|
1
|
Gluz O, Liedtke C, Gottschalk N, Pusztai
L, Nitz U and Harbeck N: Triple-negative breast cancer-current
status and future directions. Ann Oncol. 20:1913–1927.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hudis CA and Gianni L: Triple-negative
breast cancer: An unmet medical need. Oncologist. 16 (Suppl
1):S1–S11. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Burstein HJ, Curigliano G, Loibl S, Dubsky
P, Gnant M, Poortmans P, Colleoni M, Denkert C, Piccart-Gebhart M,
Regan M, et al: Estimating the benefits of therapy for early-stage
breast cancer: The St. Gallen international consensus guidelines
for the primary therapy of early breast cancer 2019. Ann Oncol.
30:1541–1557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cardoso F, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S and Senkus E:
ESMO Guidelines Committee. Early breast cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 30:1194–1220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pusztai L, Foldi J, Dhawan A, DiGiovanna
MP and Mamounas EP: Changing frameworks in treatment sequencing of
triple-negative and HER2-positive, early-stage breast cancers.
Lancet Oncol. 20:e390–e396. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang M, O'Shaughnessy J, Zhao J,
Haiderali A, Cortés J, Ramsey SD, Briggs A, Hu P, Karantza V, Aktan
G, et al: Association of pathologic complete response with
long-term survival outcomes in triple-negative breast cancer: A
meta-analysis. Cancer Res. 80:5427–5434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sikov WM, Polley MY, Twohy E, Perou CM,
Singh B, Berry DA, Tolaney SM, Somlo G, Port ER, Ma CX, et al:
CALGB (alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant
chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in
triple-negative breast cancer (TNBC). J Clin Oncol. 37 (Suppl
15)(S591)2019.
|
|
9
|
Spring LM, Fell G, Arfe A, Trippa L,
Greenup R, Reynolds K, Smith BL, Moy B, Isakoff S, Parmigiani G and
Bardia A: Abstract GS2-03: Pathological complete response after
neoadjuvant chemotherapy and impact on breast cancer recurrence and
mortality, stratified by breast cancer subtypes and adjuvant
chemotherapy usage: Individual patient-level meta-analyses of over
27,000 patients. Cancer Res. 79 (Suppl 4):GS2–03. 2019.
|
|
10
|
European Medicines Agency: The role of the
pathological complete response as an endpoint in neoadjuvant breast
cancer studies, 2014 https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-role-pathological-complete-response-endpoint-neoadjuvant-breast-cancer-studies_en.pdf.
Accessed March 20, 2014.
|
|
11
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stockmans G, Deraedt K, Wildiers H,
Moerman P and Paridaens R: Triple-negative breast cancer. Curr Opin
Oncol. 20:614–620. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Collignon J, Lousberg L, Schroeder H and
Jerusalem G: Triple-negative breast cancer: Treatment challenges
and solutions. Breast Cancer (Dove Med Press). 8:93–107.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang F, Kemp CJ and Henikoff S:
Anthracyclines induce double-strand DNA breaks at active gene
promoters. Mutat Res. 773:9–15. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Poggio F, Bruzzone M, Ceppi M, Pondé NF,
La Valle G, Del Mastro L, de Azambuja E and Lambertini M:
Platinum-based neoadjuvant chemotherapy in triple-negative breast
cancer: A systematic review and meta-analysis. Ann Oncol.
29:1497–1508. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
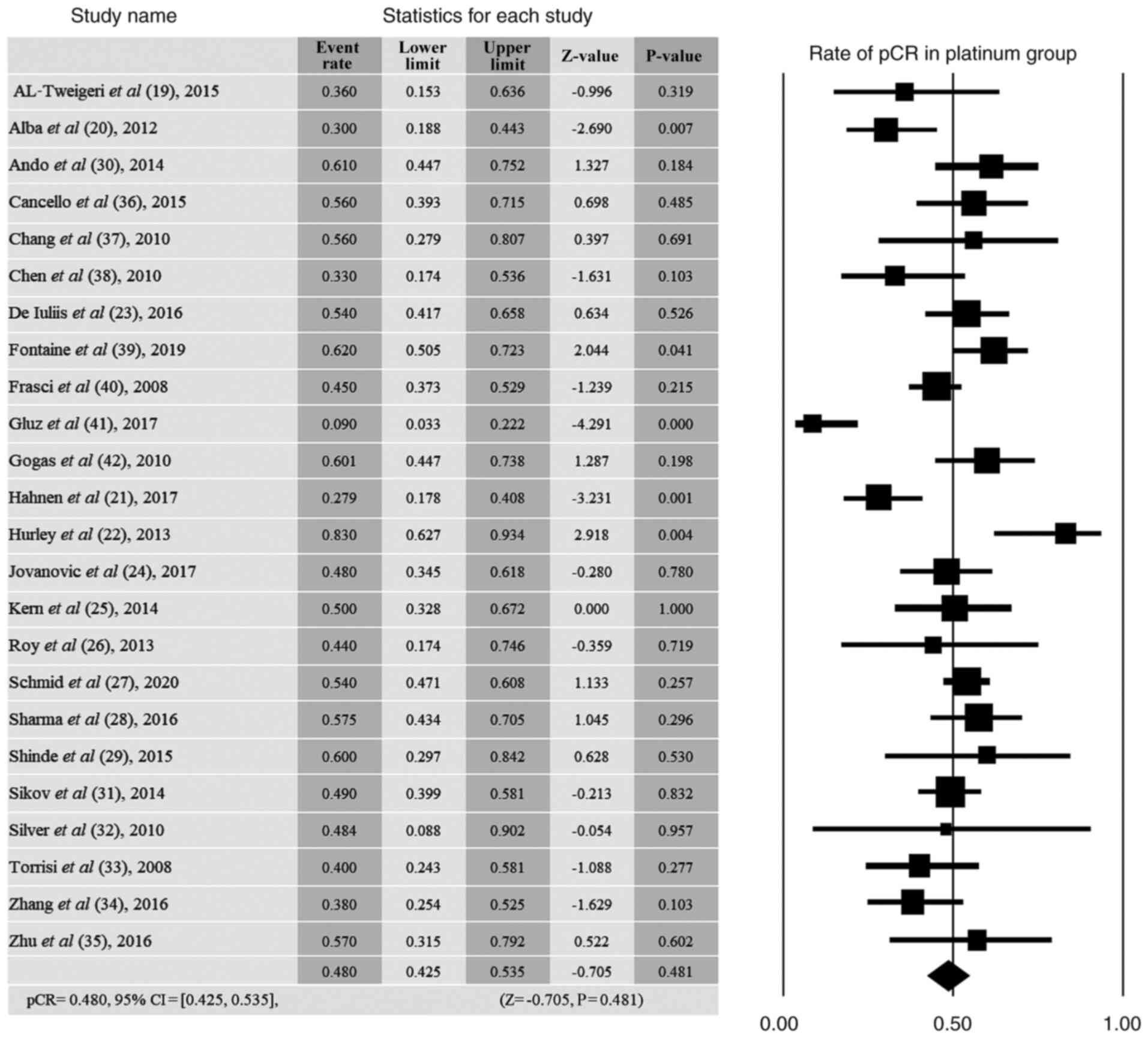
AL-Tweigeri T, AlSayed A, Alawadi S,
Ibrahim M, Ashour W, Jaafar H, Abulkhair O, AL-Abdulkarim H, Khalid
H and Ajarim D: Gulf Oncology Research Group (GORG-001). A
multicenter prospective phase II trial of neoadjuvant epirubicin,
cyclophosphamide, and 5-fluorouracil (FEC100) followed by
cisplatin-docetaxel with or without trastuzumab in locally advanced
breast cancer. Cancer Chemother Pharmacol. 77:147–153.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alba E, Chacon JI, Lluch A, Anton A,
Estevez L, Cirauqui B, Carrasco E, Calvo L, Segui MA, Ribelles N,
et al: A randomized phase II trial of platinum salts in basal-like
breast cancer patients in the neoadjuvant setting. Results from the
GEICAM/2006-03, multicenter study. Breast Cancer Res Treat.
136:487–493. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hahnen E, Lederer B, Hauke J, Loibl S,
Kröber S, Schneeweiss A, Denkert C, Fasching PA, Blohmer JU,
Jackisch C, et al: Germline mutation status, pathological complete
response, and disease-free survival in triple-negative breast
cancer: Secondary analysis of the GeparSixto randomized clinical
trial. JAMA Oncol. 3:1378–1385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hurley J, Reis IM, Rodgers SE,
Gomez-Fernandez C, Wright J, Leone JP, Larrieu R and Pegram MD: The
use of neoadjuvant platinum-based chemotherapy in locally advanced
breast cancer that is triple negative: Retrospective analysis of
144 patients. Breast Cancer Res Treat. 138:783–794. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
De Iuliis F, Salerno G, Corvino R,
D'Aniello D, Cefalì K, Taglieri L, Lanza R and Scarpa S:
Anthracycline-free neoadjuvant chemotherapy ensures higher rates of
pathologic complete response in breast cancer. Clin Breast Cancer.
17:34–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jovanovic B, Mayer IA, Mayer EL, Abramson
VG, Bardia A, Sanders ME, Kuba MG, Estrada MV, Beeler JS, Shaver
TM, et al: A randomized phase II neoadjuvant study of cisplatin,
paclitaxel with or without everolimus in patients with stage II/III
triple-negative breast cancer (TNBC): Responses and long-term
outcome correlated with increased frequency of DNA damage response
gene mutations, TNBC subtype, AR status, and Ki67. Clin Cancer Res.
23:4035–4045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kern P, Kalisch A, von Minckwitz G, Pütter
C, Kolberg HC, Pott D, Kurbacher C, Rezai M and Kimmig R:
Neoadjuvant, anthracycline-free chemotherapy with carboplatin and
docetaxel in triple-negative, early-stage breast cancer: A
multicentric analysis of rates of pathologic complete response and
survival. J Chemother. 28:210–217. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roy V, Pockaj BA, Allred JB, Apsey H,
Northfelt DW, Nikcevich D, Mattar B and Perez EA: A phase II trial
of docetaxel and carboplatin administered every 2 weeks as
preoperative therapy for stage II or III breast cancer: NCCTG study
N0338. Am J Clin Oncol. 36:540–544. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sharma P, López-Tarruella S, García-Saenz
JA, Ward C, Connor CS, Gómez HL, Prat A, Moreno F, Jerez-Gilarranz
Y, Barnadas A, et al: Efficacy of neoadjuvant carboplatin plus
docetaxel in triple-negative breast cancer: Combined analysis of
two cohorts. Clin Cancer Res. 23:649–657. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shinde AM, Zhai J, Yu KW, Frankel P, Yim
JH, Luu T, Kruper L, Vito C, Shaw S, Vora NL, et al: Pathologic
complete response rates in triple-negative, HER2-positive, and
hormone receptor-positive breast cancers after anthracycline-free
neoadjuvant chemotherapy with carboplatin and paclitaxel with or
without trastuzumab. Breast. 24:18–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ando M, Yamauchi H, Aogi K, Shimizu S,
Iwata H, Masuda N, Yamamoto N, Inoue K, Ohono S, Kuroi K, et al:
Randomized phase II study of weekly paclitaxel with and without
carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil
as neoadjuvant chemotherapy for stage II/IIIA breast cancer without
HER2 overexpression. Breast Cancer Res Treat. 145:401–409.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sikov WM, Berry DA, Perou CM, Singh B,
Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER,
et al: Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(alliance). J Clin Oncol. 33:13–21. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Silver DP, Richardson AL, Eklund AC, Wang
ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, et
al: Efficacy of neoadjuvant cisplatin in triple-negative breast
cancer. J Clin Oncol. 28:1145–1153. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Torrisi R, Balduzzi A, Ghisini R, Rocca A,
Bottiglieri L, Giovanardi F, Veronesi P, Luini A, Orlando L, Viale
G, et al: Tailored preoperative treatment of locally advanced
triple negative (hormone receptor negative and HER2 negative)
breast cancer with epirubicin, cisplatin, and infusional
fluorouracil followed by weekly paclitaxel. Cancer Chemother
Pharmacol. 62:667–672. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang P, Yin Y, Mo H, Zhang B, Wang X, Li
Q, Yuan P, Wang J, Zheng S, Cai R, et al: Better pathologic
complete response and relapse-free survival after carboplatin plus
paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant
chemotherapy for locally advanced triple-negative breast cancer: A
randomized phase 2 trial. Oncotarget. 7:60647–60656.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu T, Liu CL, Zhang YF, Liu YH, Xu FP, Zu
J, Zhang GC, Li XR, Liao N and Wang K: A phase II trial of
dose-dense (biweekly) paclitaxel plus carboplatin as neoadjuvant
chemotherapy for operable breast cancer. Breast Cancer Res Treat.
156:117–124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cancello G, Bagnardi V, Sangalli C,
Montagna E, Dellapasqua S, Sporchia A, Iorfida M, Viale G, Barberis
M, Veronesi P, et al: Phase II study with epirubicin, cisplatin,
and infusional fluorouracil followed by weekly paclitaxel with
metronomic cyclophosphamide as a preoperative treatment of
triple-negative breast cancer. Clin Breast Cancer. 15:259–265.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chang HR, Glaspy J, Allison MA, Kass FC,
Elashoff R, Chung DU and Gornbein J: Differential response of
triple-negative breast cancer to a docetaxel and carboplatin-based
neoadjuvant treatment. Cancer. 116:4227–4237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu
JS, Shao ZM, Shen ZZ and Shen KW: Weekly paclitaxel plus
carboplatin is an effective nonanthracycline-containing regimen as
neoadjuvant chemotherapy for breast cancer. Ann Oncol. 21:961–967.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fontaine C, Renard V, Van den Bulk H,
Vuylsteke P, Glorieux P, Dopchie C, Decoster L, Vanacker L, de
Azambuja E, De Greve J, et al: Weekly carboplatin plus neoadjuvant
anthracycline-taxane-based regimen in early triple-negative breast
cancer: A prospective phase II trial by the breast cancer task
force of the Belgian society of medical oncology (BSMO). Breast
Cancer Res Treat. 176:607–615. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Frasci G, Comella P, Rinaldo M, Iodice G,
Di Bonito M, D'Aiuto M, Petrillo A, Lastoria S, Siani C, Comella G
and D'Aiuto G: Preoperative weekly cisplatin-epirubicin-paclitaxel
with G-CSF support in triple-negative large operable breast cancer.
Ann Oncol. 20:1185–1192. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gluz O, Nitz U, Liedtke C, Christgen M,
Grischke EM, Forstbauer H, Braun M, Warm M, Hackmann J, Uleer C, et
al: Comparison of neoadjuvant nab-paclitaxel+carboplatin vs
nab-paclitaxel+gemcitabine in triple-negative breast cancer:
Randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst.
110:628–637. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gogas H, Pectasides D, Kostopoulos I,
Lianos E, Skarlos D, Papaxoinis G, Bobos M, Kalofonos HP, Petraki
K, Pavlakis K, et al: Paclitaxel and carboplatin as neoadjuvant
chemotherapy in patients with locally advanced breast cancer: A
phase II trial of the hellenic cooperative oncology group. Clin
Breast Cancer. 10:230–237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Petrelli F, Coinu A, Borgonovo K, Cabiddu
M, Ghilardi M, Lonati V and Barni S: The value of platinum agents
as neoadjuvant chemotherapy in triple-negative breast cancers: A
systematic review and meta-analysis. Breast Cancer Res Treat.
144:223–232. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pandy JGP, Balolong-Garcia JC,
Cruz-Ordinario MVB and Que FVF: Triple negative breast cancer and
platinum-based systemic treatment: A meta-analysis and systematic
review. BMC Cancer. 19(1065)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lokich J and Anderson N: Carboplatin
versus cisplatin in solid tumors: An analysis of the literature.
Ann Oncol. 9:13–21. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Greenup R, Buchanan A, Lorizio W, Rhoads
K, Chan S, Leedom T, King R, McLennan J, Crawford B, Kelly Marcom P
and Shelley Hwang E: Prevalence of BRCA mutations among women with
triple-negative breast cancer (TNBC) in a genetic counseling
cohort. Ann Surg Oncol. 20:3254–3258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Caramelo O, Silva C, Caramelo F, Frutuoso
C and Almeida-Santos T: The effect of neoadjuvant platinum-based
chemotherapy in BRCA mutated triple negative breast
cancers-systematic review and meta-analysis. Hered Cancer Clin
Pract. 17(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang CJ, Xu Y, Lin Y, Zhu HJ, Zhou YD, Mao
F, Zhang XH, Huang X, Zhong Y, Sun Q and Li CG: Platinum-based
neoadjuvant chemotherapy for breast cancer with BRCA mutations: A
meta-analysis. Front Oncol. 10(592998)2020.PubMed/NCBI View Article : Google Scholar
|