Introduction
Inflammatory bowel disease (IBD) is characterized by
an array of symptoms that cause inflammation of the
gastrointestinal tract and affect millions of people annually
(1). The most prevalent forms of
IBD are Crohn's disease and ulcerative colitis (UC), which are
considered recurring illnesses that cause serious health issues
(2,3). UC is an idiopathic condition of
colonic mucosa characterized by symptoms of severe abdominal pain
and bloody diarrhea (4). The
mucosal ulceration can proximally extend from rectum and thus
affects the entire colon (5). It is
important to determine the pathogenesis of UC and as its incidence
continues to increase worldwide (6). Recent advancements in UC research have
unveiled the role of long non-coding (lnc)RNAs in controlling gene
expression and epigenetics (7,8).
lncRNAs are ~200 nucleotides in length and commonly lack open
reading frames that are translated into proteins (9). Aberrant expression of lncRNAs has been
reported in several diseases, and their differential expression has
been reported in various inflammatory conditions, including
rheumatoid arthritis, osteoarthritis and asthma (10,11).
However, atypical levels of lncRNAs in UC have not yet been
investigated.
Inflammatory diseases have been functionally
characterized by upregulated levels of various inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-23, IL-12 and interferon-AS1 (IFNG-AS1), which are regulated
by lncRNAs (12,13). For example, overexpression of lncRNA
H19 directly targets the intestinal epithelial barrier function,
resulting in the development of UC (14). Similarly, IFNG-AS1 has been reported
to regulate interferon-γ inflammatory responses and contributes to
UC progression (15). A recent
study reported that ANRIL suppression may inhibit the development
of UC by regulating the miR-323b-5p/toll-like receptor 4/myeloid
differentiation primary response 88/NF-κB pathway (16). This indicates that lncRNAs can
regulate the progress of UC through a specific mechanism.
lncRNA nuclear enriched abundant transcript 1
(NEAT1) plays a crucial role in the formation of paraspeckles
(17). NEAT1 expression is
upregulated in liver diseases and different types of cancer
(18). Recently, its aberrant
expression has been reported in hepatocellular carcinoma and
fibrosis of pulmonary epithelial cells (19,20).
However, its role in UC remains unclear. Thus, it is important to
determine the underlying molecular mechanisms of lncRNA NEAT1 in
the development and progression of UC.
The present study aimed to investigate the role of
NEAT1 in UC development and determine its functional mechanism on
lipopolysaccharide (LPS)-induced injury in FHCs.
Materials and methods
Tissue samples
The clinicopathological characteristics and medical
status of all patients are presented in Table I. Tissue samples were collected from
30 patients with UC (17 males and 13 females; age range, 39-67
years; mean age, 56.1±7.8 years) and 30 healthy individuals (19
males and 11 females; age range, 42-66 years; mean age, 55.3±6.6
years) at Shanghai Tenth People's Hospital, Clinical Medical
College of Nanjing Medical University from June 2017 to October
2019. Patients were included in the current study if they had: No
other obvious complications and no therapeutic intervention applied
prior to enrolment. The exclusion criteria were as follows: i)
Patients transferred from other hospitals and ii) patients who were
diagnosed with multiple clinical disorders, such as bacterial
dysentery and intestinal tuberculosis. Following pathological
analysis, a colonic mucosa pinch biopsy of the sigmoid colon was
performed on all patients and the samples were immediately
snap-frozen in liquid nitrogen at -80˚C until subsequent
experimentation. The present study was approved by the Ethics
Committee of the Affiliated Changzhou No. 2 People's Hospital of
Nanjing Medical University (Jiangsu, China; approval no.
IR-B-2019-11-14) and written informed consent was provided by all
participants prior to the study start.
 | Table ICharacteristics of healthy
individuals and patients with UC. |
Table I
Characteristics of healthy
individuals and patients with UC.
| Characteristic | Healthy | UC |
|---|
| Number of
patients | 30 | 30 |
| Age (mean ± SD),
years | 55.3±6.6 | 56.1±7.8 |
| Age range,
years | 48-62 | 46-64 |
| Sex (male vs.
female) | 19 vs. 11 | 17 vs. 13 |
| Duration of
symptoms, months | NA | 14 |
| Medication | | |
|
5-ASA | 0 | 30 |
|
Antibiotics | 0 | 5 |
|
Steroids | 0 | 7 |
|
Immunomodulators | 0 | 2 |
|
Biologics | 0 | 1 |
Cell culture and treatment
UC is characterized by abnormal colonic epithelial
cells (21). Thus, colonic
epithelial FHCs treated with LPS were used as a cell model of UC,
as previously described (16,22).
To induce cell injury, FHCs were purchased from the American Type
Culture Collection and maintained in an equal proportion of (1:1)
DMEM (Gibco; Thermo Fisher Scientific, Inc.) and Ham F-12 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10 ng/ml
cholera toxin (Calbiochem; Merck KGaA), 25 mmol/l HEPES
[N-(2-hydroxyeth-yl) piperazine-N0 (2-ethanesulfonic acid)], 0.005
mg/ml transferrin, 100 ng/ml hydrocortisone (all from
Sigma-Aldrich; Merck KGaA), 0.005 mg/ml insulin and 10% fetal
bovine serum (GE Healthcare). Cell injury was induced following
treatment of FHCs with 0, 5, 10 and 20 ng/ml LPS (Beijing Solarbio
Science & Technology, Co., Ltd.) in accordance with a
previously described method (20).
Cell transfection
MicroRNA (miRNA/miR)-603 mimics/inhibitors (50 nM,
respectively), negative control miRNA mimics/inhibitor (miR-NC
mimics/miR-NC inhibitor), small interfering (si)-NEAT1 and si-NC
were purchased from Shanghai GeneChem Co., Ltd. The overexpression
vector pcDNA-fibroblast growth factor 9 (FGF9) was constructed by
inserting an amplified FGF9 at the HindIII and EcoRI sites of the
pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.), and
an empty vector was used as a control. Sequences of the constructed
plasmids were confirmed via Sanger sequencing (Sangon Biotech Co.,
Ltd.).
For cell transfection, FHCs were seeded into 6-well
plates and cultured until they reached 60-70% confluence.
Subsequently, cells were transfected with 4 µg overexpression
vector using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 15 min, according
to the manufacturer's instructions (11). The following sequences were used:
si-NEAT1, 5'-GGAGUCAUGCCUUAUACAATT-3'; si-NC,
5'-CAACAAGATGAAGAGCACCAA-3'; miR-603 mimics,
5'-CACACACUGCAAUUACUUUUGC-3'; miR-NC mimics,
5'-UUCUCCGAACGUGUCACGUTT-3'; mi-603 inhibitor,
5'-GCAAAAGUAAUUGCAGUGUGUG-3' and miR-NC inhibitor,
5'-CGAACGUGUCACGUTT-3'. Cells were collected 48 h post-transfection
and used for subsequent experimentation 72 h post-transfection.
MTT bioassay
For the MTT bioassay, FHCs were seeded into 96-well
plates at a density of 10,000 cells/well for further treatment.
Cells were treated with 500 mg/ml of MTT reagent (Sigma-Aldrich;
Merck KGaA) and incubated for 3 h at 37˚C. Following the MTT
incubation, the purple formazan crystals were dissolved using 200
ml dimethyl sulfoxide solution (Sigma-Aldrich; Merck KGaA) and
absorbance was measured at a wavelength of 490 nm, using a
microplate reader (Bio-Rad Laboratories, Inc.) (23).
Apoptosis analysis
For apoptosis analysis, FHCs were seeded into
96-well plates at a density of 10,000 cells/well for further
treatment. Cells were subsequently stained with propidium iodide
(PI) and FITC-Annexin V, using the FITC-Annexin V Apoptosis
Detection kit (BD Biosciences) for 30 min at 37˚C. Apoptotic cells
were subsequently analyzed using a BD FACS Calibur flow cytometer
(BD Biosciences) and FlowJo 7.6.1 software (Tree Star) (24).
Analysis of inflammatory
cytokines
The number of inflammatory cytokines, including
IL-1β (cat. no. E-EL-H0109c), TNF-a (cat. no. E-EL-H0149c), IL-6
(cat. no. E-EL-H0192c) and IL-8 (cat. no. E-EL-H6008), that were
released following infection was quantified using ELISA (Elascience
Biotechnology, Inc.) in LPS induced FHCs, according to the
manufacturer's protocol.
Bioinformatics analysis
Bioinformatics analysis was performed using StarBase
v2.0 and TargetScan (http://starbase.sysu.edu.cn/), which revealed that the
miR-603 binding sites in NEAT1 and FGF9 are potential targets for
miR-603.
Dual-luciferase reporter assay
NEAT1 and FGF9 wild-type (WT) (NEAT1-WT and FGF9-WT)
and mutant type (NEAT1-MUT and FGF9-Mut) reporter vectors were
constructed by Beijing Transgen Biotech Co., Ltd. The reporter
plasmids (GP-mirGLO) were co-transfected into FHCs with either
miR-603 mimics or NC, using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following
transfection for 36 h at 37˚C, cells were lysed and relative
luciferase activities were detected using a dual-luciferase
reporter system (Promega Corporation) following 36 h of incubation.
Relative luciferase activity was normalized to that of
Renilla luciferase as previously described (25).
Reverse transcription-quantitative
(RT-q) PCR
Cellular RNA was extracted from FHC cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNA using the Reverse
Transcription (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and Bio-Rad select cDNA synthesis (Qiagen, Inc.) kits for 60 min at
37˚C. Relative NEAT1 and FGF9 expression levels were quantified
using the Takara relative fluorescence quantification kit (One Step
TB Green® PrimeScript™ RT-PCR kit, Takara Bio, Inc.).
The following primer sequences were used for qPCR: NEAT1 forward,
5'-CCTAGCATGTTTGACAGGCG-3' and reverse,
5'-TGCCACCTGGAAAATAAAGCG-3'; FGF9 forward,
5'-GAAAGACCACAGCCGATTTG-3' and reverse, 5'-TTCATCCCGAGGTAGAGTCC-3';
and GAPDH forward, 5'-CCCACTCCTCCACCTTTGAC-3' and reverse,
5'-CATACCAGGAAATGAGCTTGACAA-3'; miR-603 forward,
5'-CAGCACACACTGCAATTAC-3' and reverse,
5'-GTCCAGTTTTTTTTTTTTTTTGCAA-3'; U6 forward,
5'-GCGCGTCGTGAAGCGTTC-3' and reverse,
5'-GTGCAGGGTCCGAGGT-3'.miR-603 expression was quantified by using
TaqMan Universal Master Mix II with TaqMan microRNA assays, in
which U6 served as the internal control. The thermocycling
conditions were as follows: Pre-denaturation at 95˚C for 1 min,
followed by 35 cycles of denaturation for 10 sec, 60˚C for 20 sec,
72˚C for 10 sec (25). The relative
expression levels were calculated using the 2-ΔΔCq
method (26).
Western blotting
Total cellular proteins from LPS-induced FHCs cells
were extracted after FHC lysates were generated using RIPA buffer
(Santa Cruz Biotechnology, Inc.) for 30 min. Protein concentration
was then determined using a Nanodrop Nd-1000 UV-Vis
Spectrophotometer (Nanodrop Technologies; Thermo Fisher Scientific,
Inc.). Next, 30 µg protein samples with equal amounts were resolved
using 10% SDS-PAGE, transferred onto PVDF membranes
(MilliporeSigma) and blocked with 5% non-fat skim milk/TBST at room
temperature for 2 h. Membranes were incubated with primary
antibodies (all, 1:1,000) against β-actin (cat. no. ab8226), Bax
(cat. no. ab32503), caspase 3 (cat. no. ab32351), caspase 9 (cat.
no. ab32539) and FGF9 (cat. no. ab206408) purchased from Abcam
overnight at 4˚C. Following the primary incubation, membranes were
incubated with secondary HRP-conjugated rabbit IgG antibodies (cat.
no. ab6702; 1:2,000; Abcam) (20),
and protein bands were visualized using chemiluminescence reagents
(Pierce; Thermo Fisher Scientific, Inc.). Quantity One software
(version 4.3.0; Bio-Rad Laboratories, Inc.) was used for
densitometric analysis.
Statistical analysis
Data were presented as the mean ± SD and analyzed
using SPSS 22.0 software (IBM Corp.). All experiments were
performed in triplicate and data are presented as the mean ±
standard deviation. Paired and unpaired Student's t-test were used
to compare differences between two groups, while one-way ANOVA with
Tukey's post hoc test was used to compare differences between
multiple groups. Pearson's correlation analysis was performed to
assess the correlation between NEAT1 and miR-603 expression.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NEAT1 expression is upregulated in
patients with UC
RT-qPCR analysis was performed to detect relative
NEAT1 expression in UC tissues of patients and healthy individuals.
The results demonstrated that NEAT1 expression was significantly
upregulated in patients with UC, suggesting its potential role in
UC development (P<0.01; Fig.
1A). FHCs were treated with different concentrations of LPS (0,
5, 10 and 20 ng/ml) for 2 h to stimulate FHCs. RT-qPCR analysis
demonstrated that NEAT1 expression significantly increased in
relation to increased LPS concentration (P<0.01; Fig. 1B). Therefore, 20 ng/ml LPS was used
to induce cells for further study. RT-qPCR analysis was also
performed to detect NEAT1 expression at different time points (0,
1, 2 and 4 h) following treatment with 20 ng/ml LPS. The results
demonstrated that NEAT1 expression significantly increased overtime
following treatment with LPS, suggesting the potential involvement
of NEAT1 in the development of UC (P<0.01; Fig. 1C). The results demonstrated that
NEAT1 played an indispensable role in UC.
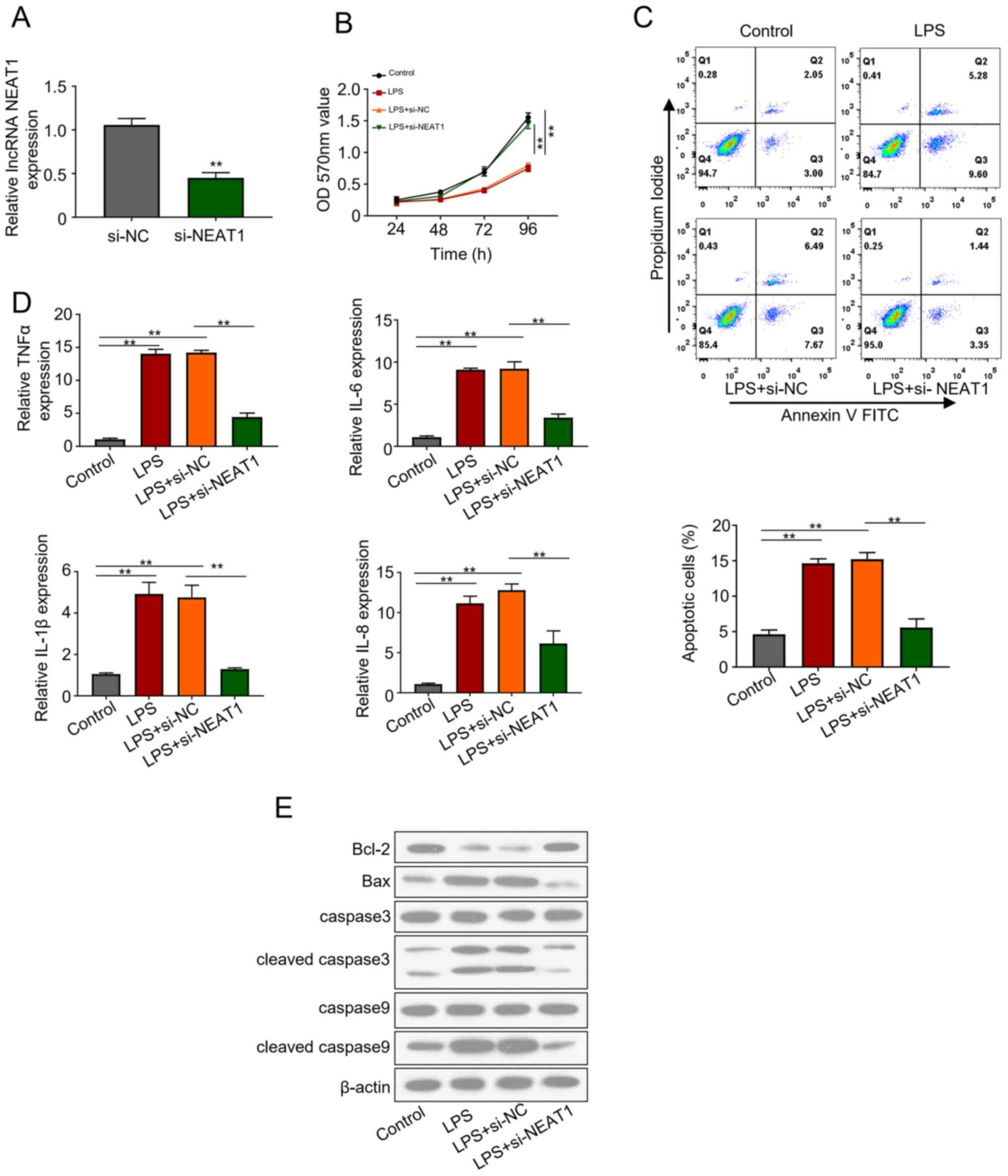
NEAT1 knockdown induces cell
viability, and suppresses cell apoptosis and the production of
cytokines in LPS-stimulated FHCs
The effects of NEAT1 knockdown on FHCs were
evaluated via transfection with si-lncRNA NEAT1 plasmid. Knockdown
efficiency of si-NEAT1 plasmid was assessed 48 h post-transfection,
which demonstrated that NEAT1 expression significantly decreased in
the transfected cells compared with the control group (P<0.01;
Fig. 2A). si-NEAT1 transfected FHCs
were treated with 20 ng/ml LPS for 2 h. The MTT bioassay was
performed to assess the viability of the control, LPS, LPS+ si-NC
and LPS + si-NEAT1 groups, revealing that si-NEAT1 significantly
recovered the decreased cell activity induced by LPS (P<0.01;
Fig. 2B). In addition, LPS treated
FHCs transfected with si-NEAT1 significantly attenuated apoptosis
levels compared with the other groups (P<0.01; Fig. 2C). Similarly, ELISA emphasized the
increased production of inflammatory cytokines, IL-1β, IL-6, IL-8
and TNFα, in LPS treated cells. However, NEAT1 knockdown in the LPS
+ si-NEAT1 group significantly inhibited cell apoptosis and
restored cell viability (P<0.01; Fig. 2D). Western blot analysis
demonstrated the notable downregulation of apoptosis regulator,
including Bax and cleaved caspase 3 and caspase 9, in FHCs
transfected with LPS + si-NEAT1, which was previously upregulated
following treatment with LPS (Fig.
2E). The results revealed that NEAT1 knockdown increased cell
viability, decreased cell apoptosis and inhibited the production of
cytokines in LPS-stimulated FHCs.
NEAT1 knockdown alleviates LPS-induced
cell injury by upregulating miR-603 expression
Bioinformatics analysis revealed that NEAT1 is a
potential target of miR-603, which was further confirmed by
generating NEAT1 mutant (Fig. 3A).
RT-qPCR analysis was performed to detect miR-603 expression in
si-NEAT1 transfected FHCs. The results demonstrated that miR-603
expression increased in the si-NEAT1 group compared with the
control group (P<0.01; Fig.
3B).
The dual-luciferase activity of miR-603 mimics was
significantly lower in cells co-transfected with miR-603 mimics +
NEAT1-WT compared with miR-NC + NEAT1-WT (P<0.01; Fig. 3C). Notably, no significant
difference was observed between NEAT1 mutant co-transfected with
miR-603 mimics or miR-NC (P<0.01; Fig. 3C). The results demonstrated that
miR-603 expression was upregulated in healthy individuals compared
with patients with UC (P<0.01; Fig.
3D). In addition, Pearson's correlation analysis demonstrated
that NEAT1 expression was negatively correlated with miR-603
expression (Fig. 3E). Notably,
miR-603 expression significantly decreased in FHCs treated with
LPS, in a concentration-dependent manner (P<0.01; Fig. 3F). Similarly, incubation with LPS
significantly decreased miR-603 expression in a time-dependent
manner (P<0.01; Fig. 3G). The
present study also investigated miR-603 expression in FHCs
transfected with miR-603 mimics and miR-603 inhibitor via RT-qPCR
analysis (Fig. S1). The results
demonstrated that miR-603 expression increased in cells transfected
with miR-603 mimics, the effects of which were reversed following
transfection with miR-603 inhibitor. The results demonstrated that
NEAT1 knockdown alleviated LPS-induced cell injury by upregulating
miR-603 expression levels.
miR-603 negatively regulates FGF9
expression and FGF9 is a target of miR-603
Potential binding sites of miR-603 in FGF9
(3'-untranslated region) were identified using the TargetScan
online database (Fig. 4A).
Co-transfection with miR-603 mimics and FGF9-WT significantly
decreased luciferase activity, while no significant difference was
observed in luciferase activity following co-transfection with
miR-603 mimics and FGF9-MUT, confirming that FGF9 is a target of
miR-603 (P<0.01; Fig. 4B).
Relative FGF9 expression was based on the transfection of FHCs with
LPS, LPS + si-NEAT1, LPS + si-NEAT1 + miR-603 inhibitor and LPS +
si-NEAT1 + FGF9. The results suggest that NEAT1 acts as a sponge
for FGF9 and treatment with LPS + si-NEAT1 significantly decreased
FGF9 expression compared with the other treatments (P<0.01;
Fig. 4C). Similarly, western blot
analysis demonstrated that treatment with LPS + si-NEAT1 notably
decreased FGF9 expression (Fig.
4C). The results of the MTT bioassay demonstrated that cell
viability was significantly enhanced in cells in the control and
LPS + si-NEAT1 groups compared with the other groups (LPS, LPS +
si-NEAT1 + miR-603 inhibitor and LPS + si-NEAT1 + FGF9 groups
(P<0.01; Fig. 4D). The levels of
inflammatory cytokines, including IL-1β, IL-6, IL-8 and TNFα,
significantly increased in LPS treated FHCs, but significantly
decreased following treatment with LPS + si-NEAT1 (P<0.01;
Fig. 4E). The results revealed that
lncRNA NEAT1 knockdown relieved UC inflammation by regulating the
miR-603/FGF9 pathway.
 | Figure 4miR-603 negatively regulates FGF9
expression and FGF9 is a target of miR-603. (A) Bioinformatics
analysis of miR-603 binding sites in FGF9. (B) Relative luciferase
activity of FHCs co-transfected with miR-NC, miR-603 mimics, and
FGF9-WT and FGF9-MUT. (C) Relative FGF9 expression in FHCs in the
different treatment groups. (D) The MTT bioassay was performed to
assess cell viability in the different treatment groups. (E)
Expression levels of different cytokines in LPS-treated FHCs. All
experiments were performed in triplicate. **P<0.01
vs. miR-NC. miR, microRNA; FGF9, fibroblast growth factor 9; FHCS,
fetal human cells; NC, negative control; WT, wild-type; MUT,
mutant; LPS, lipopolysaccharide; OD, optical density; TNF, tumor
necrosis factor; IL, interleukin. |
Discussion
Despite recent efforts, the treatment and diagnosis
of IBD remains a challenge in clinical studies (27,28).
Thus, recent studies have aimed to investigate the underlying
molecular mechanisms of IBD development and disease progression
(29-31).
lncRNAs are critical diagnostic and therapeutic biomarkers, which
act as competitive endogenous RNAs (32). These competitive endogenous RNAs
regulate gene expression and sponge miRNAs (33,34).
Recently, the role and differential expression of lncRNAs have been
investigated in the development of UC and IBD (10,35). A
total of 455 differentially expressed lncRNAs were detected in
patients with UC compared with the control group, and BC012900 was
demonstrated to modulate epithelial cell apoptosis (36). Similarly, the potential of
differentially expressed lncRNA IFNG-AS1 was validated in IBD
pathophysiology (15).
The present study aimed to investigate the
differential expression of NEAT1 in UC progression and development.
The results demonstrated that NEAT1 expression was upregulated in
patients with UC compared with healthy individuals, which is
consistent with the results of a study by Pan et al
(37) in which LncRNA NEAT1
mediated intestinal inflammation by regulating TNFRSF1B.
Mechanistically, inhibition of NEAT1 significantly decreased injury
in FHCs treated with LPS. NEAT1 also plays a role in inflammation
by regulating different signaling pathways. For example, NEAT1
ameliorates LPS-induced inflammation in MG63 cells by activating
autophagy and suppressing the NLRP3 inflammasome (38). Furthermore, NEAT1 acts as a key
regulator of cell apoptosis and the inflammatory response via the
miR-944/TRIM37 axis in acute lung injury (39). Furthermore, FGF9 was identified as a
direct target of miR-603, and miR-603 expression was negatively
correlated with FGF9. Taken together, these results suggest that
high NEAT1 expression plays a key role in the progression of UC,
and its association in the development of other diseases was
speculated.
NEAT1 expression is upregulated in glioma, and NEAT1
knockdown is associated with inhibition of cell invasion and
migration (25). In addition, NEAT1
contributes to the development of hepatocellular carcinoma by
enhancing STAT3 expression and sponging miR-485(20). Furthermore, NEAT1 has been
implicated in the disease progression of breast cancer and fibrosis
of pulmonary epithelial cells (19,40).
Increasing evidence suggest that miR-603 may serve as a tumor
suppressor to inhibit tumorigenesis by targeting several signaling
enzymes. For example, miR-603 inhibits breast cancer by targeting
elongation factor-2 kinase (41).
Furthermore, miR-603 expression is downregulated in ovarian cancer
tissues compared with paracancerous tissues, and miR-603 targets
hexokinase-2 to inhibit the malignancy of ovarian cancer (42). Genome-wide analysis demonstrated
that miR-603 acts as O6-methylguanine methyl transferase
by regulating miRNAs in glioblastomas, suggesting its potential as
a therapeutic target in the treatment of different tumors (27).
FGF9 is involved in several processes in the human
body, including mitogenic and cell survival activities, tumor
growth, tissue repair and embryonic development (43,44).
The protein is associated with the modulation of Schwann cell
myelination in developing nerves and creating a pro-inflammatory
environment (45). In addition,
inhibition of apoptosis in the PI3K/AKT signaling pathway in
ischemia is associated with upregulated FGF9 expression (14,15,46).
To the best of our knowledge, the present study was the first to
investigate the development of UC by NEAT1 through the regulation
of the miR-603/FGF9 pathway. Given the potential of FGF9 as a
downstream ligand and an activator of the PI3K/AKT pathway, it can
modulate several inflammatory responses. For example, FGF9
negatively regulates bone mass by inhibiting osteogenesis and
promoting osteoclastogenesis via the MAPK and PI3K/AKT signaling
pathway (47).
In conclusion, the results of the present study
revealed that FGF9 is a direct target of miR-603 and demonstrated
that NEAT1 knockdown alleviates LPS-induced cell injury. Taken
together, these results suggest that NEAT1 knockdown can
potentially inhibit the progression and development of UC by
regulating the miR-603/FGF9 pathway, representing a promising
therapeutic target for UC treatment and diagnosis.
Supplementary Material
Relative miR-603 expression was
detected by reverse transcription-quantitative PCR. The results
revealed that miR-603 expression increased in FHCs co-transfected
with miR-NC mimics and miR-603 mimics. However, miR-603 expression
decreased in FHCs co-transfected with miR-NC inhibitor and miR-603
inhibitor. **P<0.01 vs. miR-NC mimics/inhibitor. miR,
microRNA; NC, negative control.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and ZL designed the current study. JF collected
the data. LF analysed the data. HL and HZ performed the
experiments. SL analyzed the data and drafted the manuscript. All
the authors revised and corrected the manuscript. All authors have
read and approved the final manuscript. FL and ZL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Changzhou No.2 People's Hospital of
Nanjing Medical University (Jiangsu, China; approval no.
IR-B-2019-11-14) and written informed consent was provided by all
participants prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feuerstein JD and Cheifetz AS: Ulcerative
colitis: Epidemiology, diagnosis, and management. Mayo Clin Proc.
89:1553–1563. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feagan BG, Rutgeerts P, Sands BE, Hanauer
S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese
S, et al: Vedolizumab as induction and maintenance therapy for
ulcerative colitis. N Engl J Med. 369:699–710. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ng SC, Shi HY, Hamidi N, Underwood FE,
Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et
al: Worldwide incidence and prevalence of inflammatory bowel
disease in the 21st century: A systematic review of
population-based studies. Lancet. 390:2769–2778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Galipeau HJ, Caminero A, Turpin W,
Bermudez-Brito M, Santiago A, Libertucci J, Constante M, Raygoza
Garay JA, Rueda G, Armstrong S, et al: Novel fecal biomarkers that
precede clinical diagnosis of ulcerative colitis. Gastroenterology.
160:1532–1545. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cleynen I, Boucher G, Jostins L, Schumm
LP, Zeissig S, Ahmad T, Andersen V, Andrews JM, Annese V and Brand
S: Inherited determinants of Crohn's disease and ulcerative colitis
phenotypes: A genetic association study. Lancet. 387:156–167.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Caballol B, Gudiño V, Panes J and Salas A:
Ulcerative colitis: Shedding light on emerging agents and
strategies in preclinical and early clinical development. Expert
Opin Investig Drugs. 30:931–946. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carpenter S, Aiello D, Atianand MK, Ricci
EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M and Lawrence
JB: A long noncoding RNA mediates both activation and repression of
immune response genes. Science. 341:789–792. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chu C, Qu K, Zhong FL, Artandi SE and
Chang HY: Genomic maps of long noncoding RNA occupancy reveal
principles of RNA-chromatin interactions. Mol Cell. 44:667–678.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Constanty F and Shkumatava A: lncRNAs in
development and differentiation: From sequence motifs to functional
characterization. Development. 148(dev182741)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mirza AH, Berthelsen CH, Seemann SE, Pan
X, Frederiksen KS, Vilien M, Gorodkin J and Pociot F:
Transcriptomic landscape of lncRNAs in inflammatory bowel disease.
Genome Med. 7(39)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiao YQ, Huang ML, Xu AT, Zhao D, Ran ZH
and Shen J: LncRNA DQ786243 affects Treg related CREB and Foxp3
expression in Crohn's disease. J Biomed Sci. 20(87)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Neurath MF: Cytokines in inflammatory
bowel disease. Nat Rev Immunol. 14:329–342. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Nielsen OH and Ainsworth MA: Tumor
necrosis factor inhibitors for inflammatory bowel disease. N Engl J
Med. 369:754–762. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen SW, Wang PY, Liu YC, Sun L, Zhu J,
Zuo S, Ma J, Li TY, Zhang JL, Chen GW, et al: Effect of long
noncoding RNA H19 overexpression on intestinal barrier function and
its potential role in the pathogenesis of ulcerative colitis.
Inflamm Bowel Dis. 22:2582–2592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Padua D, Mahurkar-Joshi S, Law IK,
Polytarchou C, Vu JP, Pisegna JR, Shih D, Iliopoulos D and
Pothoulakis C: A long noncoding RNA signature for ulcerative
colitis identifies IFNG-AS1 as an enhancer of inflammation. Am J
Physiol Gastrointest Liver Physiol. 311:G446–G457. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qiao C, Yang L, Wan J, Liu X, Pang C, You
W and Zhao G: Long noncoding RNA ANRIL contributes to the
development of ulcerative colitis by miR-323b-5p/TLR4/MyD88/NF-κB
pathway. Biochem Biophys Res Commun. 508:217–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Adriaens C, Standaert L, Barra J, Latil M,
Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W,
et al: p53 induces formation of NEAT1 lncRNA-containing
paraspeckles that modulate replication stress response and
chemosensitivity. Nat Med. 22:861–868. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Bu FT, Wang A, Zhu Y, You HM, Zhang YF,
Meng XM, Huang C and Li J: LncRNA NEAT1: Shedding light on
mechanisms and opportunities in liver diseases. Liver Int.
40:2612–2626. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu H, Chen Y, Zhuang J, Zhu S, Xu B and
Hong J: The role and mechanism of lncRNA NEAT1 in the fibrosis of
pulmonary epithelial cell. Mol Cell Toxicol. 16:185–191. 2020.
|
|
20
|
Zhang XN, Zhou J and Lu XJ: The long
noncoding RNA NEAT1 contributes to hepatocellular carcinoma
development by sponging miR-485 and enhancing the expression of the
STAT3. J Cell Physiol. 233:6733–6741. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tian Y, Cui L, Lin C, Wang Y, Liu Z and
Miao X: LncRNA CDKN2B-AS1 relieved inflammation of ulcerative
colitis via sponging miR-16 and miR-195. Int Immunopharmacol.
88(106970)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu M and Xie J: LncRNA MALAT1 promotes
ulcerative colitis by upregulating lncRNA ANRIL. Dig Dis Sci.
65:3191–3196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fu C, Li D, Zhang X, Liu N, Chi G and Jin
X: LncRNA PVT1 facilitates tumorigenesis and progression of glioma
via regulation of MiR-128-3p/GREM1 axis and BMP signaling pathway.
Neurotherapeutics. 15:1139–1157. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sang Y, Chen B, Song X, Li Y, Liang Y, Han
D, Zhang N, Zhang H, Liu Y, Chen T, et al: circRNA_0025202
regulates tamoxifen sensitivity and tumor progression via
regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther.
27:1638–1652. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou K, Zhang C, Yao H, Zhang X, Zhou Y,
Che Y and Huang Y: Knockdown of long non-coding RNA NEAT1 inhibits
glioma cell migration and invasion via modulation of SOX2 targeted
by miR-132. Mol Cancer. 17(105)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cao G, Tan B, Wei S, Shen W, Wang X, Chu
Y, Rong T and Gao C: Down-regulation of MBNL1-AS1 contributes to
tumorigenesis of NSCLC via sponging miR-135a-5p. Biomed
Pharmacother. 125(109856)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hodson R: Inflammatory bowel disease.
Nature. 540(S97)2016.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Rosen MJ, Dhawan A and Saeed SA:
Inflammatory bowel disease in children and adolescents. JAMA
Pediatr. 169:1053–1060. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu JW, Rouzigu A, Teng LH and Liu WL: The
construction and comprehensive analysis of inflammation-related
ceRNA networks and tissue-infiltrating immune cells in ulcerative
progression. Biomed Res Int. 2021(6633442)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li H, Xuan J, Zhang W, An Z, Fan X, Lu M
and Tian Y: Long non-coding RNA SNHG5 regulates ulcerative colitis
via microRNA-375/Janus kinase-2 axis. Bioengineered. 12:4150–4158.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ye M, Wang C, Zhu J, Chen M, Wang S, Li M,
Lu Y, Xiao P, Zhou M, Li X and Zhou R: An NF-κB-responsive long
noncoding RNA, PINT, regulates TNF-α gene transcription by
scaffolding p65 and EZH2. FASEB J. 35(e21667)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220(e202009045)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bochenek G, Häsler R, El Mokhtari NE,
König IR, Loos BG, Jepsen S, Rosenstiel P, Schreiber S and Schaefer
AS: The large non-coding RNA ANRIL, which is associated with
atherosclerosis, periodontitis and several forms of cancer,
regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 22:4516–4527.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guo F, Tang C, Li Y, Liu Y, Lv P, Wang W
and Mu Y: The interplay of LncRNA ANRIL and miR-181b on the
inflammation-relevant coronary artery disease through mediating
NF-κB signalling pathway. J Cell Mol Med. 22:5062–5075.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu F, Zikusoka M, Trindade A, Dassopoulos
T, Harris ML, Bayless TM, Brant SR, Chakravarti S and Kwon JH:
MicroRNAs are differentially expressed in ulcerative colitis and
alter expression of macrophage inflammatory peptide-2 alpha.
Gastroenterology. 135:1624–1635.e24. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu F, Huang Y, Dong F and Kwon JH:
Ulcerative colitis-associated long noncoding RNA, BC012900,
regulates intestinal epithelial cell apoptosis. Inflamm Bowel Dis.
22:782–795. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pan S, Liu R, Wu X, Ma K, Luo W, Nie K,
Zhang C, Meng X, Tong T, Chen X, et al: LncRNA NEAT1 mediates
intestinal inflammation by regulating TNFRSF1B. Ann Transl Med.
9(773)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dai W, Wang M, Wang P, Wen J, Wang J, Cha
S, Xiao X, He Y, Shu R and Bai D: lncRNA NEAT1 ameliorates
LPS-induced inflammation in MG63 cells by activating autophagy and
suppressing the NLRP3. Int J Mol Med. 47:607–620. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen C, Zhang H, Ge M, Ye J, Li R and Wang
D: LncRNA NEAT1 acts as a key regulator of cell apoptosis and
inflammatory response by the miR-944/TRIM37 axis in acute lung
injury. J Pharmacol Sci. 145:202–212. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pang Y, Wu J, Li X, Wang C, Wang M, Liu J
and Yang G: NEAT1/miR-124/STAT3 feedback loop promotes breast
cancer progression. Int J Oncol. 55:745–754. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bayraktar R, Pichler M, Kanlikilicer P,
Ivan C, Bayraktar E, Kahraman N, Aslan B, Oguztuzun S, Ulasli M,
Arslan A, et al: MicroRNA 603 acts as a tumor suppressor and
inhibits triple-negative breast cancer tumorigenesis by targeting
elongation factor 2 kinase. Oncotarget. 8:11641–11658.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lu J, Wang L, Chen W, Wang Y, Zhen S, Chen
H, Cheng J, Zhou Y, Li X and Zhao L: miR-603 targeted hexokinase-2
to inhibit the malignancy of ovarian cancer cells. Arch Biochem
Biophys. 661:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shamsi F, Xue R, Huang TL, Lundh M, Liu Y,
Leiria LO, Lynes MD, Kempf E, Wang CH, Sugimoto S, et al: FGF6 and
FGF9 regulate UCP1 expression independent of brown adipogenesis.
Nat Commun. 11(1421)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li YH, Chen TM, Huang BM, Yang SH, Wu CC,
Lin YM, Chuang JI, Tsai SJ and Sun HS: FGF9 is a downstream target
of SRY and sufficient to determine male sex fate in ex vivo XX
gonad culture. Biol Reprod. 103:1300–1313. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Deng B, Lv W, Duan W, Liu Y, Li Z, Song X,
Cui C, Qi X, Wang X and Li C: FGF9 modulates Schwann cell
myelination in developing nerves and induces a pro-inflammatory
environment during injury. J Cell Biochem. 119:8643–8658.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yin Y and Ornitz DM: FGF9 and FGF10
activate distinct signaling pathways to direct lung epithelial
specification and branching. Sci Signal.
13(eaay4353)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tang L, Wu M, Lu S, Zhang H, Shen Y, Shen
C, Liang H, Ge H, Ding X and Wang Z: Fgf9 negatively regulates bone
mass by inhibiting osteogenesis and promoting osteoclastogenesis
Via MAPK and PI3K/AKT signaling. J Bone Miner Res. 36:779–791.
2021.PubMed/NCBI View Article : Google Scholar
|