Introduction
The endogenous amino acid 5-aminolevulinic acid
(5-ALA) is widely distributed in both animals and plants and is
synthesized by glycine condensation and succinyl-CoA via the
mitochondrial ALA synthase in animal cells (1). It is then further converted into
protoporphyrin IX (PPIX) due to well-known subsequent enzymatic
reactions (1). Next, a divalent
iron is coordinated to PPIX for heme synthesis (1). A synthesized heme binds to
corresponding apoproteins and becomes a hemeprotein (2).
Furthermore, 5-ALA hydrochloride has been orally
administered for the photodynamic diagnosis using specific
accumulation of fluorescent PPIX in tumor tissues of patients with
cancer (3-5).
Conversely, 5-ALA is metabolized to heme combined with iron in
normal tissues, producing hemeproteins that exhibit several
biological effects such as oxidoreductive activities (catalase and
oxidase), electron transport (cytochrome c), and oxygen delivery
(myoglobin and hemoglobin) (6,7).
Externally administered 5-ALA significantly affects energy
metabolism of the whole body through mitochondrial hemeproteins
synthesized from 5-ALA, which is reduced with aging (8). Ingested 5-ALA is reported to enhance
energy metabolism by activating the mitochondrial electron
transport system that promotes ATP synthesis in the mouse liver
(9). Furthermore, 5-ALA with
sodium ferrous citrate (SFC) is reported to augment exercise
efficiency in elderly women (10).
Our previous studies reported that 5-ALA with SFC exerts
anti-diabetic effects in Zucker diabetic fatty (ZDF) rats and
patients with borderline diabetes (11,12).
Orally administered 5-ALA/SFC reduces fasting blood glucose and
hemoglobin A1c levels and improves glucose tolerance in ZDF rats
without affecting insulin secretion (11). The lipid content in 3T3-L1
adipocytes is decreased by 5-ALA/SFC and glucose uptake in 3T3-L1
and L6 myotube cells is induced (13).
Glucose uptake from blood to cells is caused by
insulin secreted from the pancreas, which translocates glucose
transporter (GLUT)4 from the cytoplasm to the plasma membrane in
muscles and adipose tissues (14,15).
Overall, 14 GLUT isoforms have been detected, and their expression
levels differ among organs (16).
Because the administration of 5-ALA/SFC decreases blood glucose
level in vivo and enhances glucose uptake in vitro,
some glucose transporters in tissues are assumed to be activated;
however, no study has identified the involved tissues and
transporters.
Therefore, the present study analyzed glucose
kinetics in vivo and the expression level of GLUT isoforms
in the plasma membrane to identify a glucose transporter activated
by 5-ALA/SFC. 5-ALA/SFC was administered to high-fat diet (HFD)-fed
mice to elucidate the target tissues that reduced the blood glucose
level. Furthermore, the effect of 5-ALA/SFC on glucose uptake in
C2C12 myotube cells was examined, and the expression level and type
of glucose transporters in the plasma membranes of C2C12 cells were
investigated.
Materials and methods
Reagents
5-ALA hydrochloride was provided by SBI
Pharmaceuticals Co., Ltd. SFC was obtained from Komatsuya
Corporation. Dulbecco's modified Eagle's medium (DMEM), horse
serum, HEPES, NaCl, KCl, MgSO4,
KH2PO4, CaCl2, phloretin, Tris-HCl
and dithiothreitol (DTT) were purchased from FUJIFILM Wako Pure
Chemical Corporation.
Cell culture and treatment
C2C12 myotube cells (CRL-1772; American Type Culture
Collection) were cultured at 37˚C under 5% CO2 using
high-glucose DMEM (4,500 mg/l glucose) supplemented with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.). For myotube
differentiation, C2C12 cells were cultured for 5-7 days in
high-glucose DMEM supplemented with 2% horse serum, and
differentiated myotube cells were used for every experiment. For
2-deoxyglucose (2DG) uptake analysis, differentiated C2C12 cells
were incubated in low-glucose DMEM (1,000 mg/l glucose) containing
2% horse serum for 18 h in a 12-well plate. Subsequently, the cells
were treated with or without 5-ALA/SFC in low-glucose DMEM
containing 0.1% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA) for 6 h at 37˚C. Doses of 5-ALA (100, 250, 500 µM) and SFC
(50, 125, 250 µM) were determined from previous studies (11,13,17).
The cells were washed three times with 37˚C Krebs-Ringer's
phosphate (KRPH) buffer (20 mM HEPES, 137 mM NaCl, 4.7 mM KCl, 1 mM
MgSO4, 5 mM KH2PO4, 1 mM
CaCl2; pH 7.4) and incubated for 18 min at 37˚C in 0.1%
BSA-added KRPH buffer with or without insulin (Thermo Fisher
Scientific, Inc.), 5-ALA, SFC or 5-ALA/SFC. The amount of 2DG
uptake in C2C12 cells was measured according to the manufacturer's
protocol of the 2DG Uptake Measurement kit (Cosmo Bio Co., Ltd.).
Next, 2DG was added to a final concentration of 1 mM, and
incubation was continued for 20 min at 37˚C. This reaction was
stopped by adding an ice-cold KRPH buffer containing 200 µM
phloretin (FUJIFILM Wako Pure Chemical Corporation). Subsequently,
cells were washed three times with ice-cold KRPH buffer. Cells were
sonicated for 30 sec on ice with 10 mM Tris-HCl (pH 8.0). The
sonicated cellular solutions were heated at 85˚C for 15 min and
centrifuged at 16,000 x g for 20 min at 4˚C. Supernatants were used
to measure 2DG amounts and protein concentrations. For western
blotting analysis, differentiated C2C12 cells were cultured in a
10-cm dish.
Animals
The animal experiment protocols in the present study
were approved by the Institutional Animal Care and Use Committee of
Kobe University (approval no. 24-04-02; Kobe) and were conducted
according to the guidelines set by this institution. A total of 18
C57BL/6N male mice (19-week-old; Japan SLC, Inc.) were acclimated
at 23±3˚C and relative humidity of 50±10% in a 12 h light-dark
cycle (light from 8:00 a.m. to 8:00 p.m.), with water and a normal
diet (ND) feed (cat. no. D12450B; Research Diets, Inc.) provided ad
libitum for 1 week. After a week of ND feed, mice (body weight,
29-36 g) were categorized into three groups according to the oral
administration for 4 weeks: i) ND Group with saline administration
(ND; n=5); ii) HFD group with saline administration (HFD; 60 Kcal%
fat; cat. no. D12492; Research Diets, Inc.; n=8); and iii) HFD
group with 300 mg/kg 5-ALA and 47.1 mg/kg SFC administration (HFD +
5-ALA/SFC; n=5). Body weights, food intake and water intake of mice
were measured every day during the experiment.
On the final day, all mice were fasted for 5 h.
After fasting, mixtures of 2 g/kg glucose and 3.2 mg/kg 2DG were
intraperitoneally injected into ND, HFD and HFD + 5-ALA/SFC groups
(each group, n=5) for intraperitoneal glucose tolerance test
(IPGTT) and glucose uptake measurement. A total of three mice in
the HFD group were intraperitoneally injected with glucose only as
a negative control for 2DG. After measuring blood glucose levels,
all mice were anesthetized with an intraperitoneal injection of 60
mg/kg pentobarbital and euthanized by exsanguination until cardiac
arrest. Mortality was confirmed by respiratory arrest and reflex
activity arrest. Organ samples were collected and stored at -80˚C.
Frozen samples were analyzed for 2DG uptake measurement and western
blotting as described below.
IPGTT
IPGTT was performed after 5 h fasting (8:00 a.m. to
1:00 p.m.). Blood was collected from the tail veins of the mice.
Blood glucose concentrations were measured at 0, 15, 30, 60, 90 and
120 min after the injection using Stat Strip XP (Nipro
Corporation). The areas under the curve (AUCs) were calculated by
the linear trapezoid method (18).
Measurement of 2DG uptake in mouse
tissues
Amounts of 2DG uptake were measured according to the
protocol of 2DG uptake measurement kit (Cosmo Bio Co., Ltd.)
(19). Protein concentrations of
the samples were determined using Pierce® BCA™ Protein
Assay kit (Thermo Fisher Scientific, Inc.). Frozen mouse tissues
were homogenized with an electric homogenizer at 10-40 times the
weights of the dilution solution included in the kit. Homogenized
solutions were heated at 85˚C for 15 min, followed by
centrifugation at 16,000 x g for 20 min at 4˚C. Supernatants were
used to measure 2DG uptake.
Western blotting
Extractions of the membrane and total proteins from
C2C12 cells were performed as previously described (20). Briefly, treated cells were
homogenized in 50 mM Tris-HCl (pH 8.0), containing 0.5 mM DTT, 1%
Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, Inc.)
and phosphatase inhibitors (Thermo Fisher Scientific, Inc.)
(solution A). To prepare total cell lysate, a homogenized solution
was gently stirred with an equal volume of solution A containing
1.0% NP-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl
sulfate (SDS) on ice for 1 h. To prepare the plasma membrane,
another homogenized solution was centrifuged at 3,000 x g for 10
min at 4˚C. The washed precipitate was reacted for 1 h with gentle
stirring on ice, then centrifuged at 20,000 x g for 20 min at 4˚C.
The proteins (5-20 µg/lane) were subjected to SDS-PAGE using a
4-15% Miniprotein® TGX™ precast gel (Bio-Rad
Laboratories, Inc.) and transferred to a PVDF membrane (Bio-Rad
Laboratories, Inc.). After blocking the membrane with 5% non-fat
milk in TBS with 0.05% Tween-20 (TBST) for 1 h at room temperature,
the membrane was incubated with an anti-GLUT1 antibody (cat. no.
12939; Cell Signaling Technology, Inc.) and anti-insulin receptor β
(IR-β) antibody (cat. no. sc-711; Santa Cruz Biotechnology, Inc.)
overnight at 4˚C. After washing, the membranes were incubated with
HRP-linked donkey anti-rabbit IgG antibody (cat. no. NA934; Cytiva)
for 1 h at room temperature, and developed with Immuno Star LD
(cat. no. 292-699003; FUJIFILM Wako Pure Chemical Corporation)
using ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc.).
Image Lab software 4.0.1 (Bio-Rad Laboratories, Inc.) was used for
densitometry. GLUT4 and GAPDH detections were conducted using the
same membranes after stripping antibodies with Restore™ Western
Blot Stripping Buffer (Thermo Fisher Scientific, Inc.). The
stripped membrane was blocked with 5% milk in TBST and reacted with
anti-GLUT4 antibody (cat. no. 2213; Cell Signaling Technology,
Inc.) or anti-GAPDH antibody (cat. no. E1C604-1; EnoGene Biotech
Co, Ltd.) overnight at 4˚C. HRP-linked sheep anti-mouse IgG
antibody (cat. no. NA931; Cytiva) for GLUT4 and HRP-linked donkey
anti-rabbit IgG antibody (cat. no. NA934; Cytiva) for GAPDH were
used. IR-β was used as an internal control in the plasma membrane
according to the previous study (21), and GAPDH was used as a control for
the total protein. The data were analyzed using the ChemiDoc MP
Imaging System (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analyzed using GraphPad Prism 7 (GraphPad
Software, Inc.). Results are expressed as means ± standard error of
the mean. In vivo data were statistically analyzed by
one-way ANOVA with Tukey's test. In vitro data were
statistically analyzed by one-way ANOVA with Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
5-ALA/SFC improves glucose tolerance
in HFD-fed mice via upregulation of glucose uptake in muscle
tissues
The effects of 5-ALA/SFC on body weight and glucose
tolerance in HFD-fed mice were investigated. HFD-fed mice became
23% heavier compared with ND-fed mice, which was a significant
change (Fig. 1A; Table I). No significant differences in
food and water intakes were observed among the HFD and HFD +
5-ALA/SFC groups (data not shown). Body weights were significantly
lighter in the HFD + 5-ALA/SFC group compared with the HFD group at
4 weeks after the administration (Fig.
1A; Table I). Total white fat
weights were significantly heavier in the HFD group compared with
the ND group (Table I). By
contrast, white fat weights were not significant change between the
HFD + 5-ALA/SFC and HFD groups (Table
I). Next, the effect of 5-ALA/SFC on glucose tolerance was
confirmed (Fig. 1B). Plasma
glucose levels from 15-60 min after glucose administration were
significantly higher in the HFD group compared with the ND group
(Fig. 1B). Plasma glucose levels
were significantly lower in the HFD + 5-ALA/SFC group compared with
the HFD group at 30 min (Fig. 1B).
Moreover, the AUC of plasma glucose levels was significantly lower
in the HFD + 5-ALA/SFC group compared with the HFD group (Fig. 1C), suggesting that 5-ALA/SFC
prevented obesity and improved glucose tolerance in HFD-fed
mice.
 | Figure 15-ALA/SFC enhances glucose tolerance
in the high-fat diet fed group by increasing glucose uptake in
muscle tissues. (A) Body weight for 4 weeks after the
administration of 5-ALA/SFC or saline. (B) Plasma glucose levels
during IPGTT. (C) AUC of glucose levels during IPGTT. (D) 2DG
uptake into each tissue in the ND, HFD and HFD + 5-ALA/SFC groups.
n=5-8. *P<0.05, **P<0.01,
***P<0.01 vs. ND group; #P<0.05,
##P<0.01 vs. HFD group. AUC, area under the curve;
IPGTT, intraperitoneal glucose tolerance test; 5-ALA,
5-aminolevulinic acid; SFC, sodium ferrous citrate; 2DG,
2-deoxyglucose; ND, normal diet, HFD, high-fat diet. |
 | Table IBody and organ weights in mice fed
with ND, HFD or HFD + 5-ALA/SFC administration for 4 weeks. |
Table I
Body and organ weights in mice fed
with ND, HFD or HFD + 5-ALA/SFC administration for 4 weeks.
| Weights | ND | HFD | HFD + 5-ALA/SFC |
|---|
| Body weight, g | 32.94±0.94 |
40.38±0.95a |
36.76±0.54b,c |
| Liver, g | 1.26±0.14 | 1.26±0.09 | 1.40±0.04 |
| Spleen, mg | 71.74±7.91 | 85.58±4.41 | 67.02±4.68 |
| Kidney, g | 0.31±0.01 | 0.36±0.02 | 0.34±0.01 |
| Brown fat, g | 0.24±0.03 | 0.40±0.08 | 0.31±0.06 |
| White fat, g | 4.37±0.40 |
7.67±0.56d |
6.58±0.60b |
|
Retroperitoneal
and perirenal fat, g | 0.67±0.07 |
1.11±0.08d | 0.87±0.08 |
|
Epididymal
fat, g | 1.36±0.13 |
2.22±0.19d | 1.80±0.14 |
|
Mesenteric
fat, g | 0.54±0.04 |
0.87±0.07d | 0.67±0.06 |
|
Subcutaneous
fat, g | 1.81±0.20 |
3.44±0.39b |
3.24±0.46b |
Glucose uptakes in various tissues were investigated
in the ND, HFD and HFD + 5-ALA/SFC groups by measuring the amount
of 2DG uptake (Fig. 1D), a
non-metabolized glucose analog (20). The average amount of 2DG uptake in
gastrocnemius muscle was significantly lower in the HFD group
compared with in ND group (Fig.
1D). The uptake of 2DG was slightly lower in soleus muscle,
heart, white adipose tissues, brown adipose tissue and cerebrum in
the HFD groups compared with those in the ND groups; however, the
differences were not significant. By contrast, 2DG uptake levels in
gastrocnemius muscle and heart were significantly higher in the HFD
+ 5-ALA/SFC group compared with those in the HFD group (Fig. 1D). The uptakes of 2DG in soleus
muscle, white adipose tissues and cerebrum were markedly higher in
the HFD + 5-ALA/SFC groups compared with the HFD groups, but these
were not significant (Fig. 1D).
Glucose uptake was upregulated by treatment with 5-ALA/SFC in
gastrocnemius muscle and heart of HFD diabetic mice, followed by
the improvement in glucose tolerance. Subsequently, the cellular
mechanism underlying glucose uptake by 5-ALA/SFC was investigated
using C2C12 myotube cells.
5-ALA/SFC upregulates GLUT1
translocation to the plasma membrane of C2C12 mouse myotube
cells
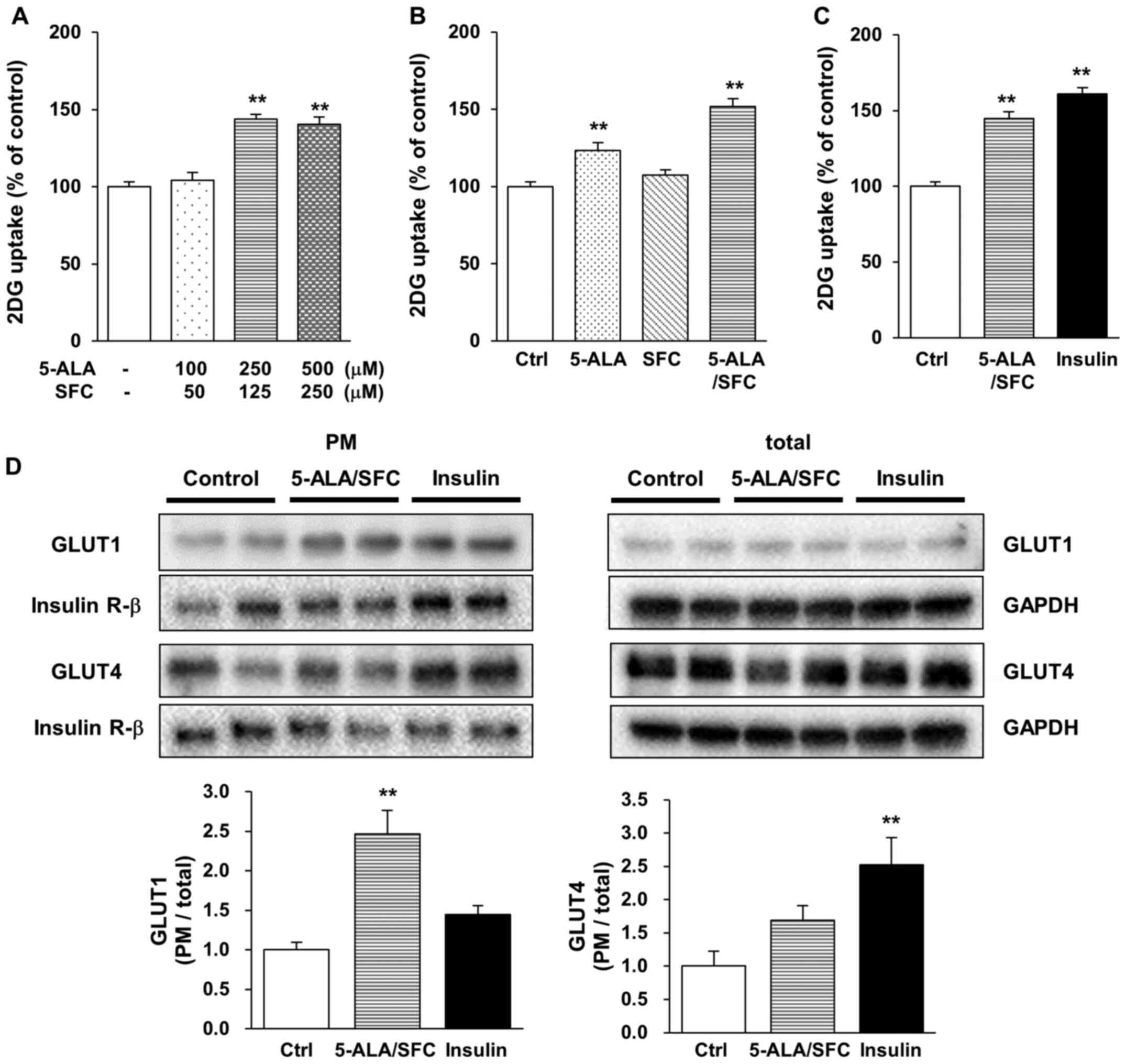
The effect of 5-ALA/SFC on glucose uptake was
investigated in C2C12 mouse myotube cells. Differentiated C2C12
cells were used to investigate the mechanism underlying 2DG uptake
by insulin stimulation. 2DG uptake was significantly increased by
treatment with a combination of >250 µM 5-ALA and >125 µM SFC
in C2C12 cells compared with that of untreated cells (Fig. 2A). Next, glucose uptake in C2C12
cells treated with 5-ALA alone, SFC alone and their combination
were examined. 5-ALA alone significantly increased 2DG uptake, but
SFC alone did not change 2DG uptake in C2C12 cells relative to that
in the untreated control cells (Fig.
2B). The average amount of 2DG uptake with 250 µM 5-ALA/125 µM
SFC was a significant increase of 145% relative to that in
untreated controls (Fig. 2C). This
increase in 5-ALA/SFC treatment was equivalent to the increase
observed in insulin treatment (Fig.
2C).
Next, GLUT1 and GLUT4 translocations in C2C12 cells
were examined using western blotting. Sodium potassium ATPase 1a in
the plasma membrane was decreased by 5-ALA/SFC (data not shown),
thus IR-β was used as a control in the plasma membrane in the
present study. GLUT1 expression in the plasma membrane was
significantly increased by 5-ALA/SFC by 2.5-fold relative to the
control (Fig. 2D). Insulin
markedly increased GLUT1 expression in the plasma membrane by
1.4-fold relative to the control, but the difference was not
statistically significant (Fig.
2D). By contrast, insulin significantly increased GLUT4
expression in the plasma membrane by 2.5-fold relative to the
control (Fig. 2D). GLUT4
expression in the plasma membrane was increased in
5-ALA/SFC-treated cells by 1.7-fold relative to that in the
control, but this change was not significant (Fig. 2D). Overall, these results suggested
that 5-ALA/SFC upregulated glucose uptake via GLUT1 translocation
in myotube cells.
Discussion
The present study investigated the mechanism
underlying the 5-ALA/SFC-mediated increase in glucose uptake using
HFD-fed diabetic mice in vivo and C2C12 myotube cells in
vitro. Administration of 5-ALA/SFC suppressed body weight gain
and recovered impaired glucose tolerance in HFD-fed mice. These
results are consistent with those of our previous report (13), which suggests that improved obesity
and glucose tolerance are directly caused by 5-ALA/SFC to reduce
fat accumulation and promote glucose uptake from blood to cells.
Additionally, this study revealed that 5-ALA/SFC enhances glucose
uptake in 3T3-L1 adipocytes by 70-90% and rat L6 myoblasts by 30%
relative to the uptake in untreated cells (13). The present study confirmed that
both 5-ALA alone and 5-ALA/SFC promoted glucose uptake in mouse
C2C12 myotube cells. Additionally, the target organs in mice with
increased glucose uptake by 5-ALA/SFC were demonstrated to mainly
be the gastrocnemius muscle and the heart. It was suggested that
the 5-ALA/SFC-mediated increase of glucose uptake in gastrocnemius
muscle and heart indicated the existence of an unknown mechanism by
which 5-ALA/SFC enhances glucose uptake specifically in these
muscles.
Muscle is a major contributor to the basal metabolic
rate of the whole body (22,23)
and is responsible for ~20% of the energy consumption in the whole
body at rest (23,24). Thus, glucose uptake in muscles can
be more energy efficient compared with glucose uptake in other
organs. The present study revealed that glucose uptake is the
highest in soleus muscle among all examined organs; however, soleus
muscle was not affected by 5-ALA/SFC. It was hypothesized that
glucose uptake in both gastrocnemius muscle and heart sufficiently
lowered systemic glucose concentrations in HFD-fed mice, because
the weight of the soleus was lighter compared with the weight of
the gastrocnemius. When calculated as the total amount of 2DG
uptake per organ, gastrocnemius muscle demonstrated greater uptake
compared with soleus muscle. Decreased blood glucose level in the
HFD + 5-ALA/SFC group was considered to be the result of increased
direct glucose uptake from blood to muscle tissues.
The current study demonstrated that the combination
treatment of 5-ALA and SFC on C2C12 cells enhanced glucose uptake.
SFC alone did not affect glucose uptake, whereas 5-ALA alone
treatment weakly enhanced glucose uptake in C2C12 cells. These
results are consistent with our previous report on glucose uptake
in rat L6 skeletal muscle cells (13), and also suggested the importance of
5-ALA metabolization to heme by adding SFC to increase glucose
uptake in muscle tissues. Presumably, treatment with 5-ALA alone
resulted in the metabolization of some 5-ALA to heme by using iron
in a culture medium, which slightly increased the glucose uptake in
C2C12 cells.
5-ALA is considered a precursor of the mitochondrial
electronic transport chain complex and upregulates ATP production
by inducing the expression levels of complex IV and ATP synthase
in vivo (9,25). Overall, the combination of 5-ALA
and SFC improves aerobic metabolism, thereby promoting cellular
glucose consumption and leading to lower blood glucose levels
(11,13). Glucose uptake in muscle tissues is
known to be caused mainly by insulin-independent GLUT1 and
insulin-dependent GLUT4 (16,26-28).
In the present study, the C2C12 cell experiment results
demonstrated an insignificant increase of GLUT1 translocation in
insulin treatment compared with controls. This indicated that a
significant induction of GLUT1 translocation may not always occur
after insulin treatment. A previous report demonstrated that GLUT1
translocation is unchanged by insulin treatment in L6 cells
(29). In the present study,
5-ALA/SFC significantly increased GLUT1 translocation from the
cytoplasm to the plasma membrane compared with that of GLUT4 in
C2C12 cells, without affecting GLUT1 expression. GLUT4 is required
for acute insulin- and contraction-induced glucose uptake in
skeletal muscle. However, the effect of GLUT1 on the skeletal
muscle glucose uptake remains unclear. GLUT1 is widely expressed in
various types of cells and tissues, such as erythrocytes and the
brain (30,31). GLUT1 is responsible for basal
glucose transport and necessary for cell survival (30). Thus, the effect of 5-ALA/SFC on the
membrane localization of GLUT1 may increase systemic glucose
metabolism. Further examination of the 5-ALA/SFC mechanisms in
diabetic mice or human muscle cells will be carried out in the
future.
The present study has some potential limitations.
First, C2C12 cells are mouse myotube cells, thus the effect of
5-ALA/SFC on GLUT1 translocation in human cell lines has not been
verified. The purpose of these cellular experiments was to clarify
the contribution of GLUT as a result of 5-ALA/SFC-induced glucose
uptake in HFD-fed mice. C2C12 cells are a well-documented model of
diabetes (32). Differentiated
C2C12 cells have myosin and glycogen, and closely mimic human
myotube (32); therefore, this
effect of 5-ALA/SFC was considered similar to humans. However, an
additional study using a human cell line should be conducted in the
future. Second, the present study has a lack of knockdown or
inhibitor experiments; therefore, the mechanism of 5-ALA/SFC on
GLUT1 translocation may not be accurately concluded from these
results. STF31, the only GLUT-1 specific inhibitor, blocks GLUT1
activity by inhibiting NAD synthase (33-35).
Thus, STF31 inhibits mitochondrial function. It is hypothesized
that 5-ALA/SFC-induced glucose uptake results from enhanced
mitochondrial function. STF31 would possibly antagonize the effect
of 5-ALA/SFC by inhibiting mitochondrial function. Therefore, STF31
is considered inappropriate to evaluate the effectiveness of
5-ALA/SFC. On the other hand, GLUT1 siRNA is likely to affect cell
survival and differentiation. Differentiated cells that express
GLUT1 and GLUT4 are essential for the glucose uptake test (19), and mixed culture of un-differential
cells cannot be evaluated. GLUT1 siRNA can affect the experimental
system itself as well as GLUT1 interference. Resolving these
limitations are still difficult to achieve and are a future
challenge.
In conclusion, the current study demonstrated that
5-ALA/SFC enhanced glucose uptake in skeletal muscles and heart,
and prevented impaired glucose tolerance in HFD-fed mice. Moreover,
it was revealed that GLUT1 translocation may be involved in this
mechanism. However, further studies are warranted to elucidate the
molecular mechanisms underlying GLUT1 translocation by 5-ALA/SFC.
The present results suggested that 5-ALA/SFC may be a useful drug
for the treatment of diabetes, particularly in patients with
insulin resistance and resistance to existing antidiabetic
drugs.
Acknowledgements
The authors thank Dr Hidemitsu Sugihara and Dr
Motowo Nakajima (SBI Pharmaceuticals Co., Ltd., Tokyo, Japan) for
their critical reading of the manuscript and helpful
discussion.
Funding
The present study was funded by SBI Pharmaceuticals Co.,
Ltd.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the commercial
restrictions of SBI Pharmaceuticals Co., Ltd., but are available
from the corresponding author on reasonable request.
Authors' contributions
YK, MI, YY and HA conceived and designed the
experiments. YK and TI performed the experiments. YK, AK and TI
confirm the authenticity of all the raw data and interpreted the
data. YK and AK statistically analyzed the data and wrote the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved by the Institutional Animal Care and Use Committee of Kobe
University (approval no. 24-04-02; Kobe, Japan).
Patient consent for publication
Not applicable.
Competing interests
YK, AK, TI and MI are employees of SBI
Pharmaceuticals Co., Ltd. YY and HA declare that they have no
competing interests.
References
|
1
|
Hendry GA and Jones OT: Haems and
chlorophylls: Comparison of function and formation. J Med Genet.
17:1–14. 1980.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Castro CE: Mechanisms of reaction of
hemeproteins with oxygen and hydrogen peroxide in the oxidation of
organic substrates. Pharmacol Ther. 10:171–189. 1980.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ: ALA-Glioma Study Group.
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Inoue K, Fukuhara H, Shimamoto T, Kamada
M, Iiyama T, Miyamura M, Kurabayashi A, Furihata M, Tanimura M,
Watanabe H, et al: Comparison between intravesical and oral
administration of 5-aminolevulinic acid in the clinical benefit of
photodynamic diagnosis for nonmuscle invasive bladder cancer.
Cancer. 118:1062–1074. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Antonini E and Brunori M: Hemoglobin and
myoglobin in their reactions with ligands. In: Frontiers of
biology. North-Holland Publication Co., Amsterdam, p436, 1971.
|
|
7
|
Sono M, Roach MP, Coulter ED and Dawson
JH: Heme-Containing Oxygenases. Chem Rev. 96:2841–2888.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Paterniti JR Jr, Lin CI and Beattie DS:
delta-Aminolevulinic acid synthetase: Regulation of activity in
various tissues of the aging rat. Arch Biochem Biophys.
191:792–797. 1978.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ogura S, Maruyama K, Hagiya Y, Sugiyama Y,
Tsuchiya K, Takahashi K, Abe F, Tabata K, Okura I, Nakajima M, et
al: The effect of 5-aminolevulinic acid on cytochrome c oxidase
activity in mouse liver. BMC Res Notes. 4(66)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Masuki S, Morita A, Kamijo Y, Ikegawa S,
Kataoka Y, Ogawa Y, Sumiyoshi E, Takahashi K, Tanaka T, Nakajima M,
et al: Impact of 5-aminolevulinic acid with iron supplementation on
exercise efficiency and home-based walking training achievement in
older women. J Appl Physiol (1985). 120:87–96. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hara T, Koda A, Nozawa N, Ota U, Kondo H,
Nakagawa H, Kamiya A, Miyashita K, Itoh H, Nakajima M, et al:
Combination of 5-aminolevulinic acid and ferrous ion reduces plasma
glucose and hemoglobin A1c levels in Zucker diabetic fatty rats.
FEBS Open Bio. 6:515–528. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Higashikawa F, Noda M, Awaya T, Tanaka T
and Sugiyama M: 5-aminolevulinic acid, a precursor of heme, reduces
both fasting and postprandial glucose levels in mildly
hyperglycemic subjects. Nutrition. 29:1030–1036. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ota U, Hara T, Nakagawa H, Tsuru E, Tsuda
M, Kamiya A, Kuroda Y, Kitajima Y, Koda A, Ishizuka M, et al:
5-aminolevulinic acid combined with ferrous ion reduces adiposity
and improves glucose tolerance in diet-induced obese mice via
enhancing mitochondrial function. BMC Pharmacol Toxicol.
18(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Klip A, McGraw TE and James DE: Thirty
sweet years of GLUT4. J Biol Chem. 294:11369–11381. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gallagher D, Belmonte D, Deurenberg P,
Wang Z, Krasnow N, Pi-Sunyer FX and Heymsfield SB: Organ-tissue
mass measurement allows modeling of REE and metabolically active
tissue mass. Am J Physiol. 275:E249–E258. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wood IS and Trayhurn P: Glucose
transporters (GLUT and SGLT): Expanded families of sugar transport
proteins. Br J Nutr. 89:3–9. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chibazakura T, Toriyabe Y, Fujii H,
Takahashi K, Kawakami M, Kuwamura H, Haga H, Ogura S, Abe F,
Nakajima M, et al: 5-Aminolevulinic acid enhances cell death under
thermal stress in certain cancer cell lines. Biosci Biotechnol
Biochem. 79:422–431. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Allison DB, Paultre F, Maggio C, Mezzitis
N and Pi-Sunyer FX: The use of areas under curves in diabetes
research. Diabetes Care. 18:245–250. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Saito K, Lee S, Shiuchi T, Toda C, Kamijo
M, Inagaki-Ohara K, Okamoto S and Minokoshi Y: An enzymatic
photometric assay for 2-deoxyglucose uptake in insulin-responsive
tissues and 3T3-L1 adipocytes. Anal Biochem. 412:9–17.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Suzuki K and Kono T: Evidence that insulin
causes translocation of glucose transport activity to the plasma
membrane from an intracellular storage site. Proc Natl Acad Sci
USA. 77:2542–2545. 1980.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiang H, Yamashita Y, Nakamura A, Croft K
and Ashida H: Quercetin and its metabolite isorhamnetin promote
glucose uptake through different signalling pathways in myotubes.
Sci Rep. 9(2690)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kinney JM and Tucker HN: Energy
Metabolism. Tissue Determinants and Cellular Corollaries. Ravan
Press, New York, NY, p562, 1992.
|
|
23
|
Zurlo F, Larson K, Bogardus C and Ravussin
E: Skeletal muscle metabolism is a major determinant of resting
energy expenditure. J Clin Invest. 86:1423–1427. 1990.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Durnin JV: Basal metabolic rate in man.
In: Joint FAO/-WHO/UNU Expert Consultation on Energy and Protein
Requirements, 1981.
|
|
25
|
Fujii C, Miyashita K, Mitsuishi M, Sato M,
Fujii K, Inoue H, Hagiwara A, Endo S, Uto A, Ryuzaki M, et al:
Treatment of sarcopenia and glucose intolerance through
mitochondrial activation by 5-aminolevulinic acid. Sci Rep.
7(4013)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mueckler M: Family of glucose-transporter
genes. Implications for glucose homeostasis and diabetes. Diabetes.
39:6–11. 1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Douen AG, Ramlal T, Rastogi S, Bilan PJ,
Cartee GD, Vranic M, Holloszy JO and Klip A: Exercise induces
recruitment of the ‘insulin-responsive glucose transporter’.
Evidence for distinct intracellular insulin- and
exercise-recruitable transporter pools in skeletal muscle. J Biol
Chem. 265:13427–13430. 1990.PubMed/NCBI
|
|
28
|
Kraegen EW, Sowden JA, Halstead MB, Clark
PW, Rodnick KJ, Chisholm DJ and James DE: Glucose transporters and
in vivo glucose uptake in skeletal and cardiac muscle: Fasting,
insulin stimulation and immunoisolation studies of GLUT1 and GLUT4.
Biochem J. 295:287–293. 1993.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yamashita Y, Okabe M, Natsume M and Ashida
H: Cacao liquor procyanidin extract improves glucose tolerance by
enhancing GLUT4 translocation and glucose uptake in skeletal
muscle. J Nutr Sci. 1(e2)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gould GW and Holman GD: The glucose
transporter family: Structure, function and tissue-specific
expression. Biochem J. 295:329–341. 1993.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Montel-Hagen A, Sitbon M and Taylor N:
Erythroid glucose transporters. Curr Opin Hematol. 16:165–172.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wong CY, Al-Salami H and Dass CR: C2C12
cell model: Its role in understanding of insulin resistance at the
molecular level and pharmaceutical development at the preclinical
stage. J Pharm Pharmacol. 72:1667–1693. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chan DA, Sutphin PD, Nguyen P, Turcotte S,
Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al:
Targeting GLUT1 and the Warburg effect in renal cell carcinoma by
chemical synthetic lethality. Sci Transl Med.
3(94ra70)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Adams DJ, Ito D, Rees MG, Seashore-Ludlow
B, Puyang X, Ramos AH, Cheah JH, Clemons PA, Warmuth M, Zhu P, et
al: NAMPT is the cellular target of STF-31-like small-molecule
probes. ACS Chem Biol. 9:2247–2254. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Garten A, Petzold S, Körner A, Imai S and
Kiess W: Nampt: Linking NAD biology, metabolism and cancer. Trends
Endocrinol Metab. 20:130–138. 2009.PubMed/NCBI View Article : Google Scholar
|