Introduction
Bile acids serve an important role in the
pathophysiology of shock (1).
Septic shock can be prevented by bile acids (2). Bile acids are an important defense
mechanism against endotoxins in microorganisms (2). Septic shock induces progressive
sclerosing cholangitis (3). Bile
acids induce platelet inhibition and fibrinolysis in hemorrhagic
shock (HS) (1). High-fat enteral
nutrition decreases endotoxins, TNF-α and intestinal permeability
in bile duct-ligated rats subjected to HS (4). As the technology becomes more
minimally invasive, biliary tract external drainage (BTED) is
widely applied in critically ill patients, such as severe acute
pancreatitis, malignant biliary obstruction (5-7),
acute cholangitis (8-11).
In our previous studies, BTED decreased multiorgan dysfunction in
HS by affecting enterohepatic circulation of bile pigments
(12-15).
Cholic acid is one of the primary components of
bile. Like cholochromes, bile acids circulate in the intestine and
liver. Bile external drainage directly decreases levels of cholic
acid in the intestinal tract and in the liver by disrupting
enterohepatic circulation (16).
Farnesoid X receptor (FXR) and Takeda G-protein coupled receptor 5
(TGR-5) are the two most common cholic acid receptors (17,18).
FXR and TGR-5 serve an important role in the enterohepatic
circulation of cholic acid. FXR, a member of the ligand-activated
nuclear receptor superfamily, is highly expressed in the liver and
gastrointestinal tract (19-21).
Bile acids are natural ligands for FXR (22,23).
Medications that activate FXR promote liver regeneration in mice
following partial hepatectomy (24-26).
TGR-5, also known as G protein-coupled bile acid receptor 1, is
expressed in the liver, lungs, intestine, placenta, gallbladder,
ovaries, macrophages, monocytes and brown adipose tissue (27,28).
TGR-5 is activated by bile acids, including cholic,
chenodeoxycholic, deoxycholic and lithocholic acid (29). TGR-5 protects the liver from bile
acid overload during liver regeneration in mice (30,31).
To the best of our knowledge, studies on changes in
bile acid receptor expression levels following BTED in shock are
limited. Since BTED is increasingly used to treat severe acute
pancreatitis and acute cholangitis and shock is common in these
patients (32), it is necessary to
clarify pathophysiological changes following BTED in shock.
Identifying the effect of BTED on expression levels of FXR and
TGR-5 in liver and intestinal during HS may provide theoretical
support for the application of BTED, as well as novel options, for
the treatment of HS. The present study aimed to investigate changes
in FXR and TGR-5 expression levels following HS by performing
reverse transcription-quantitative (RT-q)PCR, western blotting and
immunohistochemistry. The effect of BTED on FXR and TGR-5 was also
studied.
Materials and methods
Ethics statement
The present study was approved by the Institutional
Animal Care and Use Committee at Peking Union Medical College
(Beijing, China). Animal surgical procedures were performed in
strict accordance with the guidelines for the care and use of
laboratory animals established by the Animal Use and Care Committee
of the Beijing Committee on Animal Care.
The animal protocol was designed to minimize pain or
discomfort to rats. The rats were acclimatized to laboratory
conditions (25˚C, 12/12-h light/dark cycle, 50% humidity, ad
libitum access to food and water) for 1 week prior to
experimentation. All rats were intraperitoneally anesthetized with
3% sodium pentobarbital (50 mg/kg) prior to surgery and
decapitation.
Animal model
A total of 24 adult male Sprague-Dawley rats (age,
10 weeks; weight, 250-300 g) were purchased from the Fangyuan Yuan
Animal Centre of Beijing. The Chinese People's Liberation Army
Military Academy of Medical Sciences was responsible for quality
control of rats.
Rats were randomly divided into 4 groups (n=6/group)
as follows: Sham, BTED, HS and HS + BTED. Rats in the HS + BTED
group were intraperitoneally anesthetized with 3% sodium
pentobarbital (50 mg/kg). Laparotomy was performed following
shaving and sterilization. Catheters were placed in both femoral
arteries for blood pressure measurement and blood withdrawal. Bile
duct was exposed 1cm for BTED. Rats were subjected to HS by
withdrawing blood at a rate of 1 ml/min until mean arterial
pressure (MAP) of 40±5 mmHg was achieved. A catheter was inserted
into the bile duct. The distal end of the bile duct was ligated and
the catheter was passed through the rat flank to avoid bile passage
into the gut and allow the external collection of bile. The abdomen
was closed. MAP of 40±5 mmHg was maintained for 1 h. Rats were
resuscitated using shed blood and an equal volume of normal saline
at the end of the shock period. HS rats underwent pentobarbital
anesthesia, laparotomy, vascular cannulation, blood withdrawal and
suturing but no BTED. BTED rats underwent pentobarbital anesthesia,
laparotomy, vascular cannulation, bile duct cannulation and
suturing but no blood withdrawal. Sham rats underwent pentobarbital
anesthesia, laparotomy, vascular cannulation and suturing, but no
blood withdrawal or BTED. A total of 6 rats in each group was
euthanized by rapid decapitation 6 h after resuscitation. Segments
of jejunum (5 cm distal to the ligament of Treitz), ileum (2 cm
proximal to the cecum) and liver were harvested.
RT-qPCR
Jejunum, ileum and liver scrapings from all animals
were snap frozen and stored at -80˚C for RT-qPCR. Total RNA was
extracted from the jejunum, ileum and liver using
TRIzol® reagent. Aliquots (2 µg) of total RNA were used
to synthesize complementary (c)DNA. Purity and content of RNA was
determined using ultraviolet spectrophotometry. RT was performed
with 0.5 µg RNA to obtain cDNA in a mixture containing random
primers, RevertAid Reverse Transcriptase, RNase inhibitor, and
dNTPs. The PCR reaction mixture was prepared using SYBR Premix Ex
Taq (Thermo Fisher Scientific, Inc.) with specific upstream and
downstream primers. The thermocycling conditions were as follows:
Initial denaturation for 10 sec at 95˚C followed by 40 cycles of
95˚C for 5 sec and 60˚C for 20 sec in a real-time PCR system (7500;
Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative
target gene expression was quantitated according to the comparative
2-ΔΔCq method (33) and
normalized to the endogenous control gene, β-actin. The primers
used are listed in Table I.
 | Table IPrimers for reverse
transcription-quantitative PCR analysis. |
Table I
Primers for reverse
transcription-quantitative PCR analysis.
| Gene | Forward primer,
5'→3' | Reverse primer,
5'→3' |
|---|
| FXR |
GTGACAAAGAAGCCGCGAAT |
GCAGGTGAGCGCGTTGTAAT |
| TGR5 |
CCACCACTAGGGCCTGTAAC |
TCCTCGAAGCACTTGTAGCC |
| β-actin |
GCGCTCGTCGTCGACAACGG |
GTGTGGTGCCAAATCTTCTCC |
Western blotting
Scrapings of the jejunum, ileum and liver from all
animals were snap frozen and stored at -80˚C for western blotting.
RIPA lysis buffer and 5X loading buffer were prepared. Briefly,
samples were homogenized in RIPA lysis buffer supplemented with a
protease inhibitor cocktail. Tissues were frozen immediately in
liquid nitrogen and placed in a mortar for pulverization. Total
protein was extracted by centrifuging the tubes at 4˚C for 30 min
at 10,000 x g to remove debris. Protein concentration was measured
using a BCA Protein Assay kit according to the manufacturer's
instructions. A total of 10 mg protein/lane was loaded onto 10%
sodium dodecyl sulfate/polyacrylamide electrophoresis gel for
separation, and the proteins were transferred for 2 h to PVDF
membranes. Then membranes were blocked in 5% skimmed milk powder at
room temperature for 2 h. The blot was probed with primary antibody
overnight at 4˚C. Primary antibodies were rabbit polyclonal
anti-FXR (cat. no. ab28676) and anti-TGR-5 (both 1:500; cat. no.
ab72608; both Abcam) and mouse monoclonal anti-β-actin (Santa Cruz
Biotechnology, Inc.; cat. no. sc517582) (1:1,000). The blots were
incubated with horseradish peroxidase-conjugated goat anti-Rabbit
(cat. no. BS13278) or anti-mouse (cat. no. BS12471) IgG (both
1:2,000; both Bioworld Technology, Inc.) for 2 h at room
temperature and reacted with enhanced chemiluminescence substrate.
The chemiluminescence was recorded using an imaging system
(Imagequant LAS 400; GE Healthcare). The enhanced chemiluminescence
signals were digitized using Photoshop CS6 software (Adobe Systems,
Inc.) to quantify the expression levels of FXR, TGR-5 and β-actin.
Relative FXR and TGR-5 protein expression was normalized to
β-actin.
Immunohistochemistry
Samples were fixed in 4% paraformaldehyde for 24 h
at room temperature. The tissue was embedded in paraffin and
sections (4 µm) were used for immunohistochemical staining.
Sections were deparaffinized, rehydrated and incubated with 3%
hydrogen peroxide to quench any endogenous peroxidase activity.
Sections were placed in 3% citrate buffer to repair antigens. The
buffer was heated to 92-98˚C using a microwave for 10 min. Sections
were cooled to room temperature. The tissue samples were blocked
with 5% bovine serum (Wuhan Boster Biological Technology. Ltd.) for
20 min at room temperature. Sections were incubated overnight at
4˚C with optimally diluted primary antibody. Negative control
sections were incubated overnight at 4˚C with saline solution.
Primary antibodies were rabbit polyclonal anti-FXR (cat. no.
ab28676) and anti-TGR-5 (cat. no. ab72608; both 1:100; both Abcam).
Sections were washed with PBS and incubated with goat anti-Rabbit
IgG (Bioworld Technology, Inc.; cat. no. BS13278; 1:200) at room
temperature for 30 min, rewashed and incubated with
peroxidase-conjugated streptavidin at room temperature for 15 min.
Peroxidation activity was visualized via incubation with a
peroxidase substrate solution (DAB kit; Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 3 min according to the
manufacturer's instructions. Sections were counterstained with
hematoxylin at room temperature for 5 min. Image-Pro Plus software
4.0 (Media Cybernetics, Inc.) was used to determine the integrated
optical density of images. A total of six randomly selected fields
of view/section were observed under a light microscope at x100
magnification (six sections/group).
Reagents
RIPA lysis buffer, BCA Protein Assay kit and 5X
loading buffer were purchased from Beyotime Institute of
Biotechnology. Rabbit polyclonal anti-FXR (cat. no. ab28676) and
anti-TGR-5 (cat. no. ab72608) were purchased from Abcam. The mouse
monoclonal antibody to β-actin (cat. no. sc517582) was purchased
from Santa Cruz Biotechnology, Inc. The goat anti-Rabbit IgG (cat.
no. BS13278) and goat anti-Mouse IgG (cat. no. BS12471) were
purchased from Bioworld Technology, Inc. eECL Western Blot kit
(cat. no. CW0049A) was purchased from CoWin Biosciences.
RevertAidFirst Strand cDNA Synthesis kit was purchased from Thermo
Fisher Scientific, Inc. SYBR Premix Ex Taq was purchased from
Thermo Fisher Scientific, Inc. The primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.). The fluorescence
RT-qPCR kit was purchased from Takara Biotechnology, Co., Ltd.
TRIzol® and the immunohistochemistry kit were purchased
from Invitrogen (Thermo Fisher Scientific, Inc.). The 5% bovine
serum was purchased from Wuhan Boster Biological Technology.
Ltd.
Statistical analysis
Data were analyzed using SPSS 16.0 software (SPSS,
Inc.). All data are expressed as the mean ± SEM (n=6). Results were
compared by one-way analysis of variance followed by post hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
RT-qPCR
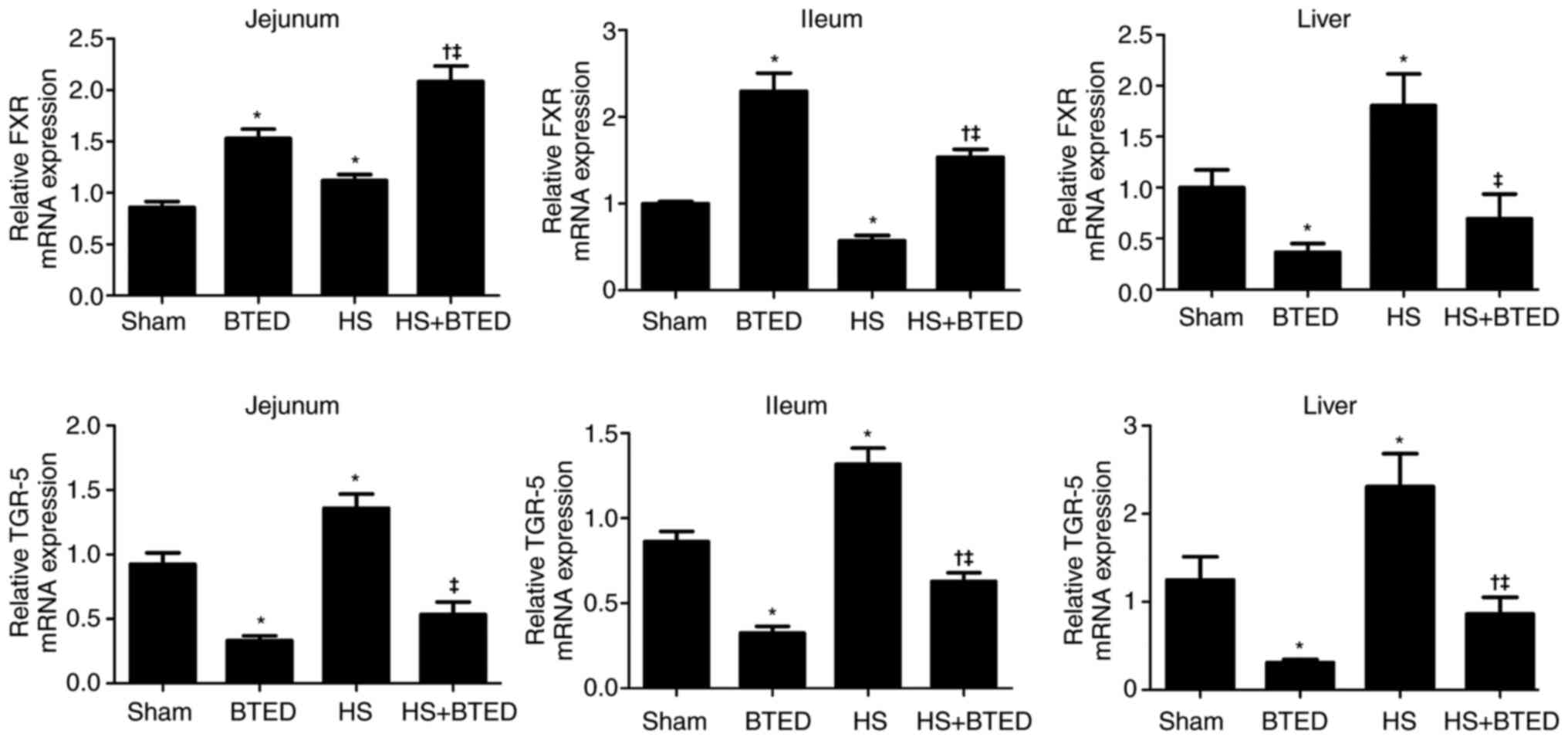
The expression levels of FXR mRNA increased
significantly in the jejunum and liver and decreased significantly
in the ileum following HS (P<0.05; Fig. 1). The expression levels of FXR
increased significantly in the jejunum and ileum and decreased
significantly in the liver following BTED (P<0.05; Fig. 1). In HS rats, BTED significantly
increased the expression levels of FXR mRNA in the jejunum and
ileum but decreased these levels in the liver (P<0.05; Fig. 1). The expression levels of TGR-5
mRNA increased significantly in the jejunum, ileum and liver
following HS (P<0.05; Fig. 1).
The expression levels of TGR-5 decreased significantly in the
jejunum, ileum and liver following BTED (P<0.05; Fig. 1). In HS rats, BTED significantly
decreased the expression levels of TGR-5 mRNA in the jejunum, ileum
and liver (P<0.05; Fig. 1).
Western blotting
The protein expression levels of FXR increased
significantly in the jejunum and liver following HS (P<0.05;
Fig. 2). The protein expression
levels of FXR increased significantly in the jejunum and ileum and
decreased significantly in the liver following BTED (P<0.05;
Fig. 2). In HS rats, BTED
significantly increased protein expression levels of FXR in the
jejunum and ileum and decreased these levels in the liver
(P<0.05; Fig. 2). The expression
levels of TGR-5 increased significantly in the jejunum, ileum and
liver following HS (P<0.05; Fig.
2). The expression levels of TGR-5 decreased significantly in
the jejunum, ileum and liver following BTED (P<0.05; Fig. 2). In HS rats, BTED significantly
decreased protein expression levels of TGR-5 in the jejunum, ileum
and liver (P<0.05; Fig. 2).
Immunohistochemistry
The protein expression levels of FXR increased
significantly in the liver and decreased significantly in the ileum
following HS (P<0.05; Fig. 3).
The protein expression levels of FXR increased significantly in the
jejunum and ileum and decreased significantly in the liver
following BTED (P<0.05; Fig. 3).
In HS rats, BTED significantly increased protein expression levels
of FXR in the jejunum and ileum and decreased these levels in the
liver (P<0.05; Fig. 3).
The protein expression levels of TGR-5 increased
significantly in the jejunum and liver following HS (P<0.05;
Fig. 4). The protein expression
levels of TGR-5 decreased significantly in the jejunum, ileum and
liver following BTED (P<0.05; Fig.
4). In HS rats, BTED significantly decreased protein expression
levels of TGR-5 in the jejunum, ileum and liver (P<0.05;
Fig. 4).
Discussion
Following fluid resuscitation in shock, cells are
liberated from ischemia and hypoxia and the body begins tissue
repair (34). The present study
showed that mRNA and protein expression levels of FXR increased
significantly in the jejunum and liver and those of TGR-5 increased
significantly in the jejunum, ileum and liver following HS. This
may be due to elimination of ischemia following fluid resuscitation
in HS. Cell repair and regeneration increase expression levels of
FXR and TGR-5. In the enterohepatic circulation, bile acid is
reabsorbed in the ileum. Increased reabsorption of bile acid during
HS may inhibit expression of FXR (35), which may partly explain why FXR was
not upregulated in the ileum following HS in the present study.
BTED is increasingly used to treat patients with
shock (36). The present study
aimed to identify pathophysiological changes following BTED in HS.
In addition to its therapeutic potential, BTED also exerts certain
negative effects. Sinusoids remain narrowed due to swelling of
activated Kupffer cells, which causes deterioration of hepatic
microcirculation during the early phase of BTED in jaundiced mice
(37). Initial liver regeneration
following major hepatectomy is decreased following biliary drainage
and is associated with decreased serum bile acid levels (38). BTED as a treatment for obstructive
jaundice markedly suppresses liver regeneration following partial
hepatectomy (39). However,
internal biliary drainage does not suppress regeneration of rat
liver following partial hepatectomy (16). In our unpublished studies, liver
damage was aggravated following BTED in a rat model of HS.
Preoperative bile replacement using large volumes of bile improves
blood coagulation and cellular immunity in patients with jaundice
treated with BTED (40). FXR and
TGR-5 serve a key role in pathophysiological changes of the liver
(41). Bile acid promotes liver
regeneration via FXR signaling in rats (42). Sirtuin1 controls liver regeneration
by regulating bile acid metabolism via FXR (43). TGR-5 regulates bile acid
hydrophobicity and stimulates bile acid excretion in urine during
liver regeneration (44). BTED may
affect liver function by decreasing cholic acid levels (39). To the best of our knowledge,
however, the number of studies on changes in bile acid receptors
following BTED in shock is limited. Therefore, the present study
aimed to assess changes in FXR and TGR-5 expression in the jejunum,
ileum and liver following BTED in HS rats. BTED significantly
decreased the expression levels of FXR and TGR-5 in the liver in HS
rats. This may be because BTED disrupts cholic acid enterohepatic
circulation, thereby decreasing levels of cholic acid in the body
(45). Due to lack of stimulation,
levels of FXR and TGR-5 decrease, resulting in inhibition of tissue
repair and liver cell regeneration The aforementioned results
suggested that bile acid supplementation may be beneficial for
patients receiving BTED during HS. In HS rats, BTED significantly
increased expression levels of FXR but decreased those of TGR-5 in
the jejunum and ileum. This may explain why BTED does not cause
injury to the jejunum and ileum.
The present study is only a preliminary observation
and the clinical significance of the effect of BTED on FXR and
TGR-5 in HS is unclear. For example, FXR and TGR-5 promote organ
regeneration (46,47). However, the effect of BTED on
expression levels of proliferation markers is unclear. The
association between BTED and organ regeneration should be
investigated in future experiments.
In summary, the expression levels of FXR and TGR-5
changed following BTED in HS. BTED significantly increased
expression levels of FXR in the jejunum and ileum but decreased FXR
expression in the liver in HS rats. BTED significantly decreased
expression levels of TGR-5 in the jejunum, ileum and liver in HS
rats.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Fund of China (grant no. 81801901).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW, HWH, XZ and YL drafted the manuscript. LW and
HWH performed surgical procedures. LW and HWH performed statistical
analysis. XZ and YL conceived and designed the study. LW and XZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee at Peking Union Medical College
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
LW, ORCID no. 0000-0001-6553-6381; HWH, ORCID no.
0000-0003-3422-3109; XZ, ORCID no. 0000-0001-8523-8082; YL, ORCID
no. 0000-0003-0274-8322.
References
|
1
|
Wiener G, Moore HB, Moore EE, Gonzalez E,
Diamond S, Zhu S, D'Alessandro A and Banerjee A: Shock releases
bile acid inducing platelet inhibition and fibrinolysis. J Surg
Res. 195:390–395. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bertók L: Role of endotoxins and bile
acids in the pathogenesis of septic circulatory shock. Acta Chir
Hung. 36:33–36. 1997.PubMed/NCBI
|
|
3
|
Engler S, Elsing C, Flechtenmacher C,
Theilmann L, Stremmel W and Stiehl A: Progressive sclerosing
cholangitis after septic shock: A new variant of vanishing bile
duct disorders. Gut. 52:688–693. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Luyer MD, Buurman WA, Hadfoune M, Jacobs
JA, Dejong CH and Greve JW: High-fat enteral nutrition reduces
endotoxin, tumor necrosis factor-alpha and gut permeability in bile
duct-ligated rats subjected to hemorrhagic shock. J Hepatol.
41:377–383. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu C, Huang XE, Wang SX, Lv PH, Sun L and
Wang FA: Comparison of infection between internal-external and
external percutaneous transhepatic biliary drainage in treating
patients with malignant obstructive jaundice. Asian Pac J Cancer
Prev. 16:2543–2546. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saettini F, Agazzi R, Giraldi E, Foglia C,
Cavalleri L, Morali L, Fasolini G, Spotti A and Provenzi M:
Percutaneous transhepatic biliary drainage in an infant with
obstructive jaundice caused by neuroblastoma. Pediatr Hematol
Oncol. 32:223–228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arkadopoulos N, Kyriazi MA, Papanikolaou
IS, Vasiliou P, Theodoraki K, Lappas C, Oikonomopoulos N and
Smyrniotis V: Preoperative biliary drainage of severely jaundiced
patients increases morbidity of pancreaticoduodenectomy: Results of
a case-control study. World J Surg. 38:2967–2972. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shinya S, Sasaki T, Yamashita Y, Kato D,
Yamashita K, Nakashima R, Yamauchi Y and Noritomi T: Procalcitonin
as a useful biomarker for determining the need to perform emergency
biliary drainage in cases of acute cholangitis. J Hepatobiliary
Pancreat Sci. 21:777–785. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
McNabb-Baltar J, Trinh QD and Barkun AN:
Biliary drainage method and temporal trends in patients admitted
with cholangitis: A national audit. Can J Gastroenterol.
27:513–518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Itoi T, Tsuyuguchi T, Takada T, Strasberg
SM, Pitt HA, Kim MH, Belli G, Mayumi T, Yoshida M, Miura F, et al:
TG13 indications and techniques for biliary drainage in acute
cholangitis (with videos). J Hepatobiliary Pancreat Sci. 20:71–80.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Abdelkader AM, Zidan AM and Younis MT:
Temporary CBD stenting with a nelaton tube is a more practical and
safer option than T-tube drainage after conventional CBD
exploration for choledocholithiasis. HPB Surg.
2018(8035164)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang L, Zhao B, Chen Y, Ma L, Chen EZ and
Mao EQ: Biliary tract external drainage protects against intestinal
barrier injury in hemorrhagic shock rats. World J Gastroenterol.
21:12800–12813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Zhao B, Chen Y, Ma L, Chen EZ and
Mao EQ: Inflammation and edema in the lung and kidney of
hemorrhagic shock rats are alleviated by biliary tract external
drainage via the heme oxygenase-1 pathway. Inflammation.
38:2242–2251. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang L, Zhao B, Chen Y, Ma L, Chen EZ and
Mao EQ: Biliary tract external drainage increases the expression
levels of heme oxygenase-1 in rat livers. Eur J Med Res.
20(61)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang L, Zhao B, Chen Y, Ma L, Chen EZ and
Mao EQ: Biliary tract external drainage alleviates kidney injury in
shock. J Surg Res. 199:564–571. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Suzuki H, Iyomasa S, Nimura Y and Yoshida
S: Internal biliary drainage, unlike external drainage, does not
suppress the regeneration of cholestatic rat liver after partial
hepatectomy. Hepatology. 20:1318–1322. 1994.PubMed/NCBI
|
|
17
|
Iracheta-Vellve A, Calenda CD, Petrasek J,
Ambade A, Kodys K, Adorini L and Szabo G: FXR and TGR5 agonists
ameliorate liver injury, steatosis, and inflammation after binge or
prolonged alcohol feeding in mice. Hepatol Commun. 2:1379–1391.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mertens KL, Kalsbeek A, Soeters MR and
Eggink HM: Bile acid signaling pathways from the enterohepatic
circulation to the central nervous system. Front Neurosci.
11(617)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang YD, Chen WD, Moore DD and Huang W:
FXR: A metabolic regulator and cell protector. Cell Res.
18:1087–1095. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee FY, Lee H, Hubbert ML, Edwards PA and
Zhang Y: FXR, a multipurpose nuclear receptor. Trends Biochem Sci.
31:572–580. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Horikawa T, Oshima T, Li M, Kitayama Y,
Eda H, Nakamura K, Tamura A, Ogawa T, Yamasaki T, Okugawa T, et al:
Chenodeoxycholic acid releases proinflammatory cytokines from small
intestinal epithelial cells through the farnesoid X receptor.
Digestion. 100:286–294. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tuominen I and Beaven SW: Intestinal
farnesoid X receptor puts a fresh coat of wax on fatty liver.
Hepatology. 62:646–648. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hou Y, Fan W, Yang W, Samdani AQ, Jackson
AO and Qu S: Farnesoid X receptor: An important factor in blood
glucose regulation. Clin Chim Acta. 495:29–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Meng Q, Chen X, Wang C, Liu Q, Sun H, Sun
P, Peng J and Liu K: Alisol B 23-acetate promotes liver
regeneration in mice after partial hepatectomy via activating
farnesoid X receptor. Biochem Pharmacol. 92:289–298.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang L, Wang YD, Chen WD, Wang X, Lou G,
Liu N, Lin M, Forman BM and Huang W: Promotion of liver
regeneration/repair by farnesoid X receptor in both liver and
intestine in mice. Hepatology. 56:2336–2343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen WD, Wang YD, Zhang L, Shiah S, Wang
M, Yang F, Yu D, Forman BM and Huang W: Farnesoid X receptor
alleviates age-related proliferation defects in regenerating mouse
livers by activating forkhead box m1b transcription. Hepatology.
51:953–962. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kawamata Y, Fujii R, Hosoya M, Harada M,
Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al:
A G protein-coupled receptor responsive to bile acids. J Biol Chem.
278:9435–9440. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Finn PD, Rodriguez D, Kohler J, Jiang Z,
Wan S, Blanco E, King AJ, Chen T, Bell N, Dragoli D, et al:
Intestinal TGR5 agonism improves hepatic steatosis and insulin
sensitivity in Western diet-fed mice. Am J Physiol Gastrointest
Liver Physiol. 316:G412–G424. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fiorucci S, Mencarelli A, Palladino G and
Cipriani S: Bile-acid-activated receptors: Targeting TGR5 and
farnesoid-X-receptor in lipid and glucose disorders. Trends
Pharmacol Sci. 30:570–580. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jourdainne V, Péan N, Doignon I, Humbert
L, Rainteau D and Tordjmann T: The bile acid receptor TGR5 and
liver regeneration. Dig Dis. 33:319–326. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Péan N, Doignon I, Garcin I, Besnard A,
Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L, et
al: The receptor TGR5 protects the liver from bile acid overload
during liver regeneration in mice. Hepatology. 58:1451–1460.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Berquist TH, May GR, Johnson CM, Adson MA
and Thistle JL: Percutaneous biliary decompression: Internal and
external drainage in 50 patients. AJR Am J Roentgenol. 136:901–906.
1981.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gordon D and Spiegel R: Fluid
resuscitation: History, physiology, and modern fluid resuscitation
strategies. Emerg Med Clin North Am. 38:783–793. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakatani T and Kobayashi K: Post-traumatic
jaundice-its mechanism from a view point of hepatic mitochondrial
function. Nihon Geka Gakkai Zasshi. 92:441–447. 1991.PubMed/NCBI(In Japanese).
|
|
36
|
Shimada H, Nakagawara G, Kobayashi M,
Tsuchiya S, Kudo T and Morita S: Pathogenesis and clinical features
of acute cholangitis accompanied by shock. Jpn J Surg. 14:269–277.
1984.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Okaya T, Nakagawa K, Kimura F, Shimizu H,
Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Ito H and Miyazaki M:
The alterations in hepatic microcirculation and Kupffer cell
activity after biliary drainage in jaundiced mice. J Hepatobiliary
Pancreat Sci. 19:397–404. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Otao R, Beppu T, Isiko T, Mima K, Okabe H,
Hayashi H, Masuda T, Chikamoto A, Takamori H and Baba H: External
biliary drainage and liver regeneration after major hepatectomy. Br
J Surg. 99:1569–1574. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Iyomasa S, Terasaki M, Kuriki H, Nimura Y,
Shionoya S, Kojima K and Yoshida S: Decrease in regeneration
capacity of rat liver after external biliary drainage. Eur Surg
Res. 24:265–272. 1992.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yoshida Y, Ajiki T, Ueno K, Shinozaki K,
Murakami S, Okazaki T, Matsumoto T, Matsumoto I, Fukumoto T, Usami
M and Ku Y: Preoperative bile replacement improves immune function
for jaundiced patients treated with external biliary drainage. J
Gastrointest Surg. 18:2095–2104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ferrell JM, Pathak P, Boehme S, Gilliland
T and Chiang JY: Deficiency of both farnesoid X receptor and takeda
G protein-coupled receptor 5 exacerbated liver fibrosis in mice.
Hepatology. 70:955–970. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ding L, Yang Y, Qu Y, Yang T, Wang K, Liu
W and Xia W: Bile acid promotes liver regeneration via farnesoid X
receptor signaling pathways in rats. Mol Med Rep. 11:4431–4437.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Garcia-Rodriguez JL, Barbier-Torres L,
Fernández-Álvarez S, Gutiérrez-de Juan V, Monte MJ, Halilbasic E,
Herranz D, Álvarez L, Aspichueta P, Marín JJ, et al: SIRT1 controls
liver regeneration by regulating bile acid metabolism through
farnesoid X receptor and mammalian target of rapamycin signaling.
Hepatology. 59:1972–1983. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fan M, Wang X, Xu G, Yan Q and Huang W:
Bile acid signaling and liver regeneration. Biochim Biophys Acta.
1849:196–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun Q, Fang F, Lu GC, Mao HH, Xu JH, Zhou
SK, Tong XM, Guo Y, Wu JF and Jiang B: Effects of different
drainage methods on serum bile acid and hepatocyte apoptosis and
regeneration after partial hepatectomy in rats with obstructive
jaundice. J Biol Regul Homeost Agents. 33:571–579. 2019.PubMed/NCBI
|
|
46
|
Jung K, Kim M, So J, Lee SH, Ko S and Shin
D: Farnesoid X receptor activation impairs liver progenitor
cell-mediated liver regeneration via the PTEN-PI3K-AKT-mTOR axis in
zebrafish. Hepatology. 74:397–410. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chiang JYL and Ferrell JM: Bile acid
receptors FXR and TGR5 signaling in fatty liver diseases and
therapy. Am J Physiol Gastrointest Liver Physiol. 318:G554–G573.
2020.PubMed/NCBI View Article : Google Scholar
|