Introduction
Cardiovascular diseases (CVDs) are a group of heart
and blood vessel disorders that cause ~17.5 million deaths each
year and have become the leading cause of death worldwide (1). Cardiac hypertrophy (CH) is an
adaptive reaction of cardiomyocytes to pressure overload and
neurohumoral and cytokine abnormalities (2), which are manifested by increased size
of cardiomyocytes and contractile protein synthesis (3,4).
However, continuous pathological stress overload results in
irreversible CH and induces cardiac maladaptation and cardiac
remodeling (5), thereby increasing
the risk of developing various CVDs such as arrhythmia, myocardial
ischemia, heart failure and sudden death.
Accumulating studies have demonstrated that, in
myocardial remodeling, the content of angiotensin II (Ang II) is
directly proportional to the degree of myocardial hypertrophy
(6-8).
Ang II directly participates in the development of myocardial
hypertrophy by generating reactive oxygen species (ROS), reducing
membrane potential and inducing mitochondrial dysfunction (6-8).
Mitochondria are critical in energy metabolism, signal transduction
and cell proliferation of cardiomyocytes (9). Mitochondrial dysfunctions are the
major cause of CH, which include mitochondrial ROS increase,
electron transport chain protein damage, mitochondrial DNA (mtDNA)
damage and a decrease in metabolic capacity (10-12).
Therefore, reducing the content of Ang II and maintaining
mitochondrial function in cardiomyocytes are potential strategies
for the treatment of pathological CH.
Traditional Chinese medicine (TCM) has a history of
>2,000 years. It is widely used in Eastern Asia to prevent or
treat a multitude of diseases, including CVDs (13). Tanshinones, berberine,
matrine/oxymatrine, qiliqiangxin and Radix puerariae are
representative compounds that can prevent or alleviate pathological
CH and cardiac remodeling (14-17).
Huoxue Qianyang Qutan recipe (HQQR) is a TCM compound consisting of
Salviae miltiorrhizae, Stone Cassia, Ligusticum
chuanxiong, Uncaria angustifolia, mulberry parasite,
hawthorn and corn whisker. A previous study demonstrated that, in
rats with obesity and hypertension (OBH), HQQR could alleviate
mitochondrial dysfunction and left ventricular hypertrophy by
modulating the sirtuin 1 (SIRT1)/peroxisome proliferator-activated
receptor-gamma coactivator-1α (PGC-1α) deacetylation pathway in
vivo (18). Furthermore, one
of our previous studies revealed that the TCM Huoxue Qianyang
decoction synergized with the activating transcription factor
6/CHOP endoplasmic reticulum stress signaling pathway and improved
heart remodeling in rats with OBH (19). However, the therapeutic effect and
the specific molecular mechanisms underlying the action of HQQR in
Ang II-induced cardiomyocyte hypertrophy remain unclear.
Therefore, the present study was conducted to
investigate the effect of HQQR on Ang II-induced cardiomyocyte
hypertrophy in isolated primary cardiomyocytes, and to evaluate the
role of HQQR in mitochondrial function to explore its underlying
mechanism.
Materials and methods
Animals and drugs
All experimental procedures involving animals were
approved by the Institutional Animal Care and Use Committee at
Yueyang Hospital of Integrated Traditional Chinese and Western
Medicine Affiliated to Shanghai University of Traditional Chinese
Medicine (approval no. 18922), according to the principles outlined
in the National Institutes of Health Guidelines for Care and Use of
Laboratory Animals.
Pregnant Sprague Dawley rats (n=2; female; 10 weeks
old; 260-270 g) purchased from the Vital River Laboratory Animal
Technology Co., Ltd. [animal license number, SCXK (Beijing)] were
fed ad libitum in an animal facility room with a 12-h
light/dark cycle at a temperature of 20-22˚C, with ~50% humidity.
After pregnant rats gave birth, neonatal rats (within 24 h of
birth; 5-6 g) were euthanized using CO2 inhalation at a
flow rate of 1.2 l/min, which displaces 30% of the cage volume per
min, followed with further cervical dislocation.
Valsartan capsules (Novartis International AG) were
dissolved in 100% DMSO as 10 mmol/l stock solutions and stored at
-80˚C before use. The compound stock solution was diluted in cell
culture medium (DMEM medium with 10% FBS and 1% double-antibiotic)
to a final concentration of 10 µmol/l (20). HQQR, consisting of Salvia
miltiorrhiza, Stone Cassia, Ligusticum chuanxiong,
Uncaria angustifolia, corn whisker, mulberry parasite and
hawthorn (5:10:3:5:10:5:5), was decocted and dried according to a
common protocol (18).
Isolation and culture of rat primary
cardiomyocytes
Primary cardiomyocytes were isolated from the
cardiac tissue of neonatal rats (within 24 h of birth) as
previously described (21).
Briefly, the hearts of the neonatal rats were collected, and the
atrium of each heart was put into 5-ml sterile centrifuge tubes to
be fully cut into small pieces. After full digestion with 0.4%
Collagenase IV (cat. no. C4-BIOC; Sigma-Aldrich; Merck KGaA) and
0.05% trypsin-EDTA (cat. no. T1300; Beijing Solarbio Science &
Technology Co., Ltd.) in 37˚C constant temperature water bath for
20 min, the cell suspension was centrifuged at room temperature for
10 min at 1,000 x g. After multiple digestion, the cell suspensions
were filtered through a 100-mesh filter and mixed, and the cells
were cultured at 37˚C in DMEM medium (cat. no. SH30243.01; Hyclone;
Cytiva) with 10% FBS (cat. no. 16000-044, Gibco; Thermo Fisher
Scientific, Inc.) and 1% double-antibiotic
(penicillin-streptomycin; cat. no. P1400; Beijing Solarbio Science
& Technology Co., Ltd.) in a 37˚C, 5% CO2 incubator.
Next, the isolated cells were subjected to flow cytometry detection
of troponin I (troponin I, TnI; 1:50; cat. no. ab196384; Abcam) to
identify the purity of primary cardiomyocytes. The morphology of
cardiomyocytes was observed, and pictures were captured with a
light microscope (magnification, x200).
Experimental procedure
Cardiomyocytes were divided into the following five
groups (5x105 cells; 6-well plate): i) Vehicle (solvent
group, DMSO); ii) Ang II + vehicle; iii) Ang II + 0.2 mg/ml HQQR;
iv) Ang II + 0.5 mg/ml HQQR; and v) Ang II + valsartan. Ang II
(cat. no. 4474-91-3) was purchased from Beijing Solarbio Science
& Technology Co., Ltd., and the corresponding concentration (1
µmol/l) was prepared according to the manufacturer's instructions.
Ang II and HQQR were added at the same time.
Fluorescent immunostaining
Cell suspensions containing 1x105 primary
rat cardiomyocytes were added to a 24-well plate and treated with
Ang II, Ang II + 0.2 mg/ml HQQR, Ang II + 0.5 mg/ml HQQR, Ang II +
10 µmol/l valsartan, or Ang II + ROS scavenger (1 mM; cat. no.
S6205; Selleck Chemicals) for 24 h. Then, slides containing
experimental primary cardiomyocytes were harvested, fixed with 4%
formaldehyde for 30 min at room temperature, and permeabilized with
0.5% Triton X-100 in PBS for 10 min at room temperature. After
blocking with 1% bovine serum albumin (BSA; cat. no. A8010; Beijing
Solarbio Science & Technology Co., Ltd.) in PBS for 30 min at
room temperature, the slides were incubated with anti-α-actinin
antibody (1:200; cat. no. ab68194; Abcam) and anti-α-smooth muscle
actin (α-SMA; 1:500; ab32575; Abcam) overnight at 4˚C in a wet-box.
Subsequently, the slides were incubated with Alexa Fluor
555-labeled donkey anti-rabbit IgG (H + L) antibody (1:500; cat.
no. A0453; Beyotime Institute of Biotechnology) for 1 h at room
temperature in a wet-box before staining the nuclei with
DAPI-containing media for 15 min at room temperature (1:500; cat.
no. C1002; Beyotime Institute of Biotechnology). Pictures were
taken using a fluorescence microscope (Nikon Corporation).
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was performed in accordance with the
manufacturer's kit protocol (Beyotime Institute of Biotechnology).
Cell suspensions containing 3x103 primary rat
cardiomyocytes were added to a 96-well plate. The cells were
treated with 1 µmol/l Ang II (22)
and gradient concentrations of HQQR (0, 0.05, 0.1, 0.2, 0.5 and 1
mg/ml) or valsartan (10 µmol/l) after 24 h of incubation. Finally,
10 µl CCK-8 solution were added into each well, and the cells were
incubated in an incubator at 37˚C with 5% CO2 for 1 h.
Cell proliferation at 0, 12, 24 and 48 h was detected by recording
the optical density at 450 nm (OD450).
Reverse transcription-quantitative
PCR
Total RNA was extracted from primary rat
cardiomyocytes using TRIzol® reagent (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Complementary DNA was synthesized
using the RevertAid First Strand cDNA Synthesis kit (Fermentas;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions and under the following conditions: 37˚C for 60 min;
85˚C for 5 min; 4˚C for 5 min. mRNA levels were quantified using
SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions and under the
following thermocycling conditions: 95˚C for 10 min; 40 cycles of
95˚C for 15 sec and 60˚C for 45 sec. Relative mRNA levels were
obtained using the 2-ΔΔCq method (23). GAPDH was used as an internal
control for normalization. The primer sequences are listed in
Table I.
 | Table IPrimers for reverse
transcription-quantitative PCR analysis. |
Table I
Primers for reverse
transcription-quantitative PCR analysis.
| Gene | Forward primer (5'
to 3') | Reverse Primer (5'
to 3') |
|---|
| ANP |
GGGCTTCTTCCTCTTCCTG |
TCTGAGACGGGTTGACTTCC |
| BNP |
TAGCCAGTCTCCAGAACAATCC |
ACCTCAGCCCGTCACAGC |
| β-MHC |
TGACAACGCCTATCAGTACATG |
CCTGGGGTCTGGTCCTTC |
| Sirt1 |
GGTTAGGTGGCGAGTATGC |
TATGAAGAGGTGTTGGTGGC |
| Nrf1 |
ACCCAAGCATTACGGACC |
CAGTACCAACCTGGATGAGC |
| Tfam |
CGCATACCCTCGCCTGTC |
GTTCTGAAACTTTTGCATCTGG |
| Ndufa13 |
CGGGGACTGTCGGGATAC |
GGAGGAGTGGCATGAGGG |
| SDHB |
TACAAATCCATTGAGCCCTATC |
GCACTCATACAATCCGTCCAG |
| COX IV |
GGCGTGACTACCCCTTGC |
CTCATTGGTGCCCTTGTTC |
| COX1 |
AACTAGGACAACCAGGAGCAC |
AATCATTAGCGGCACAAGC |
| ATPase 6 |
GAGCCGTAATTCTAGGTTTCC |
ATTGTAGCGGTTGGTGGG |
| Ppargc1a |
AACCGCAGTCGCAACATG |
GGAGGAGTCGTGGGAGGAG |
| mtDNA |
AGCTCCAGCTTTTGTTCCC |
CTTCCGGTTCGTATGTTGTG |
| GAPDH |
GGAGTCTACTGGCGTCTTCAC |
ATGAGCCCTTCCACGATGC |
Western blot analysis
The total protein of primary rat cardiomyocytes was
extracted using RIPA buffer, which contained a protease and
phosphatase inhibitor (cat. no. R0010; Beijing Solarbio Science
& Technology Co., Ltd.). Protein was then quantified using a
BCA kit (cat. no. PICPI23223; Thermo Fisher Scientific, Inc.).
Subsequently, proteins (25 µg per lane) were separated using 10%
SDS-PAGE and transferred to PVDF membranes. After blocking in 5%
non-fat milk for 1 h at room temperature, the protein bands were
incubated with primary antibodies overnight at 4˚C, followed by
incubation with the corresponding secondary antibodies [anti-rabbit
(cat. no. A0208; 1:1,000; Beyotime Institute of Biotechnology) or
anti-mouse (cat. no. A0216; 1:1,000; Beyotime Institute of
Biotechnology) labeled with HRP] at 37°C for 1 h. After
a 5-min incubation with Immobilon Western chemiluminescent HRP
substrate (cat. no. WBKLS0100; MilliporeSigma) in the dark, the
protein bands were visually measured using a chemiluminescent
imaging system (Tanon-5200, Tanon Science and Technology Co.,
Ltd.), and then quantified using ImageJ software (v1.8.0.112;
National Institutes of Health). Anti-SIRT1 (cat. no. ab110304;
1:1,000), anti-PGC-1α (cat. no. ab54481; 1:2,000), anti-nuclear
respiratory factor 1 (NRF1; cat. no. ab175932; 1:5,000),
anti-mitochondrial transcription factor A (Tfam; cat. no. ab131607;
1:2,000), anti-NADH:ubiquinone oxidoreductase subunit A13 (NDUFA13;
cat. no. ab110240; 1:1,000), anti-succinate dehydrogenase complex
iron sulfur subunit B (SDHB; cat. no. ab178423; 1:5,000),
anti-cytochrome c oxidase subunit IV (COX IV; cat. no.
ab16056; 1:500), anti-cyclooxygenase 1 (COX1; cat. no. ab695;
1:1,000), anti-ATPase 6 (cat. no. ab192423; 1:500), anti-brain
natriuretic peptide (BNP; cat. no. ab239510; 1:2,000), anti-Bax
(cat. no. ab32503; 1:5,000), anti-Bcl-2 (cat. no. ab196495;
1:1,000) antibodies were obtained from Abcam, anti-cleaved caspase
3 (cat. no. 9661; 1:1,000), anti-caspase 3 (cat. no. 9662; 1:1,000)
were obtained from CST, anti-atrial natriuretic peptide (ANP; cat.
no. RQ4453; 0.5 µg/ml) antibody was obtained from NSJ Bioreagents,
anti-β-myosin heavy chain (β-MHC; cat. no. MA1-26180; 0.5 µg/ml)
antibody was obtained from Invitrogen; Thermo Fisher Scientific,
Inc., and anti-GAPDH as a loading control (cat. no. 60004-1-1G;
1:5,000) was obtained from ProteinTech Group, Inc. The antibodies
were diluted in PBST (containing 0.05% Tween-20).
ROS detection
ROS accumulation in cardiomyocytes was quantified
using the Active Oxygen Detection kit (cat. no. S0033; Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. After treatment with 1 µmol/l Ang II and HQQR (0.2
and 0.5 mg/ml) or valsartan (10 µmol/l), DCFH-DA probe solution was
prepared and used for incubation with the harvested cell samples at
37˚C for 30 min in the dark. The cells and solution were gently
inverted every 10 min. After washing three times with DMEM without
serum, the samples were examined via flow cytometry (Accuri C6; BD
Biosciences) using FlowJo software (v10.0.7r2; Tree Star,
Inc.).
Biochemical detection
Primary rat cardiomyocytes were treated with 1
µmol/l Ang II and HQQR (0.2 and 0.5 mg/ml) or valsartan (10 µmol/l)
for 24 h, and then, the cells were collected, and the supernatant
was kept. The levels of ATP and protein carbonyl, and the
activities of glutathione peroxidase (GSH-PX), catalase (CAT),
malondialdehyde (MDA) and superoxide dismutase (SOD) were
respectively detected using the BCA (cat. no. A045-3), ATP (cat.
no. A095), protein carbonyl (cat. no. A087), GSH-PX (cat. no.
A005), CAT (cat. no. A007-1), MDA (cat. no. A003-2) and SOD (cat.
no. A001-1) kits (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's instructions, and measured using a
visible spectrophotometer. The activities of the mitochondrial
complexes I-IV and V were respectively detected using the
mitochondrial complex I (cat. no. BC0515), II (cat. no. BC3235),
III (cat. no. BC3245), IV (cat. no. BC0945) and V (cat. no. BC1445)
Activity Test kits (Beijing Solarbio Science & Technology Co.,
Ltd.) and measured using a UV spectrophotometer.
Mitochondrial potential assay
Alterations in mitochondrial membrane potential were
evaluated using the Mitochondrial Membrane Potential Detection kit
with JC-1 (cat. no. C2006; Beyotime Institute of Biotechnology).
According to the manufacturer's instructions, the cell suspension
was prepared and detected using a flow cytometer (Accuri C6; BD
Biosciences) via the software of Flow Jo (v10.0.7r2 version). JC-1
is an ideal fluorescent probe widely used to detect mitochondrial
membrane potential. In high mitochondrial membrane potential, JC-1
aggregates in the matrix of mitochondria and forms polymer
(J-aggregates), which produces red fluorescence. When the
mitochondrial membrane potential is low, JC-1 cannot aggregate in
the matrix of mitochondria. At this time, JC-1 is a monomer and can
produce green fluorescence.
Cell apoptosis
Primary rat cardiomyocyte cells after 24 h treatment
with Ang II, Ang II + 0.2 mg/ml HQQR, Ang II + 0.5 mg/ml HQQR, Ang
II + 10 µmol/l valsartan were stained using Annexin V Apoptosis kit
(C1062; Beyotime Institute of Biotechnology) according to the
manufacturer's instructions and a Beckman CytoFLEX Flow Cytometer
(Beckman Coulter, Inc.) with FlowJo software (v10.0.7r2; Tree Star,
Inc.) was used to analyze apoptosis.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism 7.0 software (GraphPad Software, Inc.). Data are presented as
the mean ± SD. One-way ANOVA followed by Tukey's post hoc test was
applied for comparisons between groups. Results are representative
of at least three repeated experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
HQQR attenuates the inhibitory effect
of Ang II on rat cardiomyocyte proliferation
To investigate the effect of HQQR on Ang II-induced
cardiomyocyte hypertrophy, rat primary cardiomyocytes were
isolated. After detection using TnI, the results of flow cytometry
analysis confirmed that ~95% of the isolated cells were primary
cardiomyocytes (Fig. 1A).
Microscopic observations (light microscope) also indicated that the
isolated cell morphology was consistent with the morphological
characteristics of primary cardiomyocytes (Fig. 1B), including a short columnar form
with generally only one nucleus being mostly located in the middle
of the cell, while the cell shape is ellipse- or rectangle-like,
and its long axis is in the same direction as in myofibrils. The
cytotoxicity of HQQR was detected using a CCK-8 assay (Fig. S1), the results of which revealed
that 0.2 and 0.5 mg/ml HQQR had no cytotoxicity and the 24 h after
treatment had the best effect. Cell proliferation experiments
revealed that, compared with normally cultured (medium without
other treatment) primary cardiomyocytes, Ang II (1 µmol/l)
treatment significantly inhibited the proliferation of primary
cardiomyocytes, which was markedly abolished by incubation with
valsartan (10 µmol/l), the antagonist of angiotensin receptor used
as a positive control. The addition of HQQR solution ameliorated
the inhibitory effect of Ang II on primary cardiomyocyte
proliferation in a dose-dependent manner (Fig. 1C). On the basis of the present
results, Ang II treatment for 24 h followed by 0.2 and 0.5 mg/ml
HQQR concentrations were selected as treatment conditions for
subsequent experiments. Valsartan was used as a positive
control.
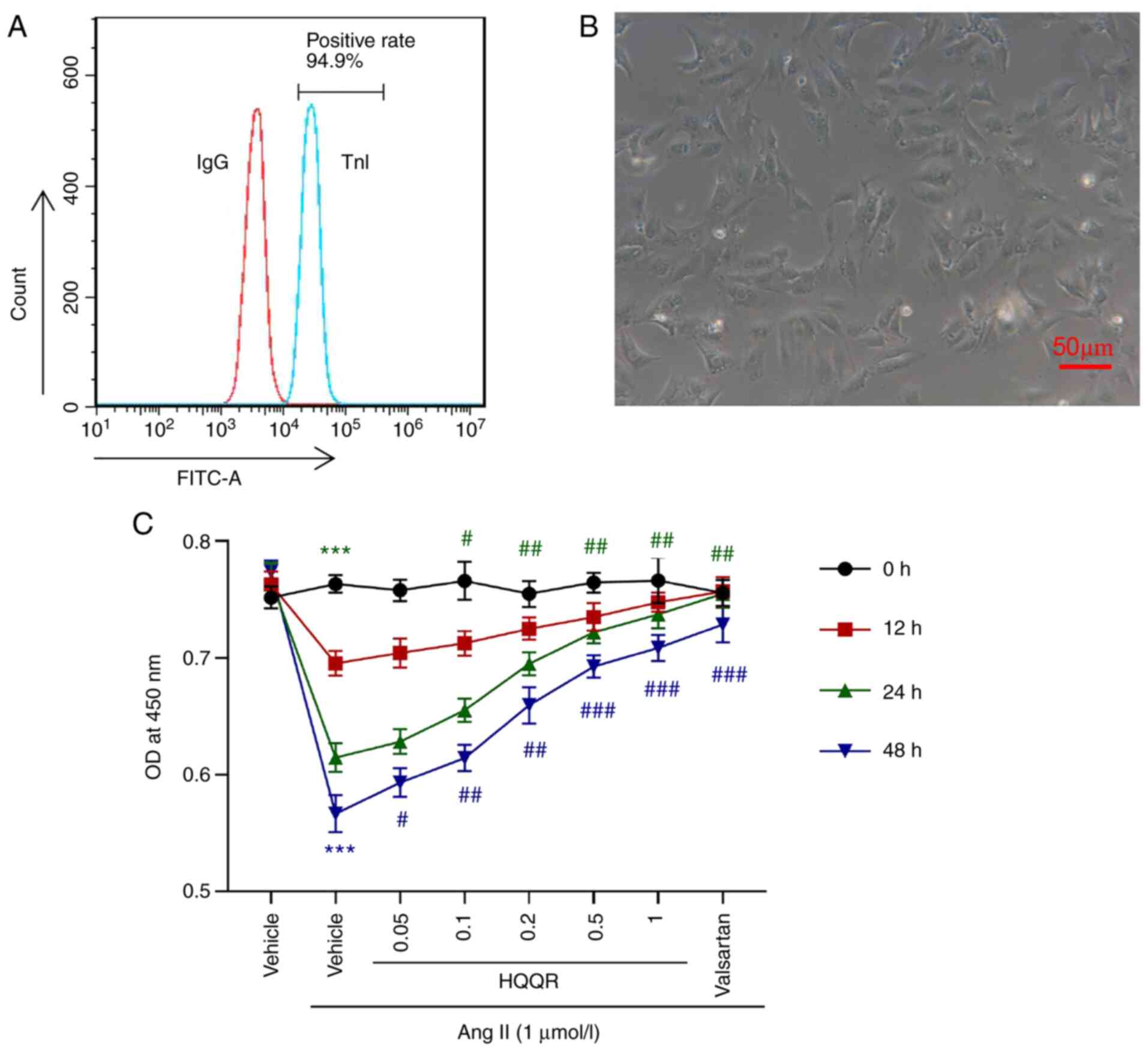 | Figure 1HQQR attenuates the inhibitory effect
of Ang II on rat cardiomyocyte proliferation. (A) TnI was detected
using flow cytometry analysis to identify the percentage of primary
cardiomyocytes. (B) Image of isolated primary rat cardiomyocytes
(magnification, x200; scale bar, 50 µm). (C) Cell Counting Kit-8
assays were used to examine the proliferation of primary
cardiomyocytes at 0, 12, 24 and 48 h after treatment with Ang II (1
µmol/l) and HQQR powder (0, 0.05, 0.1, 0.2, 0.5 and 1 mg/ml).
Vehicle and valsartan were used as negative and positive controls,
respectively. ***P<0.001 vs. the vehicle group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the Ang II + vehicle group. HQQR,
Huoxue Qianyang Qutan recipe; Ang II, angiotensin II; TnI, troponin
I; OD, optical density. |
HQQR improves AngII-induced
cardiomyocyte hypertrophy
To further examine the role of HQQR in Ang
II-induced cardiomyocyte hypertrophy, primary cardiomyocytes were
divided into the following five groups: i) Vehicle; ii) Ang II (1
µmol/l) + vehicle; iii) Ang II (1 µmol/l) + HQQR (0.2 mg/ml); iv)
Ang II (1 µmol/l) + HQQR (0.5 mg/ml); and v) valsartan + Ang II (1
µmol/l). The immunofluorescence of α-actinin revealed that,
compared with that in the vehicle group, cardiomyocyte hypertrophy
was observed in the Ang II-treated group, indicating that Ang II
induced cardiomyocyte hypertrophy. However, treatment with HQQR
could significantly inhibit Ang II-induced cardiomyocyte
hypertrophy (Fig. 2A) and reduce
the Ang II-induced expression of the myocardial hypertrophy markers
ANP, BNP and β-MHC (Fig. 2B and
C). Valsartan was used as a
positive control.
 | Figure 2HQQR improves Ang II-induced
cardiomyocyte hypertrophy. (A) Immunofluorescence experiments were
performed using anti-α-actinin antibodies to analyze cardiomyocyte
hypertrophy (magnification, x200; scale bar, 50 µm). (B) mRNA
expression levels of the myocardial hypertrophy markers ANP, BNP
and β-MHC were examined using reverse transcription-quantitative
PCR. (C) Protein expression levels of the myocardial hypertrophy
markers ANP, BNP and β-MHC were examined using western blotting.
**P<0.01 and ***P<0.001 vs. the vehicle
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the Ang II + vehicle group. HQQR,
Huoxue Qianyang Qutan recipe; Ang II, angiotensin II; ANP, atrial
natriuretic peptide; BNP, brain natriuretic peptide; β-MHC, β-major
histocompatibility complex. |
HQQR alleviates AngII-induced
cardiomyocyte hypertrophy by modulating ROS accumulation
Ang II treatment markedly enhanced ROS accumulation,
whereas treatment with HQQR significantly inhibited ROS
accumulation in Ang II-treated primary cardiomyocytes, in a
dose-dependent manner (Fig. 3A).
HQQR could also rescue the effect of Ang II treatment on the levels
of MDA, SOD, ATP, CAT, GSH-Px and protein carbonyl in primary
cardiomyocytes (Fig. 3B-G).
Consistent with these results, the immunofluorescence of α-SMA
revealed that ROS was directly involved in AngII-induced
cardiomyocyte hypertrophy, and that the ROS scavenger alleviated
AngII-induced cardiomyocyte hypertrophy (Fig. 3H). The present findings suggested
that HQQR treatment could alleviate Ang II-induced cardiomyocyte
hypertrophy, potentially by modulating ROS accumulation. Valsartan
was used as a positive control.
 | Figure 3HQQR alleviates AngII-induced
cardiomyocyte hypertrophy by modulating ROS accumulation. (A) Flow
cytometry analysis to detect the changes in ROS accumulation.
Levels of (B) ATP and (C) protein carbonyl content, and activities
of (D) MDA, (E) SOD, (F) CAT and (G) GSH-Px were quantified. (H)
Immunofluorescence experiments were performed using anti-α-SMA
antibodies to analyze cardiomyocyte hypertrophy (magnification,
x200; scale bar, 50 µm). **P<0.01 and
***P<0.001 vs. the vehicle group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the Ang II + vehicle group. HQQR,
Huoxue Qianyang Qutan recipe; Ang II, angiotensin II; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-PX,
glutathione peroxidase; α-SMA, α-smooth muscle actin. |
HQQR reverts cardiomyocyte
hypertrophy-induced membrane potential reduction and apoptosis
increase
Ang II treatment notably reduced mitochondrial
membrane potential (Fig. 4A) and
increased cell apoptosis (Fig.
4B). In Ang II-stimulated cardiomyocytes, treatment with HQQR
significantly reversed the hypertrophy-induced membrane potential
reduction and apoptosis in a dose-dependent manner. Moreover, Ang
II induced the expression of apoptosis-related proteins Bax and
cleaved caspase 3, which were significantly decreased upon HQQR
treatment, while anti-apoptotic Bcl-2 was significantly decreased
by Ang-II and increased by HQQR (Fig.
S2). Valsartan was used as a positive control.
HQQR increases the activity of
mitochondrial electron transport chain complexes in Ang II-treated
rat cardiomyocytes
To further explore the mechanism by which HQQR
affects Ang II-induced cardiomyocyte hypertrophy, the activities of
the mitochondrial complexes I-IV and V were studied 24 h after
treatment. As presented in Fig.
5A, Ang II markedly inhibited the activity of the mitochondrial
complexes I-IV and V, whereas the addition of HQQR or valsartan
partially rescued their activities. Furthermore, HQQR or valsartan
treatment markedly inhibited Ang II-induced mtDNA leakage (Fig. 5B). The mRNA and protein expressions
of genes encoding the mitochondrial complex and involved in
mitochondrial biogenesis were subsequently evaluated. As indicated
in Figs. 5C and S3, Ang II treatment reduced both mRNA
and protein levels of SIRT1, PGC-1α, NRF1, Tfam, NDUFA13, COX IV,
COX1 and ATPase 6, and elevated the expression of SDHB. These
effects were notably rescued by treatment with HQQR or valsartan,
which was used as a positive control.
 | Figure 5HQQR increases the activity of
mitochondrial electron transport chain complexes in Ang II-treated
rat cardiomyocytes. (A) The activities of the mitochondrial
complexes I-IV and V were determined. (B) The level of mtDNA was
quantified using RT-qPCR. (C) Protein levels of SIRT1, PGC-1α,
NRF1, Tfam, NDUFA13, SDHB, COX IV, COX1 and ATPase 6 in primary
cardiomyocytes were examined by western blotting.
**P<0.01 and ***P<0.001 vs. the vehicle
group; #P<0.05 and ##P<0.01 vs. the Ang
II + vehicle group. HQQR, Huoxue Qianyang Qutan recipe; Ang II,
angiotensin II; SIRT1, sirtuin 1; mtDNA, mitochondrial DNA;
RT-qPCR, reverse transcription-quantitative PCR; PGC-1α, peroxisome
proliferator-activated receptor-gamma coactivator-1α; NRF1, nuclear
respiratory factor 1; Tfam, mitochondrial transcription factor A;
NDUFA13, NADH:ubiquinone oxidoreductase subunit A13; SDHB,
succinate dehydrogenase complex iron sulfur subunit B; COX IV,
cytochrome c oxidase subunit IV; COX1, anti-cyclooxygenase
1; RT-qPCR, reverse transcription-quantitative PCR. |
Discussion
CH is generally considered to be a major inducer of
several types of CVDs (24). There
is a need to explore effective therapeutic targets with low
toxicity for the clinical treatment of CH and CVDs. Because of its
low toxicity, TCM has been used to treat chronic diseases in East
Asia throughout history. Several types of TCM have been
demonstrated to be effective for CH treatment by affecting multiple
signaling pathways or physiological activities. For instance,
Bu-Shen-Jiang-Ya decoction suppressed left ventricular hypertrophy
by regulating the extracellular signal-regulated kinase signaling
pathway in spontaneously hypertensive rats (25). Moreover, QiShenYiQi pills
ameliorated fatigue-induced CH through the regulation of energy
metabolism (26). Qiliqiangxin was
also found to attenuate phenylephrine-induced CH by upregulating
peroxisome proliferator-activated receptor γ and PGC-1α (27). Combined with the role of HQQR
(18,19) in hypertensive rats, these findings
provide evidence that TCM or a combination therapy of TCM and
western medicine has the potential to treat CH. It is necessary to
investigate TCM targeting CH treatment further, along with the
potential mechanisms to prevent drug resistance and improve
therapeutic effectiveness.
In the present study, the CCK-8 and
immunofluorescence experiments confirmed that Ang II could induce
CH (6). Functional analysis of
HQQR in the treatment of CH revealed that HQQR significantly
alleviated Ang II-induced CH in primary cardiomyocytes, thereby
suggesting the significance of HQQR in CH treatment.
The mechanism by which HQQR abolished Ang II-induced
CH was investigated. Mitochondria are double-membrane organelles
that support a variety of physiological activities, including
energy production, cell death and oxidative metabolism (9). Mitochondrial dysfunction has been
implicated in the occurrence and development of cardiomyocyte
hypertrophy (28). Mitochondrial
biogenesis is essential for sustaining mitochondrial function and
is related to ROS production (29). Although acute or mild oxidative
stress can trigger mitochondrial biogenesis due to the involvement
of mitochondrial quality control (30), the present study reported that Ang
II treatment promoted the accumulation of ROS in cardiomyocytes and
induced the disturbance of mitochondrial biogenesis, which may
further result in cardiomyocyte injury and hypertrophy. HQQR
treatment attenuated oxidative stress and restored the levels of
mitochondrial transcription factors in cardiomyocytes. The
inhibition of ROS accumulation and the normal activity of
mitochondrial electron transport chain are crucial for alleviating
mitochondrial-related diseases (10). After Ang II-induced ROS production,
ROS activate several critical signaling pathways related to cell
death and fibrosis, such as the mitogen-activated protein kinase
pathway, the extracellular regulated protein kinase signaling
pathway, protein kinase C signaling and NF-κB; these signaling
pathways participate in the process of CH (31-33).
Therefore, drugs that directly or indirectly target ROS production
might inhibit the occurrence or development of CH. As anticipated,
HQQR treatment significantly reduced the accumulation of ROS and
the indicators of oxidative stress, and elevated the levels of
scavenging system indicators, such as SOD, CAT and GSH-PX, thus
suggesting that HQQR partially prevented Ang II-induced CH by
reducing the accumulation of ROS and repressing oxidative stress in
myocardial cells. Ang II reduced the activity of the mitochondrial
electron transport chain and suppressed the expression of
mitochondrial complex subunits in cardiomyocytes; however, this
impact was significantly reverted by HQQR treatment, indicating
that HQQR could also exert its therapeutic effect by maintaining
normal electron transfer.
HQQR treatment increased the ATP content in
cardiomyocytes and elevated the levels of NRF1 and Tfam, which are
critical for mitochondrial biogenesis. SIRT1 is a deacetylase that
plays a key role in cell proliferation, differentiation,
senescence, apoptosis and metabolism (34). The SIRT1/PGC-1α pathway contributes
to mitochondrial dysfunction and mitochondrial biogenesis (35,36).
Inhibition of PGC-1α or PGC-1α deacetylation significantly
abolishes the process leading to CH (27). The present study indicated that
HQQR rescued Ang II-inhibited SIRT1 and PGC-1α expression, thus
implying that HQQR protected cardiomyocytes from the development of
CH by enhancing the SIRT1/PGC-1α regulatory pathway. It can be
speculated that HQQR improves mitochondrial biosynthesis, leading
to the reduction of oxidative stress.
Currently, there are relatively few studies on the
efficacy of HQQR in CVDs. The present study provided evidence for
the protective role of HQQR in CH, enriching the strategies for CH
treatment. Furthermore, our previous study (18) confirmed the effect of HQQR in
glucolipid metabolism and blood pressure regulation, thereby
demonstrating the potential of HQQR in treating metabolic diseases
and CVDs.
However, the effect of HQQR on the development of CH
was only investigated at the cellular level. Therefore, in
vivo experiments are required to further confirm the
therapeutic effect of HQQR on CH and further explore underlying
molecular mechanisms to expand the clinical application of
HQQR.
The present study provided evidence that Ang II
treatment could induce CH in primary cardiomyocytes, and that HQQR
could significantly prevent Ang II-induced CH by preventing
mitochondrial dysfunction and oxidative stress, reducing ROS
accumulation and protecting the electron transport chain. Although
additional studies are required to further verify the function and
mechanisms of HQQR in the development of CH, the present study
implied that HQQR could be considered a novel approach for CH
treatment.
Supplementary Material
HQQR cytotoxicity. (A) CCK-8 assays
were performed to examine the proliferation of primary
cardiomyocytes at 0 and 24 h after treatment with various doses of
HQQR solution (0, 0.05, 0.1, 0.2, 0.5 and 1 mg/ml).
*P<0.05, **P<0.01 and * *
*P<0.001, vs. the 0 mg/ml HQQR group;
#P<0.05 and ##P<0.01 vs. 0.1 mg/ml HQQR
group. (B) CCK-8 assays to examine the proliferation of primary
cardiomyocytes at 0, 12, 24 and 48 h after treatment with 0.2 and
0.5 mg/ml HQQR solution. *P<0.05,
**P<0.01 and ***P<0.001 vs. the 0 mg/ml
HQQR group. OD, optical density; HQQR, Huoxue Qianyang Qutan
recipe; CCK-8, Cell Counting Kit-8.
Protein expression levels of
apoptosis-related proteins Bax, Bcl-2 and cleaved caspase 3 were
examined using western blotting. ***P<0.001 vs. the
vehicle group; #P<0.05, # #P<0.01 and
# # #P<0.001 vs. the Ang II + vehicle group. HQQR,
Huoxue Qianyang Qutan recipe; Ang II, angiotensin II.
(A) mRNA levels of SIRT1, PGC-1α,
NRF1, Tfam, NDUFA13, SDHB, COX IV, COX1 and ATPase 6 in primary
cardiomyocytes were examined via reverse transcription-quantitative
PCR. (B) Histogram of protein levels quantification was shown.
**P<0.01 and ***P<0.001 vs. the vehicle
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the Ang II + vehicle group. HQQR,
Huoxue Qianyang Qutan recipe; Ang II, angiotensin II; SIRT1,
sirtuin 1; mtDNA, mitochondrial DNA; PGC-1α, peroxisome
proliferator-activated receptor-gamma coactivator-1α; NRF1, nuclear
respiratory factor 1; Tfam, mitochondrial transcription factor A;
NDUFA13, NADH:ubiquinone oxidoreductase subunit A13; SDHB,
succinate dehydrogenase complex iron sulfur subunit B; COX IV,
cytochrome c oxidase subunit IV; COX1, anti-cyclooxygenase
1.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 81774111 and 81803892) and
Scientific research projects of Shanghai Science and Technology
Commission (grant no. 19401970400).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DF conceived and designed the study. MG, LY, BL, JW,
XZ, JL and ZD performed the experiments. DF wrote the manuscript.
DF and MG have confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All the experiments conducted in the present study
were approved by the Ethics Committee of Yueyang Hospital of
Integrated Traditional Chinese and Western Medicine Affiliated to
Shanghai University of Traditional Chinese Medicine (approval no.
18922, Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Badimon L, Chagas P and Chiva-Blanch G:
Diet and cardiovascular disease: Effects of foods and nutrients in
classical and emerging cardiovascular risk factors. Curr Med Chem.
26:3639–3651. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu L, Su Y, Zhao Y, Sheng X, Tong R, Ying
X, Gao L, Ji Q, Gao Y, Yan Y, et al: Melatonin differentially
regulates pathological and physiological cardiac hypertrophy:
Crucial role of circadian nuclear receptor RORα signaling. J Pineal
Res. 67(e12579)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shimizu I and Minamino T: Physiological
and pathological cardiac hypertrophy. J Mol Cell Cardiol.
97:245–262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Heineke J and Molkentin JD: Regulation of
cardiac hypertrophy by intracellular signalling pathways. Nat Rev
Mol Cell Biol. 7:589–600. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Nakamura M and Sadoshima J: Mechanisms of
physiological and pathological cardiac hypertrophy. Nat Rev
Cardiol. 15:387–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsuruda T, Sekita-Hatakeyama Y, Hao Y,
Sakamoto S, Kurogi S, Nakamura M, Udagawa N, Funamoto T, Sekimoto
T, Hatakeyama K, et al: Angiotensin II stimulation of cardiac
hypertrophy and functional decompensation in
osteoprotegerin-deficient mice. Hypertension. 67:848–856.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Luo YX, Tang X, An XZ, Xie XM, Chen XF,
Zhao X, Hao DL, Chen HZ and Liu DP: SIRT4 accelerates Ang
II-induced pathological cardiac hypertrophy by inhibiting manganese
superoxide dismutase activity. Eur Heart J. 38:1389–1398.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Y, Jiao R, Ma ZG, Liu W, Wu QQ, Yang
Z, Li FF, Yuan Y, Bian ZY and Tang QZ: Sanguinarine inhibits
angiotensin II-induced apoptosis in H9c2 cardiac cells via
restoring reactive oxygen species-mediated decreases in the
mitochondrial membrane potential. Mol Med Rep. 12:3400–3408.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Murphy E, Ardehali H, Balaban RS, DiLisa
F, Dorn GW II, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, et
al: Mitochondrial function, biology, and role in disease: A
scientific statement from the American heart association. Circ Res.
118:1960–1991. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peoples JN, Saraf A, Ghazal N, Pham TT and
Kwong JQ: Mitochondrial dysfunction and oxidative stress in heart
disease. Exp Mol Med. 51:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cai J, Shi G, Zhang Y, Zheng Y, Yang J,
Liu Q, Gong Y, Yu D and Zhang Z: Taxifolin ameliorates DEHP-induced
cardiomyocyte hypertrophy via attenuating mitochondrial dysfunction
and glycometabolism disorder in chicken. Environ Pollut.
255(113155)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tian H, Yu D, Hu Y, Zhang P, Yang Y, Hu Q
and Li M: Angiotensin II upregulates cyclophilin A by enhancing ROS
production in rat cardiomyocytes. Mol Med Rep. 18:4349–4355.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hao P, Jiang F, Cheng J, Ma L, Zhang Y and
Zhao Y: Traditional Chinese medicine for cardiovascular disease:
Evidence and potential mechanisms. J Am Coll Cardiol. 69:2952–2966.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tan X, Li J, Wang X, Chen N, Cai B, Wang
G, Shan H, Dong D, Liu Y, Li X, et al: Tanshinone IIA protects
against cardiac hypertrophy via inhibiting calcineurin/NFATc3
pathway. Int J Biol Sci. 7:383–389. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang XY and Chen CX: Effect of
oxymatrine, the active component from Radix Sophorae flavescentis
(Kushen), on ventricular remodeling in spontaneously hypertensive
rats. Phytomedicine. 20:202–212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Y, Liang S, Bu P, Liang E, Yan F, Xing
Y and Zhang P: Radix Puerariae rebalances vasomotor factors
and improves left ventricular diastolic dysfunction in patients
with essential hypertension. Exp Ther Med. 20:705–713.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang J, Dong ZH, Gui MT, Yao L, Li JH,
Zhou XJ and Fu DY: HuoXue QianYang QuTan recipe attenuates left
ventricular hypertrophy in obese hypertensive rats by improving
mitochondrial function through SIRT1/PGC-1α deacetylation pathway.
Biosci Rep. 39(BSR20192909)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou X, Lu B, Fu D, Gui M, Yao L and Li J:
Huoxue Qianyang decoction ameliorates cardiac remodeling in obese
spontaneously hypertensive rats in association with ATF6-CHOP
endoplasmic reticulum stress signaling pathway regulation. Biomed
Pharmacother. 121(109518)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vaskova E, Ikeda G, Tada Y, Wahlquist C,
Mercola M and Yang PC: Sacubitril/valsartan improves cardiac
function and decreases myocardial fibrosis via downregulation of
exosomal miR-181a in a rodent chronic myocardial infarction model.
J Am Heart Assoc. 9(e015640)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ehler E, Moore-Morris T and Lange S:
Isolation and culture of neonatal mouse cardiomyocytes. J Vis Exp.
(50154)2013.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Xing L and Li Z: Angiotensin II induced
myocardial hypertrophy in neonatal rats could be attenuated by
activated κ-opioid receptor via modulating the calcineurin signal
pathways. Zhonghua Xin Xue Guan Bing Za Zhi. 43:254–258.
2015.PubMed/NCBI(In Chinese).
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li H, Sureda A, Devkota HP, Pittalà V,
Barreca D, Silva AS, Tewari D, Xu S and Nabavi SM: Curcumin, the
golden spice in treating cardiovascular diseases. Biotechnol Adv.
38(107343)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiong X, Yang X, Duan L, Liu W, Zhang Y,
Liu Y, Wang P, Li S and Li X: Traditional Chinese medicine
suppresses left ventricular hypertrophy by targeting extracellular
signal-regulated kinases signaling pathway in spontaneously
hypertensive rats. Sci Rep. 7(42965)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang R, Cui YC, Wei XH, Pan CS, Li Q, He
SY, Fan JY and Han JY: A novel traditional Chinese medicine
ameliorates fatigue-induced cardiac hypertrophy and dysfunction via
regulation of energy metabolism. Am J Physiol Heart Circ Physiol.
316:H1378–H1388. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gao RR, Wu XD, Jiang HM, Zhu YJ, Zhou YL,
Zhang HF, Yao WM, Li YQ and Li XL: Traditional Chinese medicine
Qiliqiangxin attenuates phenylephrine-induced cardiac hypertrophy
via upregulating PPARγ and PGC-1α. Ann Transl Med.
6(153)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zou R, Tao J, Qiu J, Shi W, Zou M, Chen W,
Li W, Zhou N, Wang S, Ma L and Chen X: Ndufs1 deficiency aggravates
the mitochondrial membrane potential dysfunction in pressure
overload-induced myocardial hypertrophy. Oxid Med Cell Longev.
2021(5545261)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thirupathi A and de Souza CT:
Multi-regulatory network of ROS: The interconnection of ROS, PGC-1
alpha, and AMPK-SIRT1 during exercise. J Physiol Biochem.
73:487–494. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
St-Pierre J, Drori S, Uldry M, Silvaggi
JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al:
Suppression of reactive oxygen species and neurodegeneration by the
PGC-1 transcriptional coactivators. Cell. 127:397–408.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rababa'h AM, Guillory AN, Mustafa R and
Hijjawi T: Oxidative stress and cardiac remodeling: An updated
edge. Curr Cardiol Rev. 14:53–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gallo S, Vitacolonna A, Bonzano A,
Comoglio P and Crepaldi T: ERK: A key player in the pathophysiology
of cardiac hypertrophy. Int J Mol Sci. 20(2164)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Singh RM, Cummings E, Pantos C and Singh
J: Protein kinase C and cardiac dysfunction: A review. Heart Fail
Rev. 22:843–859. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee Y, Jeong GS, Kim KM, Lee W and Bae JS:
Cudratricusxanthone A attenuates sepsis-induced liver injury via
SIRT1 signaling. J Cell Physiol. 233:5441–5446. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou
W, Zhang T, Yuan H, Zhao J, Zhang L, et al: Erythropoietin
activates SIRT1 to protect human cardiomyocytes against
doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol
Lett. 275:28–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang T, Chi Y, Ren Y, Du C, Shi Y and Li
Y: Resveratrol reduces oxidative stress and apoptosis in podocytes
via Sir2-related enzymes, sirtuins1 (SIRT1)/peroxisome
proliferator-activated receptor γ co-activator 1α (PGC-1α) axis.
Med Sci Monit. 25:1220–1231. 2019.PubMed/NCBI View Article : Google Scholar
|



















