell chamber was purchased from
BD Biosciences. Antibodies against phosphorylated (p)-FAKTyr397,
total (t)-FAK, p-SrcTyr416, p-SrcTyr527, and t-Src were obtained
from Abcam. Crystal violet was obtained from Beyotime Institute of
Biotechnology.
Cell culture and treatment
A549 and H1299 cells (The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences) were cultured in
DMEM supplemented with 10% FBS and 1% penicillin/streptomycin.
Cells were maintained in a 37˚C incubator supplemented with 5%
CO2. β-elemene was diluted to 25, 50, 100 and 200 µg/ml
with complete medium. A vehicle control consisting of 0.2% DMSO was
used (0 µg/ml β-elemene).
MTT assay
In total, 3,000 cells were plated into each well of
a 96-well plate. After incubating overnight, different
concentrations of β-elemene (0, 50, 100 and 200 µg/ml) were added
with fresh medium to each well. Subsequently, cells were washed
with phosphate-buffered saline (PBS) and further incubated with 10
µl MTT for 4 h. Subsequently, the MTT was aspirated and the cells
were washed with PBS. DMSO (100 µl) was added to dissolve the
formazan crystals. The absorbance at 570 nm was measured with a
microplate reader (Molecular Devices SpectraMax i3; Bio-Rad
Laboratories, Inc.). Cell numbers were normalized to the 0 µg/ml
group.
Migration assay
A549 and H1299 cells were plated into 12-well plates
(2x105 each well). Once cells reached 80-90% confluence,
a 200-µl pipette tip was used to generate a scratch. Floating cells
were washed with PBS. Then, different concentrations of β-elemene
(0 and 50 µg/ml) with fresh serum-free medium were added to each
well. The wound gap was imaged with a light microscope
(magnification, x4 and x10; XDS-1A; Precision Instruments) after 0,
12 and 24 h of incubation. The migration ability was determined by
measuring the width (the shortest measurement horizontally across
the gap) of the scratch and was normalized to the 0-µg/ml
group.
Invasion assay
The invasion capacity of cells was determined using
a Matrigel-coated Transwell chamber (BD Biosciences). After
treating with 0 or 50 µg/ml β-elemene with serum-free medium, 5x106
cells were plated on the top chamber of the Transwell insert and
stimulated with 500 µl medium with 50% FBS added to the bottom
chamber. After 24 h of incubation (5% CO2 at 37˚C), a
cotton swab was used to remove the non-invasive cells on the inside
of the upper chamber. The invasive cells on the underside of the
upper chamber were fixed using 4% paraformaldehyde (PFA) for 10 min
at room temperature and stained with 2% crystal violet (20 min at
room temperature). Random fields were imaged (magnification, x100)
and measured with a light microscope.
Cell adhesion assay
After treatment with 0 or 50 µg/ml β-elemene, cells
were digested and resuspended in complete medium. Then, the cells
(2,000 cells/well) were plated in each well of a 96-well plate
precoated with 20 µl Matrigel for 1 h. The cells were subsequently
gently washed with PBS. The remaining cells were fixed with 4% PFA
for 10 min at room temperature and stained with 0.2% crystal violet
for 20 min at room temperature. The plates were placed directly
under a light microscope (magnification, x100) to measure the
adhesive cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549 and H1299 cells
treated with β-elemene (0 or 50 µg/ml) using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Reverse transcription was performed
with a RevertAid First Strand cDNA Synthesis kit (cat. no. K1622;
Thermo Fisher Scientific, Inc.) using 100 ng of total RNA (60 min
at 42˚C, 5 min at 25˚C followed by 60 min at 42˚C and 5 min at
70˚C). The primers used to amplify (Table I) RhoA, Rac1,
Cdc42, matrix metalloproteinase (MMP)2 and
MMP9 were designed with Primer Premier 5.0 software (BBI
Life Sciences). The thermocycling parameters for RT-qPCR using
SybrGreen qPCR Master mix (cat. no. F-415XL; Thermo Fisher
Scientific, Inc.) were: 94˚C for 10 min followed by 40 cycles of
94˚C for 15 sec, 55˚C for 30 sec, and 72˚C for 30 sec. Relative
changes in mRNA expression were calculated using the
2-ΔΔCq method with β-actin as the internal
control (19).
 | Table IPrimer sequences for PCR
amplification. |
Table I
Primer sequences for PCR
amplification.
| Names | Sequences, 5' to
3' |
|---|
|
RhoA-Human-RT-F |
GGAAAGCAGGTAGAGTTGGCT |
|
RhoA-Human-RT-R |
GGCTGTCGATGGAAAAACACAT |
|
Rac1-Human-RT-F |
ATGTCCGTGCAAAGTGGTATC |
|
Rac1-Human-RT-R |
CTCGGATCGCTTCGTCAAACA |
|
Cdc42-Human-RT-F |
CCATCGGAATATGTACCGACTG |
|
Cdc42-Human-RT-R |
CTCAGCGGTCGTAATCTGTCA |
|
MMP2-Human-RT-F |
GATACCCCTTTGACGGTAAGGA |
|
MMP2-Human-RT-R |
CCTTCTCCCAAGGTCCATAGC |
|
MMP9-Human-RT-F |
GGGACGCAGACATCGTCATC |
|
MMP9-Human-RT-R |
TCGTCATCGTCGAAATGGGC |
|
β-actin-Human-RT-F |
AGCGAGCATCCCCCAAAGTT |
|
β-actin-Human-RT-R |
GGGCACGAAGGCTCATCATT |
Western blotting
A549 and H1299 cells were treated with 50 µg/ml of
β-elemene for 0, 1, 3, 6, 12 and 24 h. Then,
radioimmunoprecipitation assay lysis buffer and
phenylmethylsulfonyl fluoride were added to the cells, and
incubated on ice for 2 h. After centrifuging at 161 x g for 10 min
at 4˚C, 30 µg of total protein was loaded on 12% gels for sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
the gel was run for 1 h at 120 V. The proteins were transferred to
polyvinylidene difluoride membranes and run at 100 V for 1 h.
Subsequently, the membranes were blocked with 5% nonfat milk in
TBST at room temperature for 1 h. Next, the membranes were
incubated with specific primary antibodies in blocking buffer
against p-FAKTyr397 (Abcam; cat. no. ab81298; 1:1,000),
t-FAK (Abcam; cat. no. ab40794; 1:2,000), p-SrcTyr416
(Abcam; cat. no. ab4066; 1:1,000), p-SrcTyr527 (Abcam;
cat. no. ab32078; 1:5,000), and t-Src (Abcam; cat. no. ab109381;
1:10,000) at 4˚C overnight. The membranes were then washed with
TBST three times and incubated with anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (Biosharp Life Sciences;
cat. no. BL003A; 1:4,000) in blocking buffer for 1 h at room
temperature. The membranes were washed with TBST three times and
the bands were visualized using an electrochemiluminescence system
(Bio-Rad Laboratories, Inc.) in a dark room according to the
manufacturer's instructions. The expression levels of specific
proteins in A549 and H1299 cells were normalized to those in cells
of the 0 µg/ml group.
Statistical analysis
All data are shown as the mean ± standard error of
the mean. More than three independent replicates were performed for
each set of experiments. All statistical analyses were performed
using GraphPad Prism (version 5; GraphPad Software, Inc.). The
differences between treatment groups were compared using the
Student's t-test and one-way analysis of variance (ANOVA) with
Dunnett's post hoc test. P<0.05 was considered to indicate
statistically significant difference.
Results
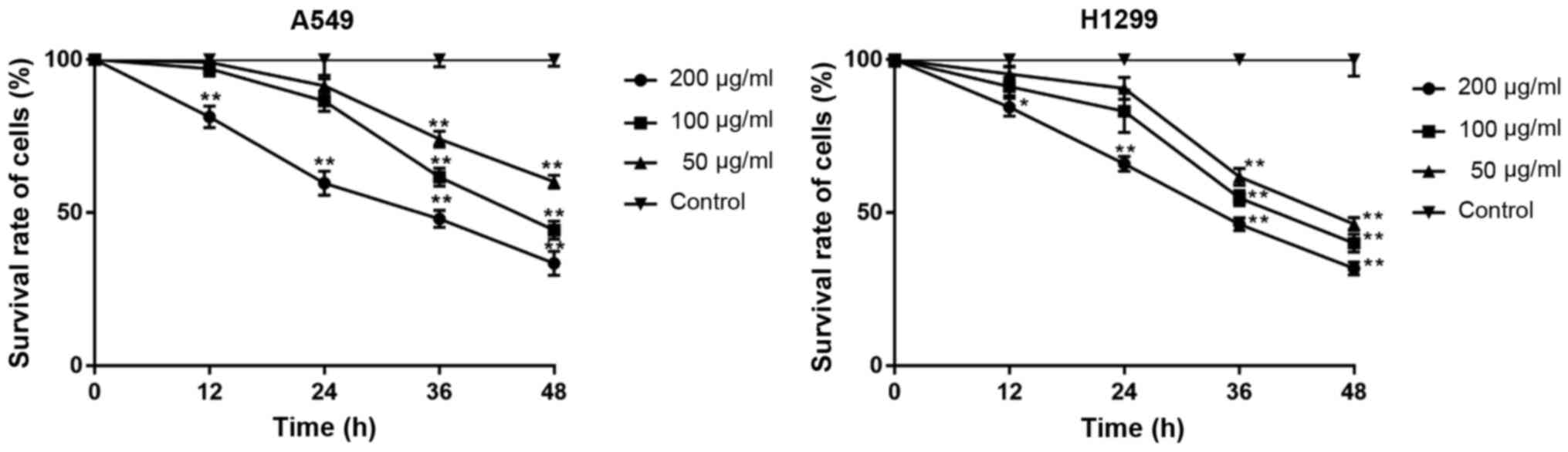
β-elemene decreases the viability of
A549 and H1299 cells
To investigate the effects of β-elemene on cell
viability and determine the suitable concentration for subsequent
experiments, A549 and H1299 cells were treated with different doses
of β-elemene (0-200 µg/ml) for 0-48 h. Cell viability was
determined with the MTT assay. A concentration-dependent decrease
in viability was observed in both cell lines after exposure to
β-elemene (Fig. 1). Treatment with
50 µg/ml β-elemene for 24 h showed no inhibitory effect on either
cell line and was selected for subsequent experiments.
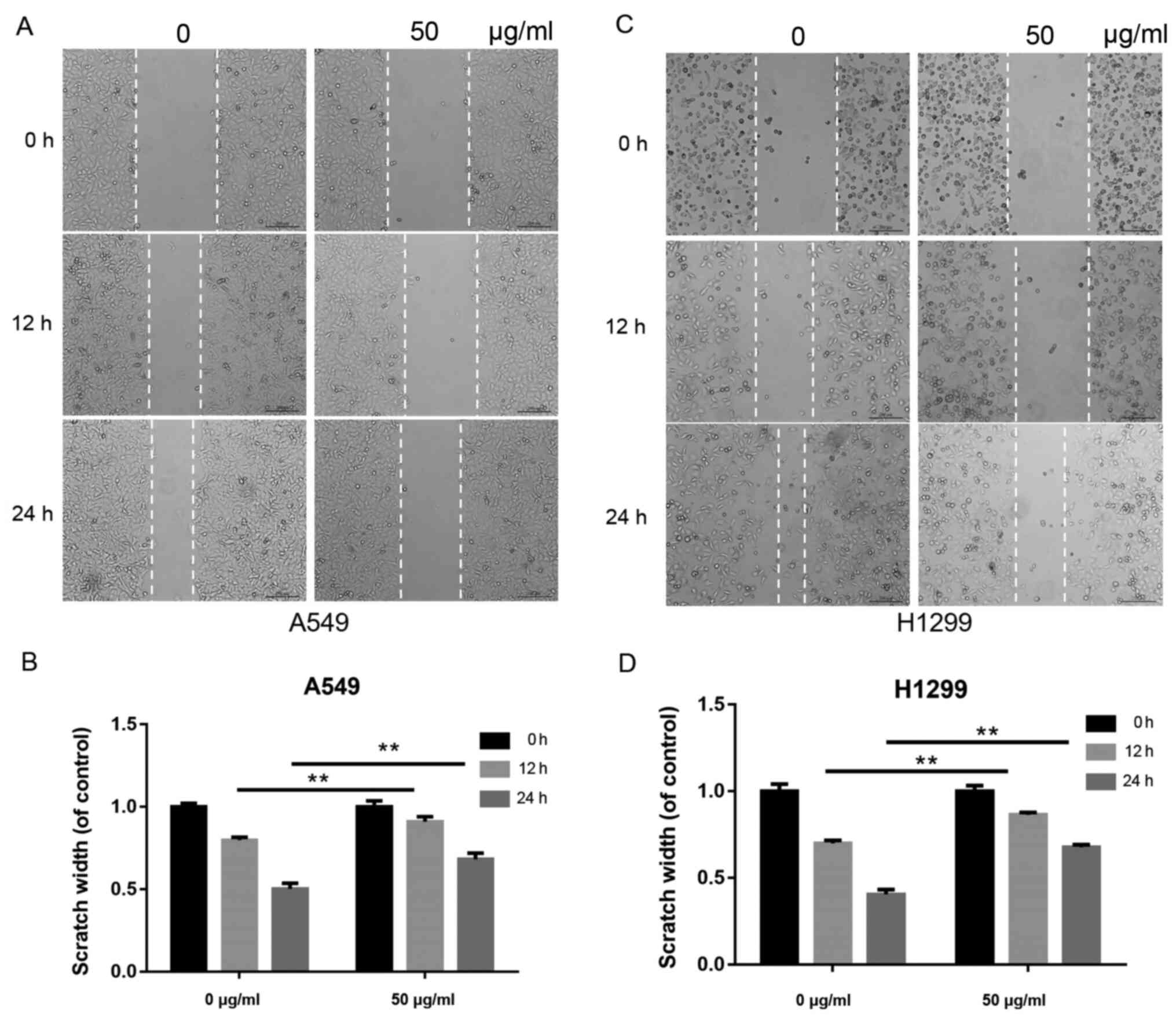
β-elemene suppresses the migration of
A549 and H1299 cells
Tumor cell migration is a critical step in
metastasis. To examine the effects of β-elemene on the migration of
A549 and H1299 cells, a scratch wound-healing assay was performed.
Cells were inoculated with 0 and 50 µg/ml of β-elemene for 0, 12
and 24 h. As shown in Fig. 2A and
B, the scratch width was longer in
the β-elemene-treated A549 cells compared with the control group.
Similarly, a longer scratch width was observed in the H1299 cells
treated with β-elemene (Fig. 2C and
D).
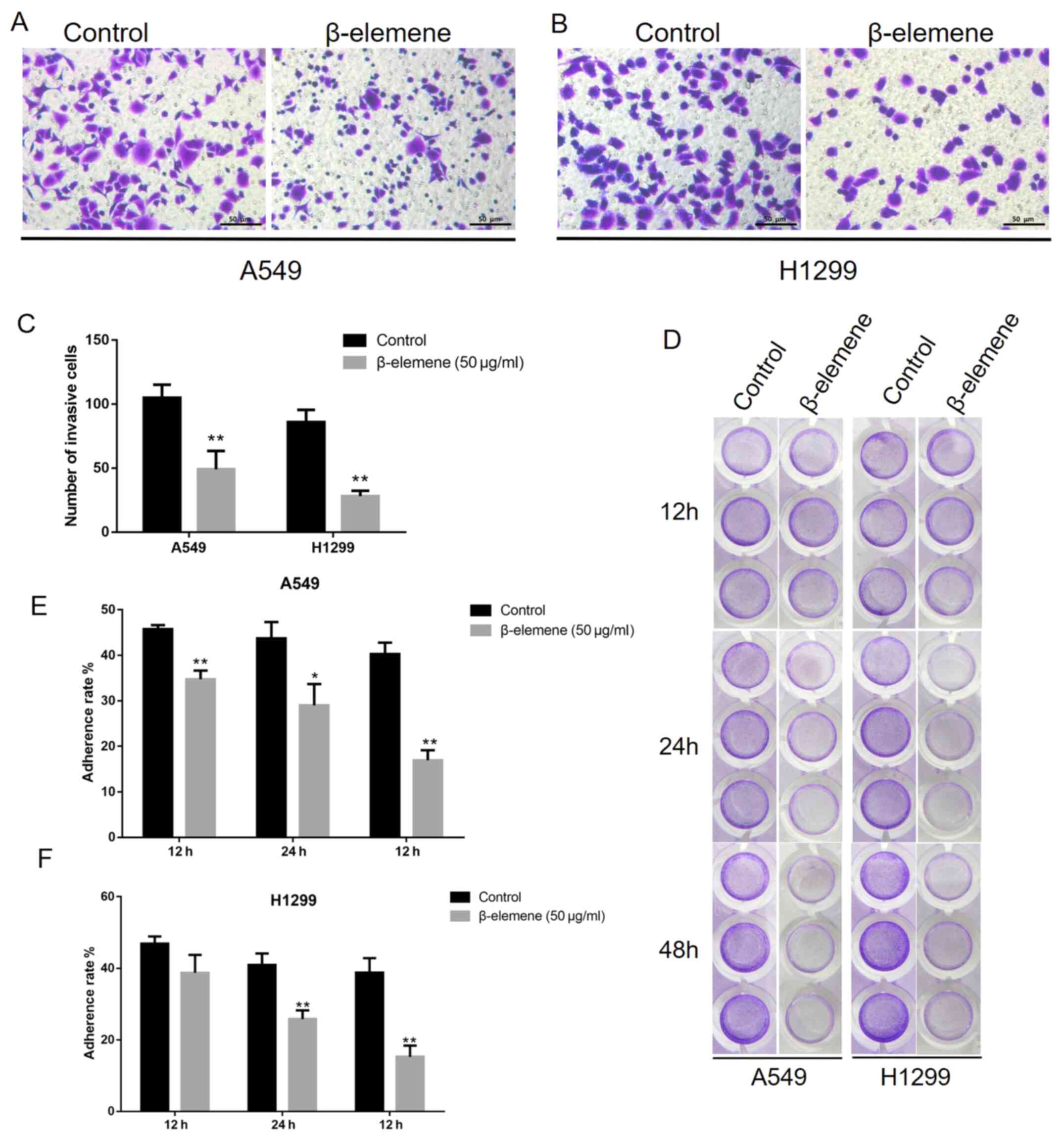
β-elemene inhibits the invasion and
adhesion of A5469 and H1299 cells
To investigate the effect of β-elemene on NSCLC cell
invasion, a Matrigel-coated Transwell assay was performed. A549 and
H1299 cells were inoculated with 50 µg/ml of β-elemene for 24 h. As
shown in Fig. 3A-C, inoculation
with β-elemene significantly decreased invasive cells, represented
by crystal violet staining. Next, the anti-adhesion effects of
β-elemene on A549 and H1299 cells were assessed using an adhesion
assay. Cells were treated with 50 µg/ml of β-elemene for 12, 24 and
48 h. Cells that adhered to the Matrigel were stained with crystal
violet. Compared with untreated cells (0 µg/ml), β-elemene exposure
resulted in a decrease in the quantity of adherent cells in a
time-dependent manner (Fig. 3D-F).
Overall, these results indicate that β-elemene inhibits invasion
and adhesion in A549 and H1299 cells.
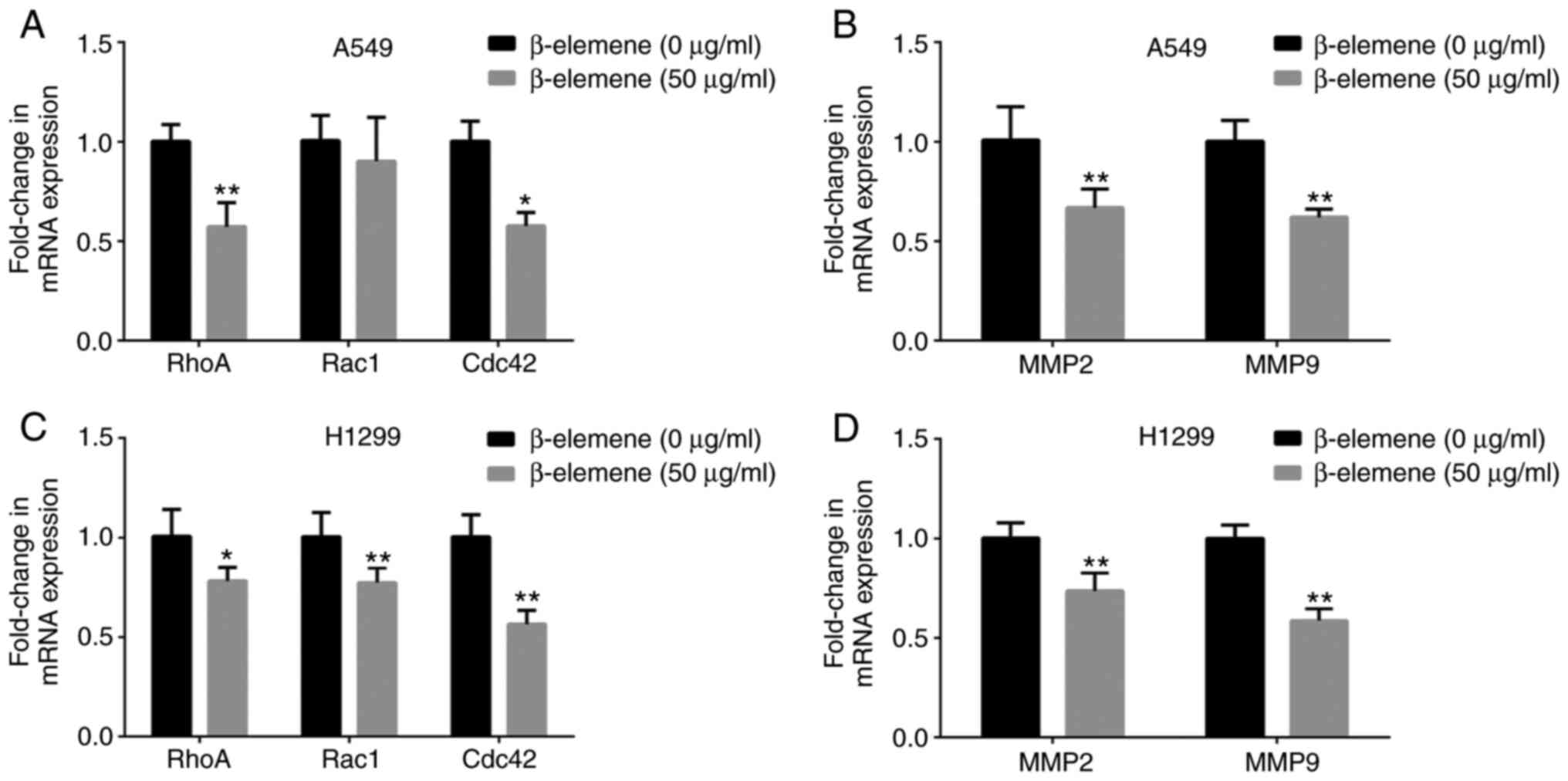
β-elemene decreases the mRNA
expression of motility-associated genes in A5469 and H1299
cells
RhoA, Rac1 and Cdc42, which are small GTP-binding
proteins in the Rho family, have been reported to regulate the
cellular cytoskeleton and cell migration (20). To determine whether β-elemene
regulates the expression of genes associated with migration and
invasion, the mRNA expression of RhoA, Rac1 and
Cac42 was compared in A549 and H1299 cells by RT-qPCR,
following inoculation with 0 and 50 µg/ml of β-elemene for 24 h.
β-elemene treatment remarkably decreased the mRNA expression levels
of RhoA, Rac1 and Cac42 (Fig. 4A and C). MMP2 and MMP9 are key members of the
MMP family, which may also facilitate cell migration and invasion
(21,22). As shown in Fig. 4B and D, the mRNA levels of MMP2 and
MMP9 were decreased after incubation with β-elemene. The
data suggest that β-elemene inhibits the expression of genes
associated with cell motility.
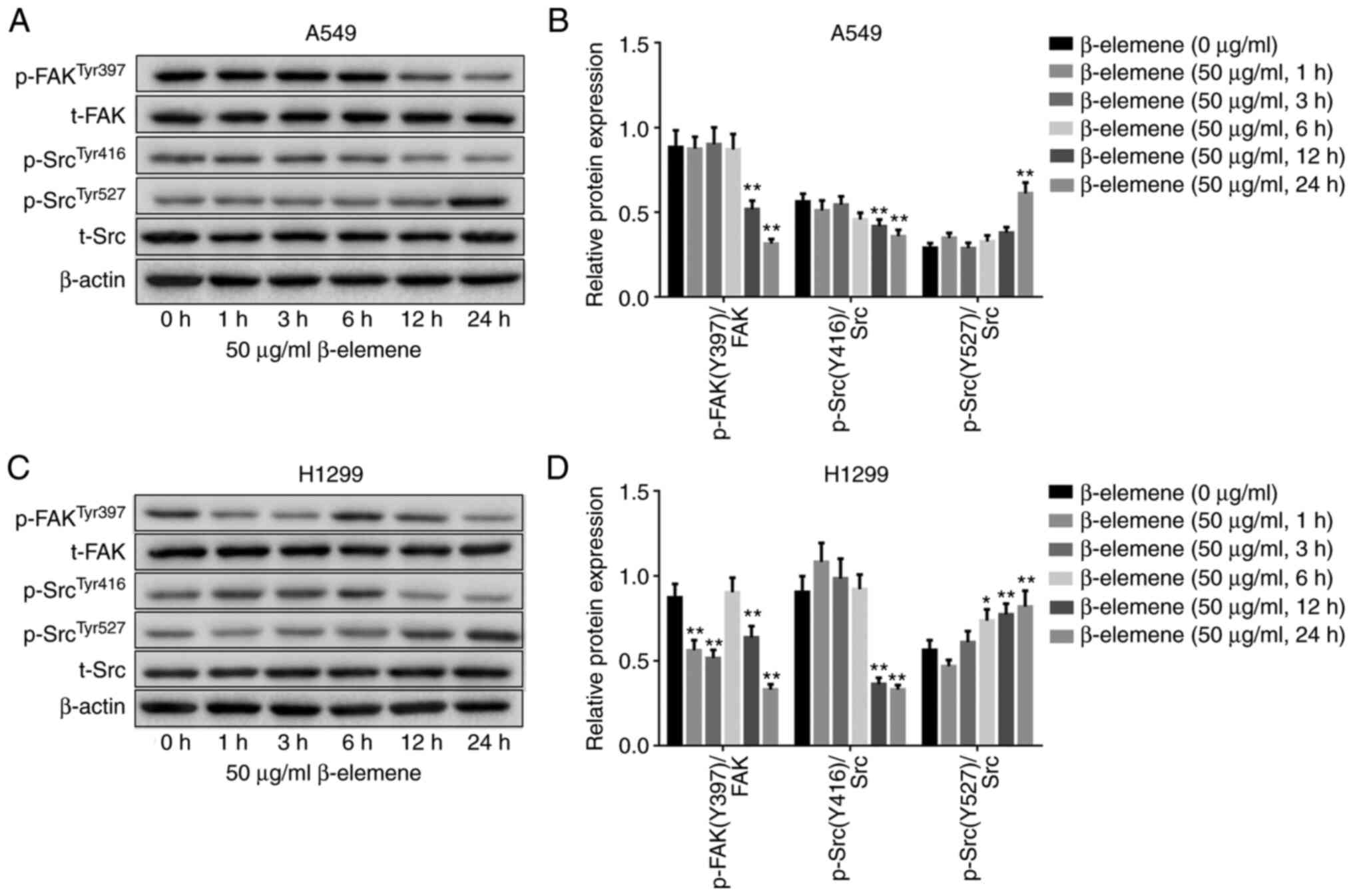
β-elemene inhibits FAK-Src activation
in A5469 and H1299 cells
To elucidate whether FAK-Src signaling was involved
in the anti-migration effect of β-elemene on NSCLC cells, the
activity of FAK and Src was evaluated by western blotting. As shown
in Fig. 5A-D, treatment with 50
µg/ml β-elemene decreased the phosphorylation of FAK (p-FAK) on
Y397 and the phosphorylation of Src (p-Src) on Y416 in both A549
and H1299 cells. However, p-Src on Y527 was increased after
β-elemene inoculation. Overall, β-elemene may inhibit the activity
of the FAK-Src pathway in lung cancer cells.
Discussion
Metastasis is a multi-step process that includes the
following: i) Tumor cells detach from the primary tumor by altering
cell-cell adhesion; ii) tumor cells infiltrate adjacent tissues;
iii) tumor cells migrate into the vasculature; iv) tumor cells
survive in the circulation; and v) tumor cells extravasate and
proliferate in a new tissue (23).
Hence, migration capacity is critical for successful metastasis. In
the present study, the role of β-elemene in the motility of NSCLC
cells was investigated. The data indicate that β-elemene treatment
inhibited the viability, migration, invasion and adhesion of A549
and H1299 cells. Moreover, β-elemene decreased the mRNA expression
levels of motility-associated genes, including RhoA,
Rac1, Cdc42, MMP2 and MMP9. Finally, it
was demonstrated that the anti-migratory and anti-invasive effects
of β-elemene might be regulated by the FAK-Src signaling
pathway.
β-elemene, an organic compound extracted from
Curcuma Rhizoma, has attracted scientific interest due to its good
performance in anti-cancer treatment. β-elemene has been reported
to inhibit cell growth, induce apoptosis, and block the EMT in
different types of cancer cells (12,14,24).
Using the MTT assay, it was also demonstrated that β-elemene
treatment inhibited the cell viability of NSCLC cells. In A549 and
H460 cells, β-elemene induced cell death through G2-M
regulation and apoptosis-modulating proteins, including Cdc2, Bcl-2
and cleaved caspase-9(25). In
H1299 cells, β-elemene inhibited cell growth through the AMPKα- and
ERK1/2-regulated inhibition of Sp1(26). β-elemene also inhibited the
migration and the invasive ability of tumor cells in gastric cancer
(27). In the present study, it was
also found that migration was inhibited in A549 and H460 cells
following β-elemene treatment.
FAK and Src are key regulators of integrin-mediated
cell adhesion and migration. FAK upregulation and
hyperphosphorylation have been demonstrated to increase invasive
capability in a variety of human cancer types including gastric
cancer and breast cancer (28). The
phosphorylation of FAK on Try397, which is the major site of
phosphorylation, led to the progression of tumor cells by promoting
migration and invasion (29). FAK
phosphorylation on Try397 creates a high-affinity binding site for
the recognition of the SH2 domain in Src family kinases. Moreover,
phosphorylation of FAK on Tyr397 is important for the activation
and recruitment of Src via the formation of the FAK-Src complex
(30). Numerous studies have
demonstrated that FAK-Src signaling is important in the regulation
of cell migration (4). Here, we
found that β-elemene treatment decreased the phosphorylation of FAK
on Tyr397 and of Src on Tyr416 in both A549 and H460 cells, while
the phosphorylation of Src on Tyr527 was elevated. The
phosphorylation of Src on Tyr527 decreases its recruitment to FAK,
while the phosphorylation of Src on Tyr416 activates its
recruitment (31). In a previous
study, breast cancer cells with FAK-Src inactivation showed
decreased metastatic potential (10). The present results suggest that the
suppression of FAK-Src signaling by β-elemene may decrease NSCLC
metastasis by decreasing cell migration.
The formation and remodeling of FAK-Src-Paxillin
contributes to cell migration in a dynamic process, under the
regulation of GTPases in the Rho family. Cell movement is dependent
on the dynamic organization of the protrusion of filopodia and
lamellipodia, whose formation is regulated by Cdc42 and
Rac1(32). RhoA also acts to
promote tension in the organization of actin. In addition, the
regulation of Rho, Rac, and Cdc42 is coordinated within cells. RhoA
is downstream of Rac1, which in turn is downstream of Cdc42. In the
present study, treatment with β-elemene led to a remarkable
decrease in the mRNA expression of RhoA, Rac1 and
Cdc42, suggesting that filopodia formation might be
disrupted by β-elemene. MMPs are required for degradation of the
ECM, which is required for the dissemination of tumor cells. Among
the MMPs, MMP2 and MMP9 are highly associated with cell migration.
It has been shown that the inhibition of MMP2 and
MMP9 decreased angiogenesis and the migration of
retinoblastoma cells (33). The
present study shows that β-elemene repressed the mRNA expression of
MMP2 and MMP9 in A549 and H1299 cells. These results suggest that
β-elemene inhibited the expression of cell motility-associated
genes.
Although the current study was the first, to the
best of our knowledge, to demonstrate that β-elemene inhibited
non-small cell lung cancer cell migration and invasion by
inactivating the FAK-Src pathway, the exact mechanism for these
inhibitory effects is yet to be fully elucidated, such as the
molecular target of β-elemene in the FAK-Src signaling pathway.
Future studies should therefore elucidate the exact molecular
target of β-elemene in non-small cell lung cancer cells and
evaluate the therapeutic effects of β-elemene in vivo.
Overall, β-elemene exposure inhibits NSCLC cell
migration and invasion by suppressing the activity of FAK-Src
signaling. Moreover, β-elemene leads to dysregulated expression of
motility-associated Rho GTPases and MMPs. The present results
suggest that β-elemene holds promise as an anti-metastatic therapy
to prevent tumor cell migration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed in this study are
available from the corresponding authors on reasonable request.
Authors' contributions
The study was conceived and designed by LQ and HZ.
HZ and SL conducted most of the experiments with assistance from
JB, NG and FH. The manuscript was written by HZ and SL, with
contributions from LQ. All authors read and approved the final
manuscript. HZ and LQ confirm the authenticity of all raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Paul CD, Mistriotis P and Konstantopoulos
K: Cancer cell motility: Lessons from migration in confined spaces.
Nat Rev Cancer. 17:131–140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Graham ZA, Gallagher PM and Cardozo CP:
Focal adhesion kinase and its role in skeletal muscle. J Muscle Res
Cell Motil. 36:305–315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Parsons JT, Martin KH, Slack JK, Taylor JM
and Weed SA: Focal adhesion kinase: A regulator of focal adhesion
dynamics and cell movement. Oncogene. 19:5606–5613. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dy GK, Ylagan L, Pokharel S, Miller A,
Brese E, Bshara W, Morrison C, Cance WG and Golubovskaya VM: The
prognostic significance of focal adhesion kinase expression in
stage I non-small-cell lung cancer. J Thorac Oncol. 9:1278–1284.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu C, Li Y, Xing Y, Cao B, Yang F, Yang
T, Ai Z, Wei Y and Jiang J: The interaction between cancer stem
cell marker CD133 and Src protein promotes focal adhesion kinase
(FAK) phosphorylation and cell migration. J Biol Chem.
291:15540–15550. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baquero P, Jiménez-Mora E, Santos A, Lasa
M and Chiloeches A: TGFβ induces epithelial-mesenchymal transition
of thyroid cancer cells by both the BRAF/MEK/ERK and Src/FAK
pathways. Mol Carcinog. 55:1639–1654. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Lee JJ, van de Ven RAH, Zaganjor E, Ng MR,
Barakat A, Demmers JJ, Finley LWS, Gonzalez Herrera KN, Hung YP,
Harris IS, et al: Inhibition of epithelial cell migration and
Src/FAK signaling by SIRT3. Proc Natl Acad Sci USA. 115:7057–7062.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ward KK, Tancioni I, Lawson C, Miller NL,
Jean C, Chen XL, Uryu S, Kim J, Tarin D, Stupack DG, et al:
Inhibition of focal adhesion kinase (FAK) activity prevents
anchorage-independent ovarian carcinoma cell growth and tumor
progression. Clin Exp Metastasis. 30:579–594. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chikara S, Lindsey K, Borowicz P,
Christofidou-Solomidou M and Reindl KM: Enterolactone alters
FAK-Src signaling and suppresses migration and invasion of lung
cancer cell lines. BMC Complement Altern Med. 17(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Logue JS, Cartagena-Rivera AX and Chadwick
RS: c-Src activity is differentially required by cancer cell
motility modes. Oncogene. 37:2104–2121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li P, Zhou X, Sun W, Sheng W, Tu Y, Yu Y,
Dong J, Ye B, Zheng Z and Lu M: Elemene induces apoptosis of human
gastric cancer cell line BGC-823 via extracellular signal-regulated
kinase (ERK)1/2 signaling pathway. Med Sci Monit. 23:809–817.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pan Y, Wang W, Huang S, Ni W, Wei Z, Cao
Y, Yu S, Jia Q, Wu Y, Chai C, et al: Beta-elemene inhibits breast
cancer metastasis through blocking pyruvate kinase M2 dimerization
and nuclear translocation. J Cell Mol Med. 23:6846–6858.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang Z, Gao M, Zhang W, Song L, Jia Y and
Qin Y: Beta-elemene treatment is associated with improved outcomes
of patients with esophageal squamous cell carcinoma. Surg Oncol.
26:333–337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zou S, Wang C, Cui Z, Guo P, Meng Q, Shi
X, Gao Y, Yang G and Han Z: β-Elemene induces apoptosis of human
rheumatoid arthritis fibroblast-like synoviocytes via reactive
oxygen species-dependent activation of p38 mitogen-activated
protein kinase. Pharmacol Rep. 68:7–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo Z, Liu Z, Yue H and Wang J:
Beta-elemene increases chemosensitivity to 5-fluorouracil through
down-regulating microRNA-191 expression in colorectal carcinoma
cells. J Cell Biochem. 119:7032–7039. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiang Z, Jacob JA, Loganathachetti DS,
Nainangu P and Chen B: β-Elemene: Mechanistic studies on cancer
cell interaction and its chemosensitization effect. Front
Pharmacol. 8(105)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu X, Xu M, Li N, Li Z, Li H, Shao S, Zou
K and Zou L: β-elemene inhibits tumor-promoting effect of M2
macrophages in lung cancer. Biochem Biophys Res Commun.
490:514–520. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Machacek M, Hodgson L, Welch C, Elliott H,
Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM and Danuser G:
Coordination of Rho GTPase activities during cell protrusion.
Nature. 461:99–103. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu L, Zhao KQ, Wang W, Cui LN, Hu LL,
Jiang XX, Shuai J and Sun YP: Nuclear receptor coactivator 6
promotes HTR-8/SVneo cell invasion and migration by activating
NF-κB-mediated MMP9 transcription. Cell Prolif.
53(e12876)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu Q, Huang Y, Wu J, Guan Y, Du M, Wang F,
Liu Z, Zhu Y, Gong G, Hou H, et al: T-cadherin inhibits invasion
and migration of endometrial stromal cells in endometriosis. Hum
Reprod. 35:145–156. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang X, Li Y, Zhang Y, Song J, Wang Q,
Zheng L and Liu D: Beta-elemene blocks epithelial-mesenchymal
transition in human breast cancer cell line MCF-7 through
Smad3-mediated down-regulation of nuclear transcription factors.
PLoS One. 8(e58719)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao SY, Wu J, Zheng F, Tang Q, Yang LJ,
Li L, Wu WY and Hann SS: β-elemene inhibited expression of DNA
methyltransferase 1 through activation of ERK1/2 and AMPKα
signalling pathways in human lung cancer cells: the role of Sp1. J
Cell Mol Med. 19:630–641. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deng M, Zhang Y, Liu B, Chen Y, Song H, Yu
R, Che X, Qu X, Liu Y, Hu X, et al: β-Elemene inhibits peritoneal
metastasis of gastric cancer cells by modulating FAK/Claudin-1
signaling. Phytother Res. 33:2448–2456. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ,
Lee JC and Shen TL: Phosphorylation of focal adhesion kinase at
Tyr397 in gastric carcinomas and its clinical significance. Am J
Pathol. 177:1629–1637. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tungsukruthai S, Sritularak B and
Chanvorachote P: Cycloartobiloxanthone inhibits migration and
invasion of lung cancer cells. Anticancer Res. 37:6311–6319.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dwyer SF and Gelman IH:
Cross-phosphorylation and interaction between Src/FAK and
MAPKAP5/PRAK in early focal adhesions controls cell motility. J
Cancer Biol Res. 2(2)2014.PubMed/NCBI
|
|
32
|
Aspenström P: The intrinsic GDP/GTP
exchange activities of Cdc42 and Rac1 are critical determinants for
their specific effects on mobilization of the actin filament
system. Cells. 8(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Webb AH, Gao BT, Goldsmith ZK, Irvine AS,
Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC,
et al: Inhibition of MMP-2 and MMP-9 decreases cellular migration,
and angiogenesis in in vitro models of retinoblastoma. BMC Cancer.
17(434)2017.PubMed/NCBI View Article : Google Scholar
|