Introduction
Osteoarthritis (OA) is a degenerative disease that
usually occurs in the hands, hips and knees (1). The main causes are mechanical
stimulation between bones and aging (2,3).
Cartilage damage is accompanied bone lesions,
synovial inflammation and chronic pain, which are the main symptoms
of OA (4). In clinical studies,
adults >25 years old show a prevalence rate of 13.9% and adults
>65 years old show a prevalence rate of 33.6% in the US
(3,5,6). For
adults >60, there is a prevalence rate of 9.6% in males and
18.0% in females, worldwide (7).
Patients with anterior cruciate ligament (ACL) injury can develop
early degeneration of joints (3).
Reconstruction surgery can relieve symptoms and reestablish
stability of the knee joint, but will not reduce the probability of
damage to the ACL progressing to joint degeneration (6). Therefore, OA could be caused by trauma
leading to chondrocyte apoptosis and chronic pain through
biological mechanisms (3). The ACL
resection model simulates the development of OA that results from
ACL damage (8). This model could be
used to assess the health benefits of drugs or supplementary
products in relation to degenerative joint disease (9,10).
Moreover, this model can be used to study the related factors of OA
and drug mechanisms (11,12).
In patients with OA, chronic inflammation primarily
occurs in synovial tissue by releasing and increasing the levels of
inflammatory factors, such as IL-1β and TNF-α, causing the
infiltration of immune cells and hyperplasia of the synovial
membrane (13). The effects of
inflammatory cytokines could cause proteolytic enzymes to degrade
type II collagen, proteoglycans and hyaluronic acid on the
extracellular matrix of cartilage (14). The extracellular matrix of cartilage
is primarily formed through cartilage secretions and plays a
critical role in the balance and control of chondrocyte function in
cartilage (15,16). Therefore, chondrocyte apoptosis
could change the formation of the extracellular matrix of cartilage
and become one of the pathological symptoms of OA (16). Chondrocyte apoptosis is mainly
induced by signaling pathways that are activated by inflammatory
factors, such as IL-1β and TNF-α (17,18).
TNF-α induces the TNF-related apoptosis-inducing ligand pathway, in
which binding to the death receptors DR4 (TRAIL-RI) and DR5
(TRAIL-RII) induces caspase-3/8 expression in the intrinsic pathway
(19,20). Meanwhile, the extrinsic pathway is
mediated by mitochondria with caspase-3/9 regulation (17). However, current treatment of OA
mainly focuses on relieving pain symptoms and maintaining or
improving joint function (21).
Therefore, preliminary treatment of OA uses drugs, such as Panadol
or non-steroidal anti-inflammatory drugs (22). Furthermore, moderate OA is treated
with a combination of glucosamine and chondroitin (22). During severe pain or late-stage of
the disease, relief of symptoms requires opioids, corticosteroids
and hyaluronic acid (22). Finally,
consideration is given to surgical methods for artificial joint
replacements (22). Annual
expenditure for the treatment for OA exceeds $20 million in the US
(23). Hence, it is important to
explore treatment methods that can alleviate the disease course of
OA.
The present study used the probiotics,
Streptococcus thermophilus (TCI633), that can produce
hyaluronic acid and be isolated from healthy human breast milk.
Previously, TCI633 was identified as a novel strain which was
discovered from human breast milk (24). TCI633 can effectively replicate in
the gastrointestinal tract (24).
Past studies have demonstrated that related bacterial species can
suppress allergic reactions, have anti-inflammatory effects and can
mediate the immune response (25,26).
Moreover, the ability of TCI633 to produce hyaluronic acid is
superior to that of known S. thermophilus YIT2084(27). Previously, we demonstrated that
TCI633 effectively produces hyaluronic acid in the gastrointestinal
tract for absorption and increases hyaluronic acid level in the
blood (24). Similarly, a previous
study also reported that TCI633 has the potential for the treatment
of osteoporosis in vivo (27). Past studies have indicated that
hyaluronic acid is a primary glycosaminoglycan in the extracellular
matrix where it binds with proteoglycans to lubricate joint
cavities and increase the viscosity of synovial fluid (28,29).
In the synovial fluid of individuals without OA, the average
molecular weight of hyaluronic acid is 4-5 million with a
concentration of 2.5-4 mg/ml. Meanwhile, in the synovial fluid of
patients with OA, there is an evident decrease in the concentration
and molecular weight of hyaluronic acid (28). In previous animal experiments,
hyaluronic acid can effectively reduce joint pain and delay disease
progression (30,31). Clinical studies have also
demonstrated that intra-articular injection of hyaluronic acid can
relieve joint pain and improve joint function (32,33).
Hyaluronic acid can also regulate the inflammation of OA through
reducing neutrophil chemotaxis, macrophage proliferation and
angiogenesis (34,35). Hyaluronic acid can also eradicate
proinflammatory cytokines and free radicals from the joint space to
lymphatics and reduce cartilage damage (36). Past animal experimentation has also
demonstrated that hyaluronic acid can effectively delay the
progression of OA in early stage after ACLT (8). Therefore, the purpose of the present
study was to investigate the possible therapeutic effects of TCI633
in increasing the level of hyaluronic acid, and to determine if
this could alleviate joint pain and swelling and improve joint
function in ACLT-treated rats.
Materials and methods
TCI633, excipient and glucosamine
sulfate salt preparation
TCI633 were provided by TCI Co., Ltd. (https://www.tci-bio.com/) and preserved at the
Bioresource Collection and Research Center (BCRC), the Food
Industry Research and Development Institute (Taiwan) (Species
preservation number: BCRC 910636). The excipient and glucosamine
sulfate salt (D-glucosamine sulfate salt; cat. no. MG052630801)
were also provided by TCI Co., Ltd. The excipient, TCI633 and
glucosamine sulfate salt were diluted and suspension in RO water
for oral administration.
Preparation of animals
In total, 63 male Wistar rats (8-weeks old; body
weight 320-330 g) were obtained from the National Laboratory Animal
Centre in Taiwan. The rats were maintained in plexiglass cages in a
temperature-controlled (24±1˚C) room under a 12-h light/dark cycle
and given free access to food and water. Each rat was used only
once for the experiment. ACLT surgery was performed under 2.5%
isoflurane anesthesia. The use of the animals accorded to the
Guiding Principles in the Care and Use of Animals of the American
Physiology Society and was approved by the Institutional Animal
Care and Use Committee of National Sun Yat-sen University
(Kaohsiung, Taiwan; approval no. 10417).
Surgical technique for induction of
OA
The method for the rat ACLT model of osteoarthritis
was performed according to Ghosh et al (36) and Yang et al (37). A parapatellar incision was made at
the right knee and arthrotomy was performed. The ACL was exposed
and transected. For the left knee operation, the ACL was exposed,
but not transected (37). The pain
behaviors and knee swelling were tested once a week. TIC633 was
administered from 8th to 20th week for every day after ACLT
surgery. TCI633 was no longer administered from weeks 21 to 24.
Experimental design
Rats were allocated randomly to six groups: Control
(n=9), ACLT (n=8), ACLT+TCI633 (5x1011 CFU/kg/day)
(n=8), ACLT+TCI633 (5x1010 CFU/kg/day) (n=9),
ACLT+TCI633 (5x109 CFU/kg/day) (n=9) and
ACLT+glucosamine sulfate (250 mg/kg) group (n=9). TCI633 and
placebo were provided by TCI Co., Ltd. The control group did not
receive any treatment. The ACLT group was treated with placebo
(oral) with suspension in RO water and ACLT+TCI633 or glucosamine
sulfate groups were treated with TCI633 (5x1011,
5x1010 and 5x109 CFU/kg/day suspension in RO
water, oral) and glucosamine sulfate (250 mg/kg in RO water, oral)
every day from week 8 to week 20 after ACLT surgery. The
glucosamine sulfate group was considered as a positive control.
Weight-bearing distribution
The weight bearing distribution for the ACLT-rats
was measured using a dual channel weight averager (Singa Technology
Corporation), which can independently measure weight bearing of
each hind paw (10,37). The change of weight bearing
distribution was analyzed as described previously (9,10,38).
Briefly, each rat was placed in an angled plexiglass chamber
positioned so that each hind paw rested on a separate force plate.
The force exerted on each hind paw (measured in grams) was averaged
over a 5-sec period and three measurements were repeated. The
changes of hind paw weight distribution are expressed as the
difference in weight bearing between the contralateral and
ipsilateral (39).
Mechanical allodynia
The method for assessing mechanical allodynia on the
rat anterior cruciate ligament transected (ACLT) model of OA was
modified from Chaplan et al (39,40).
Briefly, rats were placed in compartments of clear plastic cages on
top of an elevated metal mesh floor, permitting easy access to
paws. A series of von Frey filaments of logarithmically incremental
stiffness was applied to the mid-plantar region of the hind paw
from below the mesh floor using the ‘up-down’ method, involving
alternate larger and smaller fibers to determine the closest
filament to the threshold of pain response (licking or
withdrawal).
Measurement of knee width
Knee width was measured to determine the amount of
tissue swelling as an index of inflammation. Rats were anesthetized
briefly with 2% isoflurane and then the width of the knee joint was
measured every week by using after the ACLT surgery.
Samples collected
Rats were sacrificed by inducing deep anesthesia
with 2% isoflurane. Each rat was then perfused intracardially with
ice-cold phosphate-buffered saline (PBS) and 4% paraformaldehyde at
week 24. The knee samples were collected from rats and fixed in 10%
neutral buffered formalin at room temperature for 3 days, and then
decalcified for 2 weeks in buffered 12.5% EDTA with PBS. The
specimens were embedded in paraffin and sliced into 1-µm sections.
The sections were for hematoxylin/eosin (H&E) staining at 25˚C
(2 min staining with 10 mg/ml hematoxyli; Mecrk; 15 sec staining
with 1% eosin, Muto Pure Chemicals) and safranin O/fast green
staining at 25˚C (10 min staining with 0.1% safranin O and 5 min
staining with 0.1% fast green, Sigma-Aldrich; Merck KGaA). Each
section was examined under an upright light microscope (DM 6000B;
Leica Microsystems, Inc.) and digital-image output system (SPOT
idea 5.0 Mp Color Digital Camera; Diagnostic Instruments,
Inc.).
Histopathological findings of
joints
The sections were stained with Safranin-O/fast green
and H&E to assess the general morphology and matrix
proteoglycan of the joint tissue. Articular cartilage was graded
under microscopic examination according to the Osteoarthritis
Research Society International (OARSI) grading system (41). This system comprises of six
histological grades and four histological stages. The total score
(score = grade x stage) ranges from 1 point (control articular
cartilage) to 24 points (no repair) (41). Synovial tissue was scored with for
histological assessment (31). This
score assessed i) the synovial tissue, including hyperplasia of the
synovial lining cells (0-3 points), hypertrophy of the synovial
lining layer (0-3 points) and the infiltration of inflammatory
cells (0-3 points), and ii) the sub-synovial tissue, including the
proliferation of granulation tissue (0-3 points), vascularization
(0-3 points) and infiltration of inflammatory cells (0-3 points).
The maximum score is 18 points. The synovitis scores were divided
into three stages: 0-6 Points (mild synovitis), 7-12 points
(moderate synovitis) and ≥13 or more points (severe synovitis).
Higher scores indicated greater damage (31).
Immunohistochemical stain for collagen
II
Knee specimens were processed for
immunohistochemical analysis, as described in previous studies
(9,10). Briefly, the specimens were
de-paraffinized with xylene and rehydrated with alcohol, and the
endogenous peroxidase activity was quenched by 0.3% hydrogen
peroxide. Antigen retrieval was performed using 20 mM proteinase K
(Sigma-Aldrich; Merck KGaA) in PBS for 40 min, and slides were
incubated with primary antibody, polyclonal anti-rabbit
anti-collagen II (1:200; cat. no. 234187; Calbiochem; Merck KGaA).
Then, the sections were incubated with second antibody anti-rabbit
IgG (Vector Laboratories, Inc.). Specimens were labeled with the
avidin-biotin complex technique using an ABC kit (Vectastain ABC
kit; Vector Laboratories Inc.). Finally, specimens were treated
with 3,30-diaminobenzidine tetrahydrochloride (DAB; peroxidase
substrate kit; Vector Laboratories Inc.) for 2 min. Slides were
analyzed under a light microscope with a microscope digital image
output system. The quantitation of type II collagen
immunoreactivity in cartilage was analyzed by ImageJ version 1.51j8
(National Institutes of Health) with x200 magnification images, and
the results were averaged.
TUNEL staining
The sections were taken from the knee joint for
apoptosis dyeing of chondrocytes. Briefly, the paraffin was removed
and sections were rehydrated by soaking in xylene (100%), alcohol
(100, 95, 75 and 50%), and then proteinase K (20 mM; Sigma-Aldrich;
Merck KGaA) was added for 30 min and rinsed with PBS. Hydrogen
peroxide (3%) solvent was added for 10 min. Sections were
permeabilized with permeabilization solution containing 0.1% Triton
X-100 and 0.1% sodium citrate for a 30 min reaction at room
temperature and rinsed with PBS. Horse serum (3%, Jackson
ImmunoResearch Inc.) was added for 60 min and the TUNEL reaction
mixture agent (TUNEL reaction mixture; In Situ Cell Death
Detection kit, Fluorescein; cat. no. 11684795910, Sigma-Aldrich;
Merck KGaA) for a 90-min reaction at 37˚C. Samples were stained
with DAB dye at 25˚C for 5 min, and then soaked in 10 mg/ml
hematoxylin stai (Merck KGaA) at 25˚C for nuclear staining for 90
sec. Slides were analyzed under a light microscope with a
microscope digital image output system. The percentage of
TUNEL-positive cells were quantified by determining the number of
positive chondrocytes with x200 magnification images, as previously
described (42).
Statistical analysis
All data are expressed as mean ± standard error of
the mean in triplicate, unless otherwise specified. For statistical
analysis, all data were analyzed the differences between the
experimental groups using a standard two-way ANOVA and one-way
ANOVA followed Tukey's post hoc test at the same time-point for
multiple comparisons (SigmaPlot 11.0 for Windows; Systat Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
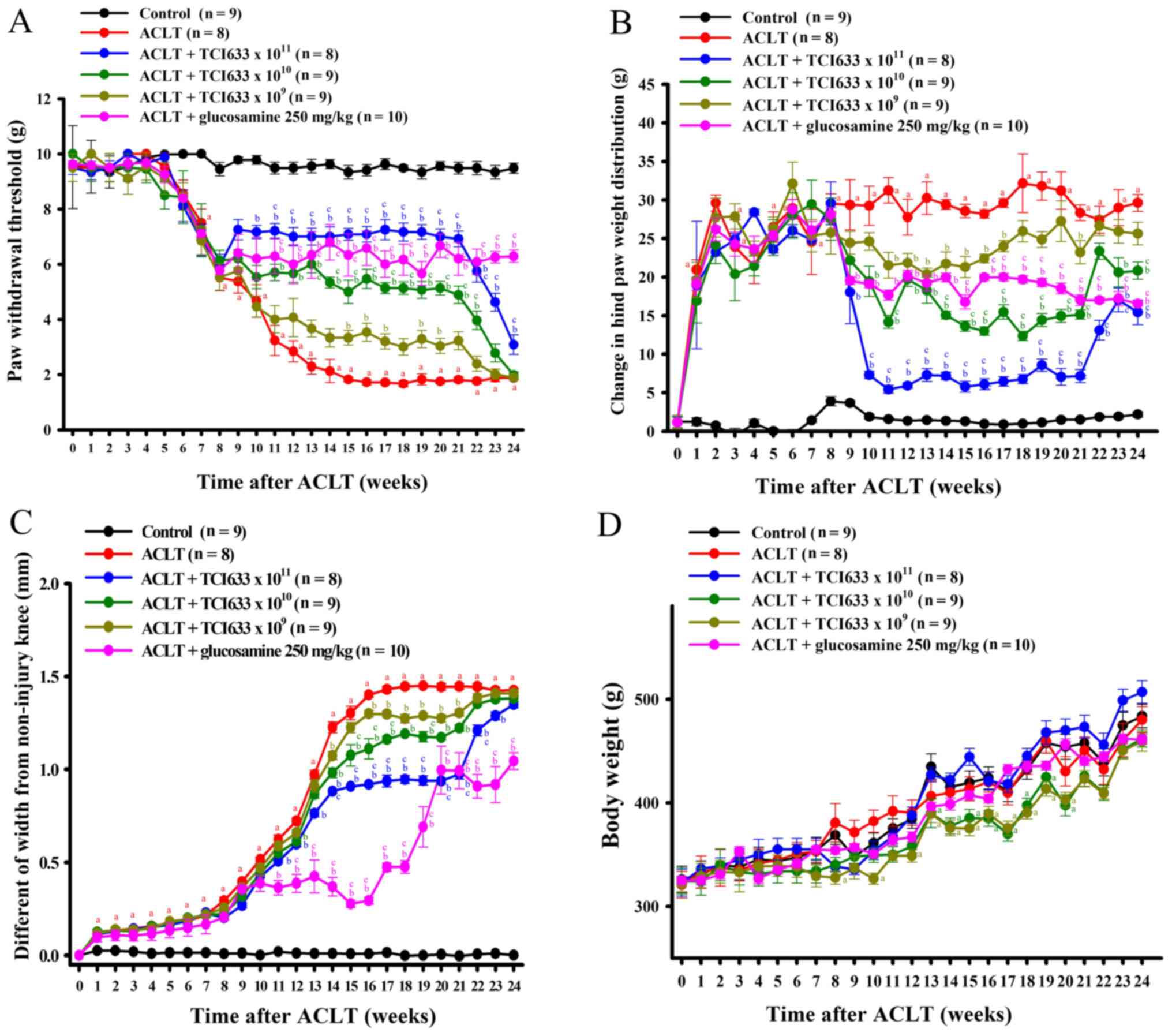
Effects of TCI633 on mechanical
allodynia
Evaluation of the treatment effects of TCI633 in
ACLT-induced mechanical allodynia showed that ACLT significantly
induced mechanical allodynia with the significant difference of
reduction threshold between the ACLT group and Control group
between weeks 7 to 24 (Fig. 1A).
The changes of mechanical allodynia were analyzed using ANOVA for
repeated measures, and the results showed significant effects in
the treatment groups and time. There was a statistically
significant interaction between treatment and time (data not
shown). Comparison of the ACLT+TCI633 (5x1011
CFU/kg/day) and ACLT group showed a significant increase of paw
withdrawal threshold between weeks 10 to 24 after ACLT surgery
(Fig. 1A). Comparison of the
ACLT+TCI633 (5x1010 CFU/kg/day) group and ACLT group
showed a significant increase of threshold between weeks 11 and 22
(Fig. 1A). Comparison of the
ACLT+TCI633 (5x109 CFU/kg/day) group and ACLT group
showed a significant increase of threshold between weeks 15 to 17
and 19 to 20 (Fig. 1A). Comparison
of the ACLT+glucosamine and ACLT group showed a significant
increase of threshold between weeks 11 to 24 after ACLT surgery
(Fig. 1A). Moreover, comparison of
the ACLT+TCI633 (5x1010 or 5x1011 CFU/kg/day)
group and ACLT ACLT+TCI633 (5x109 CFU/kg/day) group
showed a significant increase of threshold. Therefore, these
results suggested that TCI633 improves the paw withdrawal threshold
of ACLT-induced mechanical allodynia with dose-dependent effects.
Once TCI633 was no longer administered (weeks 21 to 24), there was
a downward trend for pain relief effects on mechanical allodynia in
all ACLT+TCI633 groups.
Effects of TCI633 on weight
bearing
Evaluation of the treatment effects of TCI633 on
ACLT-induced differences of weight bearing showed that ACLT
significantly induced differences of weight bearing distribution
between the ACLT group and Control group between weeks 1 to 24
(Fig. 1B). The changes of weight
bearing were analyzed using ANOVA for repeated measures, and the
results showed significant effects in the treatment groups and
time. There was a statistically significant interaction between
treatment and time. Comparison of the ACLT+TCI633
(5x1011 CFU/kg/day) and ACLT group showed the
significant decrease of the difference of weight bearing between
weeks 9 to 24 after ACLT surgery (Fig.
1B). Comparison of the ACLT+TCI633 (5x1010
CFU/kg/day) and ACLT groups showed a significant decrease in the
difference of weight bearing between weeks 10 to 21 and weeks 22 to
24 (Fig. 1B). Comparison of the
ACLT+TCI633 (5x109 CFU/kg/day) and ACLT groups showed a
significant decrease in the difference of weight bearing between
weeks 11 to 17 and week 21 (Fig.
1B). Comparison of the ACLT+glucosamine (250 mg/kg) and ACLT
groups showed a significant decrease in the difference of weight
bearing between weeks 9 to 24 (Fig.
1B). Moreover, comparison of the ACLT+TCI633 (5x1010
or 5x1011 CFU/kg/day) and ACLT+TCI633 (5x109
CFU/kg/day) groups showed a significant decrease in the difference
of weight bearing. Therefore, these results demonstrated that
TCI633 improved the effects of ACLT on weight bearing in a
dose-dependent manner. Once TCI633 was no longer administered
(weeks 21 to 24), there was an upward trend for pain relief effects
on weight bearing in all ACLT+TCI633 groups.
Effects of STCI633 on knee
swelling
Evaluation of the treatment effects of TCI633 in
ACLT-induced knee swelling showed that ACLT significantly induced
the swelling of the knee compared with the Control group between
weeks 1 to 24 (Fig. 1C). Comparison
of the ACLT+TCI633 (5x1011 CFU/kg/day) and ACLT groups
showed a significant decrease of swelling of the knee between weeks
11 to 23 after ACLT surgery (Fig.
1C). The changes of knee swelling were analyzed by ANOVA for
repeated measures, and the results showed significant effects in
the treatment groups and time. There was a statistically
significant interaction between treatment and time. Comparison of
the ACLT+TCI633 (5x1010 CFU/kg/day) ACLT group showed a
significant decrease of swelling of the knee between weeks 12 and
weeks 14 to 21 (Fig. 1C).
Comparison of the ACLT+TCI633 (5x109 CFU/kg/day) and
ACLT groups showed a significant decrease of swelling of the knee
between weeks 14 and weeks 16 to 21 (Fig. 1C). Comparison of the
ACLT+glucosamine (250 mg/kg) and ACLT groups showed a significant
decrease of swelling of the knee between weeks 11 to 24 (Fig. 1C). Moreover, comparison of the
ACLT+TCI633 (5x1010 or 5x1011 CFU/kg/day) and
ACLT ACLT+TCI633 (5x109 CFU/kg/day) groups showed a
significant decrease of swelling of the knee. In summary, these
results verified that TCI633 improved the effects on ACLT-induced
swelling of the knee with dose-dependent effects. Once TCI633 was
no longer administered (weeks 21 to 24), there was an upward trend
for pain relief effects on knee swelling from all ACLT+TCI633
groups.
Effects of TCI633 on body weight
change
The effect of TCI633 on body weight changes in ACLT
models (Fig. 1D) was analyzed using
ANOVA for repeated measures. The results showed significant effects
in the treatment groups and time. There was also a statistically
significant interaction between treatment and time. Results showed
that in terms of weight change, the ACLT and Control groups were
not significantly different from weeks 0 to 24, although a
time-dependent increase in weight was observed. After ACLT surgery,
comparison of the ACLT+TCI633 (5x1011 CFU/kg/day) and
ACLT groups showed no significant changes in weight from weeks 0 to
24 with a time-dependent increase in weight in both groups.
Comparison of the ACLT+TCI633 (5x1010 CFU/kg/day) and
Control groups showed a significant decrease in weight from weeks
13 to 20 (Fig. 1D). Comparison of
the ACLT+TCI633 (5x109 CFU/kg/day) and Control groups
showed a significant weight decrease from weeks 8 to 10 and weeks
13 to 20 (Fig. 1D). Comparison of
the ACLT+glucosamine and ACLT groups showed no significant weight
change from weeks 0 to 24. In summary, these results verified the
effect of TCI633 on weight change in ACLT models, with a slight
delay in weight increase in the ACLT+TCI633 (5x1010 and
5x109 CFU/kg/day) groups.
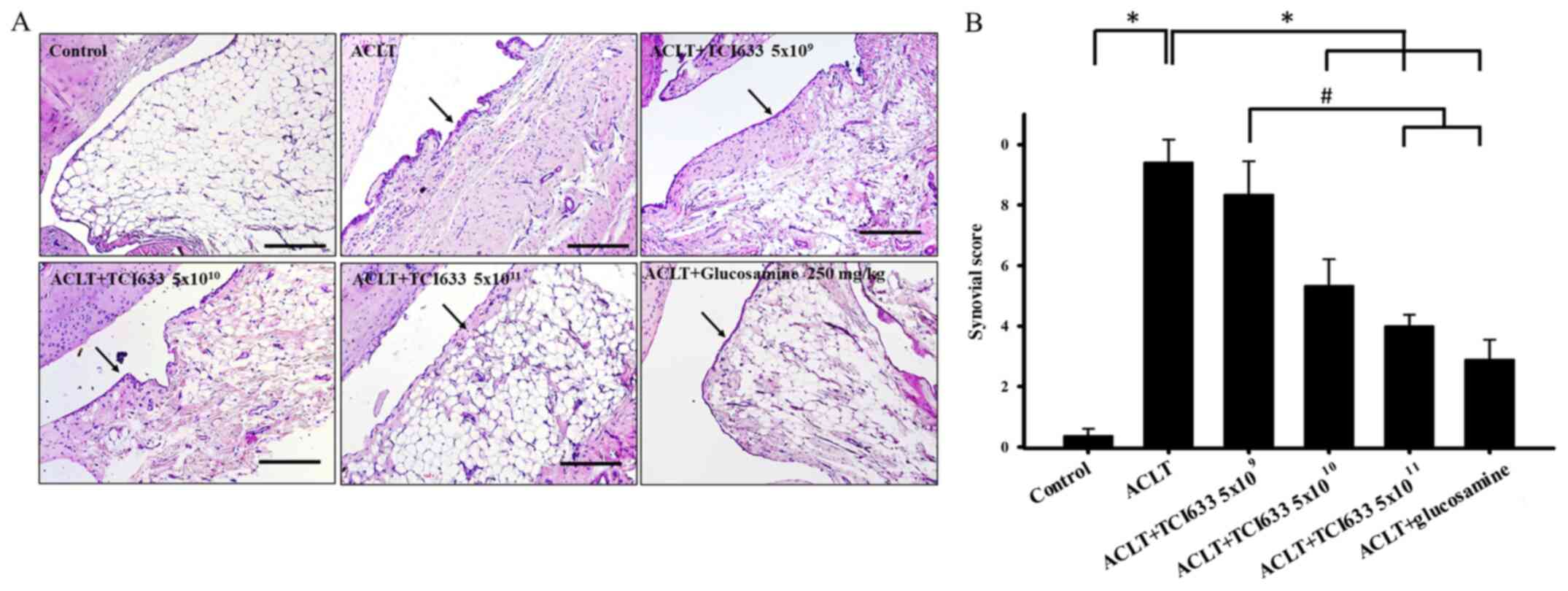
Effect of TCI633 on synovial
inflammation
Evaluation of the effect of TCI633 on synovial
inflammation in ACLT-treated rats compared with Control rats showed
infiltration of inflammatory cells, tissue hyperplasia and
hypertrophy (black arrows) in synovial tissue in week 24 (Fig. 2A). Comparison of the ACLT+TCI633
(5x109 CFU/kg/day) and ACLT groups demonstrated a
similar phenomenon. Comparison of the ACLT+TCI633
(5x1011 or 5x1010 CFU/kg/day) and ACLT groups
revealed slight inflammation with fewer inflammatory cells and
improvement of synovial tissue hyperplasia and hypertrophy at week
24 after ACLT (Fig. 2A). When
evaluating the effects of TCI633 on synovial inflammation of ACLT
rats using histopathological analysis, the ACLT group showed a
significant increase in synovial score compared with the Control
group (Fig. 2B). The ACLT+TCI633
(5x1011and 5x1010 CFU/kg/day) and
ACLT+glucosamine groups showed a significant decrease in synovial
score compared with ACLT group (Fig.
2B). Moreover, there was a significant decrease in synovial
score in ACLT+TCI633 (5x1011 CFU/kg/day) and
ACLT+glucosamine compared with the ACLT+TCI633 (5x109)
group (Fig. 2B). In summary, these
results verified that TCI633 and glucosamine significantly reduced
the effects of synovial tissue inflammation after ACLT.
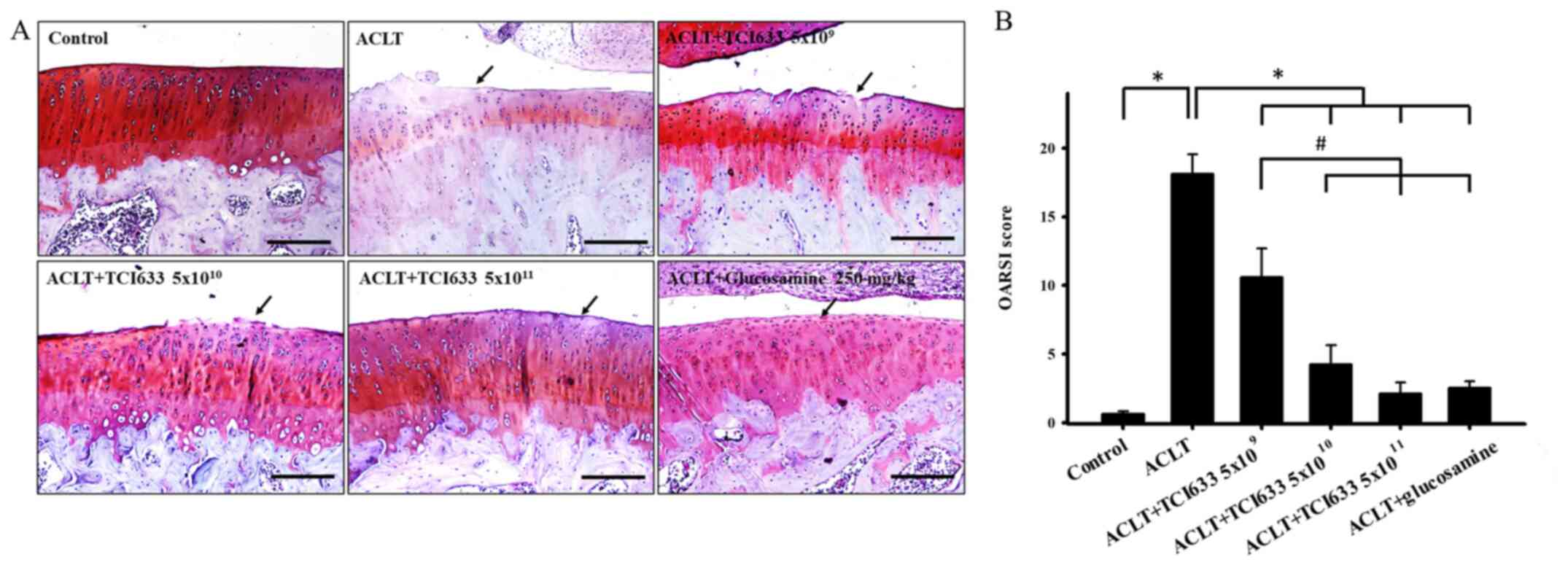
Effects of TCI633 on cartilage
damage
Evaluation of the effects of TCI633 on the cartilage
of knee after ACLT showed that, compared with the Control group,
ACLT resulted in obvious damage to surface of cartilage and
reduction of chondrocyte number. Safranin O/fast green staining
showed decreased signals (red) reflecting the intensity of the
cartilage layer in week 24 (Fig.
3A). While comparing the ACLT+TCI633 or ACLT+glucosamine groups
with the ACLT group, there were slight irregularities on the
surface of cartilage and a slight decrease in the intensity of
staining signal in cartilage was observed (Fig. 3). There was a significant increase
in OARSI score between the ACLT and Control groups (Fig. 3B). There was a significant decrease
in OARSI score in ACLT+TCI633 and glucosamine groups with compared
with ACLT group. Moreover, there was a significant decrease in
OARSI score in ACLT+TCI633 (5x1010 or 5x1011
CFU/kg/day) and ACLT+glucosamine groups compared with the
ACLT+TCI633 (5x109) group. These results verified that
TCI633 reduced the damage of cartilage after ACLT.
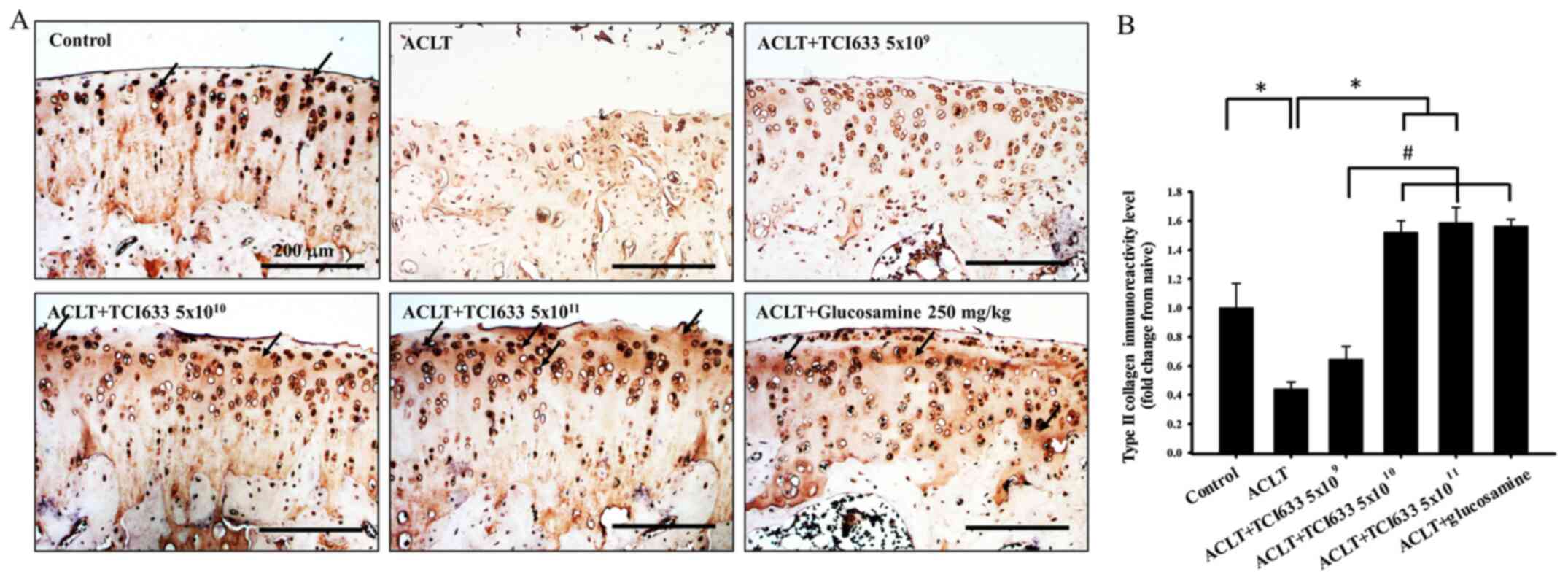
Effect of TCI633 in type II collagen
expression of cartilage
The effects of TCI633 on type II collagen expression
in the cartilage of ACLT-treated rats were evaluated. Comparison of
the ACLT and Control groups showed a notable reduction in type II
collagen expression in the cartilage after ACLT (Fig. 4). The ACLT+TCI633 (5x109
CFU/kg/day) group also displayed similar conditions compared with
the ACLT group (Fig. 4). In the
ACLT+TCI633 (5x1010 or 5x1011 CFU/kg/day) and
ACLT+glucosamine groups, there was an increasing trend in type II
collagen expression compared with the ACLT group. Quantitative
analysis of type II collagen expression in cartilage demonstrated
that the ACLT group had a significant decrease in immunoreactivity
compared with the Control group (Fig.
5B). In the ACLT+TCI633 (5x1010 or 5x1011
CFU/kg/day) and ACLT+glucosamine groups, there was a significant
increase in immunoreactivity compared with the ACLT group.
Moreover, there was a significant increase in immunoreactivity in
the ACLT+TCI633 (5x1010 or 5x1011 CFU/kg/day)
and ACLT+glucosamine groups compared with the ACLT+TCI633
(5x109 CFU/kg/day) group (Fig. 4B). In the ACLT+glucosamine group
there was a similar effect compared with the ACLT+TCI633
(5x1011 CFU/kg/day) group (Fig. 4B). These results suggested that
TCI633 increased type II collagen expression in the cartilage of
ACLT-rats.
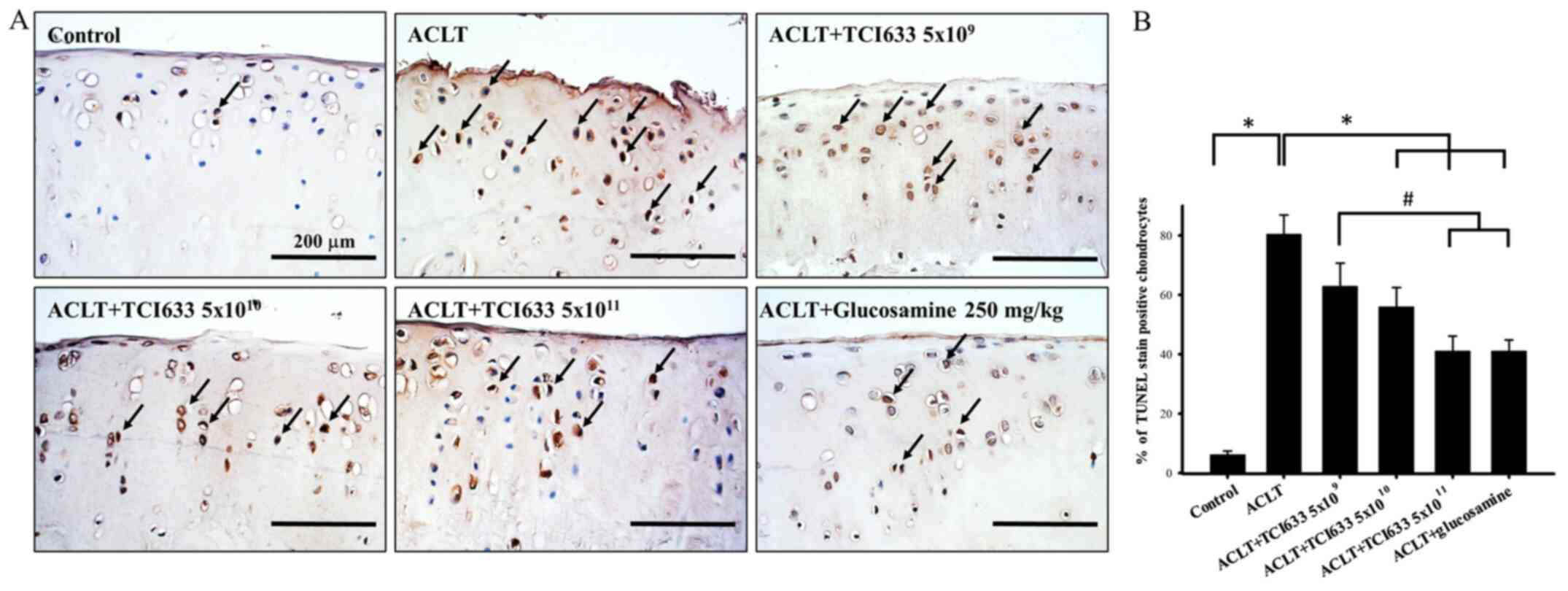
Effects of TCI633 on chondrocyte
apoptosis of cartilage
The effect of TCI633 in protecting chondrocytes in
the ACLT model was evaluated (Fig.
5). Under Control conditions, only a small ratio of
chondrocytes undergo apoptosis (Fig.
5B) The results showed that there was an evident condition of
chondrocyte apoptosis in week 24 after ACLT. With comparing with
the ACLT group, there was a reduction of chondrocyte apoptosis
observed in TCI633 (5x1010 or 5x1011
CFU/kg/day) and glucosamine treatment groups (Fig. 5B). Quantitative percentage analysis
showed that there was a clear increase in the percentage of
TUENL-positive chondrocytes in the ACLT group with compared the
Control groups in week 24. When comparing the TIC633 and
glucosamine treatment groups with the ACLT group, both show a clear
reduction in the percentage of TUNEL-positive cells. When comparing
the three different TIC633 dose groups with the ACLT group, there
is the dose-dependent effect of the reduction in the percentage of
TUNEL-positive cells. Moreover, there was a significant decrease in
the percentage of TUNEL-positive cells in the ACLT+TCI633
(5x1011 CFU/kg/day) groups and ACLT+glucosamine groups
compared with the ACLT+TCI633 (5x109 CFU/kg/day) group
(Fig. 5B). These results suggested
that TCI633 significantly protected chondrocytes against apoptosis
in the ACLT model.
Discussion
The purpose of the present study was to examine the
possible effects of TCI633 on OA using an ACLT-induced OA rat
model. A previous in vivo study have demonstrated that
earlier administration of hyaluronic acid could delay the
progression of OA in an ACLT rat model (8). Previous studies have reported that
TCI633 can effectively produce hyaluronic acid in the
gastrointestinal tract for absorption through the intestines,
resulting in increased hyaluronic acid content in the blood and
showing potential for treating osteoporosis (24,27).
The present results showed that TCI633 can effectively alleviate
the pain symptoms associated with OA in ACLT-induced rats. The
histopathological analysis also showed that oral administration of
TCI633 resulted in significant improvement of synovial
inflammation, cartilage damage and chondrocytes apoptosis after
ACLT surgery. Moreover, oral administration of TCI633 effectively
increased the presentation of type II collagen in the cartilage
after ACLT. Therefore, it was demonstrated that oral administration
of TCI633 could improve disease progression of OA in ACLT-treated
rats.
Past studies have demonstrated that the progression
of OA is accompanied by pain (1,3,43).
Previous in vivo studies have verified that the ACLT model
allows clear observation of mechanical allodynia and changes in
bipedal weight balance dispersion (9,10,44).
Moreover, there will be swelling accompanying the injured knee
joint with disease progression after ACLT (10,44).
Past studies have verified that hyaluronic acid injections can
effectively reduce arthritis pain and has therapeutic effects on OA
(22,45,46).
In vivo studies have also reported that administering
hyaluronic acid can effectively alleviate the disease progression
of arthritis caused by ACLT (5,38). A
previous study demonstrated that TCI633 can effectively produce
hyaluronic acid in the gastrointestinal tract and in blood content
(24), and the present study
observed that oral administration of TCI633 in ACLT models can
alleviate the pain and swelling. Moreover, the TCI633
(5x1010 or 5x1011 CFU/kg/day) groups and
glucosamine treatment groups had similar pain relief effects.
Therefore, it was demonstrated that TCI633 can effectively reduce
pain and inflammation after ACLT.
The primary causes for OA are long-term mechanical
stimulation and the aging process (2,3).
Symptoms such as cartilage damage, bone lesions, osteoporosis and
synovial tissue inflammation accompany the progression of OA
(6). In this study, the
histopathological staining showed observations of similar symptoms
of human OA after ACLT. The result showed that the synovial and
OARSI score were upregulated in the ACLT group. Thus, oral
administration of TCI633 and glucosamine could significantly
downregulate the index of synovial inflammation and OARSI scores
after ACLT. Moreover, administration medium or high doses of TCI633
and glucosamine had superior treatment effects compared with low
dose TCI633. Past studies demonstrated that hydrolysis of type II
collagen in cartilage would further exacerbate joint destruction of
OA (15,47,48).
The current study observed that type II collagen expression in
cartilage was decreased after ACLT, and administration of TCI633
and glucosamine could increase type II collagen expression in
cartilage. Injection of hyaluronic acid has been considered an
effective treatment to reduce synovial inflammation and increase
collagen II in cartilage after ACLT (49). Previous studies have also reported
that the TCI633 administration leads to a delaying bone loss and
strengthening bones (24,27). However, it is worth noting that
TCI633 also inhibits the gene expression of cathepsin K that plays
important role in the hydrolysis of type II collagen (27,50).
Therefore, the present study demonstrated that TIC633 can
effectively reduce the progression of OA and severity of associated
symptoms, and increase type II collagen expression in cartilage
after ACLT.
In OA disease progression, there is an association
between chondrocyte apoptosis and extracellular matrix loss of
cartilage tissue. The main association is that chondrocyte
apoptosis changes the synthesis, degeneration and breakage of
extracellular matrix in cartilage to form a vicious cycle in OA
disease progression (16,17,51).
Past studies have demonstrated that increasing type II collagen can
affect the survival rate of chondrocytes and injection of
hyaluronic acid could improve the rate of chondrocyte apoptosis
induced by ACLT (52,53). The present comparisons of the ACLT
and Control groups showed a significant increase in the amount of
chondrocyte apoptosis. Oral administration of TCI633 and
glucosamine effectively reduced the amount of chondrocyte
apoptosis. The administration of high doses of TCI633 and
glucosamine showed a significant difference with the low dosage
TCI633 group. Therefore, the present study demonstrated that oral
administration of TCI633 increased type II collagen expression in
cartilage and reduced chondrocyte apoptosis to alleviate the
effects of OA after ACLT.
Overall, the current study utilized ACLT to induce
OA to investigate the therapeutic effects of TCI633 in OA.
Nociceptive behaviors and joint swelling were induced in rats by
ACLT. The results showed that oral administration of TCI633
effectively alleviated pain and knee joint swelling after ACLT.
Simultaneously, medium to high doses of TCI633 showed similar pain
relief effects compared with the glucosamine group.
Histopathological analysis showed that oral administration of
TCI633 can effectively improve the symptoms of OA, including
synovial inflammation and cartilage damage with significant
therapeutic effects. Moreover, via analyzing type II collagen and
chondrocyte apoptosis, oral administration of TCI633 effectively
increased type II collagen expression in cartilage and reduced
chondrocyte apoptosis to slow the symptoms of OA after ACLT. Thus,
the present study demonstrated that oral administration of TCI633
could be useful for suppressing pain and reducing joint swelling
through reducing synovial inflammation and cartilage damage in OA.
In conclusion, oral administration of TCI633 for long-term use
could be used alleviate the symptoms of OA in humans.
Acknowledgements
Not applicable.
Funding
This study was supported by TCI Co., Ltd.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YYL, NFC and ZHW conceived and designed the
experiments. YYL, PCC, CWF and YWL performed the experiments. YYL
and ZHW confirm the authenticity of all the raw data. SNY, YHJ, HMK
and YCL analyzed the data. YYL, NFC, YCL and ZHW wrote the paper.
The raw data of this study are available from first author and the
corresponding author. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Animal
Care and Use Committee of National Sun Yat-sen University (approval
no. 10417).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Litwic A, Edwards MH, Dennison EM and
Cooper C: Epidemiology and burden of osteoarthritis. Br Med Bull.
105:185–199. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neogi T: The epidemiology and impact of
pain in osteoarthritis. Osteoarthritis Cartilage. 21:1145–1153.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Castrogiovanni P, Di Rosa M, Ravalli S,
Castorina A, Guglielmino C, Imbesi R, Vecchio M, Drago F,
Szychlinska MA and Musumeci G: Moderate physical activity as a
prevention method for knee osteoarthritis and the tole of
dynoviocytes as niological key. Int J Mol Sci.
20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Naito K, Watari T, Furuhata A, Yomogida S,
Sakamoto K, Kurosawa H, Kaneko K and Nagaoka I: Evaluation of the
effect of glucosamine on an experimental rat osteoarthritis model.
Life Sci. 86:538–543. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cohen-Solal M, Funck-Brentano T and Hay E:
Animal models of osteoarthritis for the understanding of the bone
contribution. Bonekey Rep. 2(422)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carmona FD, González-Gay MA and Martín J:
Genetic component of giant cell arteritis. Rheumatology (Oxford).
53:6–18. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsai WY, Wu JL, Liu CC, Cherng CH, Tsai
RY, Jean YH and Wong CS: Early intraarticular injection of
hyaluronic acid attenuates osteoarthritis progression in anterior
cruciate ligament-transected rats. Connect Tissue Res. 54:49–54.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wen ZH, Tang CC, Chang YC, Huang SY, Chen
CH, Wu SC, Hsieh SP, Hsieh CS, Wang KY, Lin SY, et al:
Intra-articular injection of the selective cyclooxygenase-2
inhibitor meloxicam (Mobic) reduces experimental osteoarthritis and
nociception in rats. Osteoarthritis Cartilage. 21:1976–1986.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wen ZH, Tang CC, Chang YC, Huang SY, Lin
YY, Hsieh SP, Lee HP, Lin SC, Chen WF and Jean YH: Calcitonin
attenuates cartilage degeneration and nociception in an
experimental rat model of osteoarthritis: Role of TGF-β in
chondrocytes. Sci Rep. 6(28862)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhen G, Wen C, Jia X, Li Y, Crane JL,
Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al: Inhibition
of TGF-β signaling in mesenchymal stem cells of subchondral bone
attenuates osteoarthritis. Nat Med. 19:704–712. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Giunta S, Castorina A, Marzagalli R,
Szychlinska MA, Pichler K, Mobasheri A and Musumeci G: Ameliorative
effects of PACAP against cartilage degeneration. Morphological,
immunohistochemical and biochemical evidence from in vivo and in
vitro models of rat osteoarthritis. Int J Mol Sci. 16:5922–5944.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rengel Y, Ospelt C and Gay S: Proteinases
in the joint: Clinical relevance of proteinases in joint
destruction. Arthritis Res Ther. 9(221)2007.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Eyre D: Collagen of articular cartilage.
Arthritis Res. 4:30–35. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Poole AR, Kobayashi M, Yasuda T, Laverty
S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, et al:
Type II collagen degradation and its regulation in articular
cartilage in osteoarthritis. Ann Rheum Dis. 61 (Suppl 2):ii78–ii81.
2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu L and Liu Z: The molecular mechanisms
of preventing apoptosis of cartilage chondrocyte to target
osteoarthritis. Future Med Chem. 9:537–540. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Musumeci G, Castrogiovanni P, Loreto C,
Castorina S, Pichler K and Weinberg AM: Post-traumatic caspase-3
expression in the adjacent areas of growth plate injury site: A
morphological study. Int J Mol Sci. 14:15767–15784. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Musumeci G, Loreto C, Carnazza ML and
Martinez G: Characterization of apoptosis in articular cartilage
derived from the knee joints of patients with osteoarthritis. Knee
Surg Sports Traumatol Arthrosc. 19:307–313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lespasio MJ, Piuzzi NS, Husni ME, Muschler
GF, Guarino A and Mont MA: Knee Osteoarthritis: A Primer. Perm J.
21:16–183. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sinusas K: Osteoarthritis: Diagnosis and
treatment. Am Fam Physician. 85:49–56. 2012.PubMed/NCBI
|
|
23
|
Ma VY, Chan L and Carruthers KJ:
Incidence, prevalence, costs, and impact on disability of common
conditions requiring rehabilitation in the United States: Stroke,
spinal cord injury, traumatic brain injury, multiple sclerosis,
osteoarthritis, rheumatoid arthritis, limb loss, and back pain.
Arch Phys Med Rehabil. 95:986–995.e1. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin YH, Su HL and Yu CH: Method of
enhancing hyaluronic acid secretion using probiotic strain. US
Patent US9289455B2. Filed March 26, 2015; issued October 15,
2015.
|
|
25
|
Ogita T, Nakashima M, Morita H, Saito Y,
Suzuki T and Tanabe S: Streptococcus thermophilus ST28
ameliorates colitis in mice partially by suppression of
inflammatory Th17 cells. J Biomed Biotechnol.
2011(378417)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ai C, Zhang Q, Ren C, Wang G, Liu X, Tian
F, Zhao J, Zhang H, Chen YQ and Chen W: Genetically engineered
Lactococcus lactis protect against house dust mite allergy
in a BALB/c mouse model. PLoS One. 9(e109461)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lin YH, Chen IH, Shih HH and Lee CY:
Method for preventing and/or treating osteoporosis by using
Streptococcus thermophilus TC1633 strain and its
metabolites. US Patent US10149871B2. Filed June 21, 2017; issued
December 21, 2017.
|
|
28
|
Balazs EA and Denlinger JL:
Viscosupplementation: A new concept in the treatment of
osteoarthritis. J Rheumatol Suppl. 39:3–9. 1993.PubMed/NCBI
|
|
29
|
Shimizu C, Yoshioka M, Coutts RD, Harwood
FL, Kubo T, Hirasawa Y and Amiel D: Long-term effects of hyaluronan
on experimental osteoarthritis in the rabbit knee. Osteoarthritis
Cartilage. 6:1–9. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Abatangelo G, Botti P, Del Bue M, Gei G,
Samson JC, Cortivo R, De Galateo A and Martelli M: Intraarticular
sodium hyaluronate injections in the Pond-Nuki experimental model
of osteoarthritis in dogs. I. Biochemical results. Clin Orthop
Relat Res. 241:278–285. 1989.PubMed/NCBI
|
|
31
|
Yoshimi T, Kikuchi T, Obara T, Yamaguchi
T, Sakakibara Y, Itoh H, Iwata H and Miura T: Effects of
high-molecular-weight sodium hyaluronate on experimental
osteoarthrosis induced by the resection of rabbit anterior cruciate
ligament. Clin Orthop Relat Res. 298:296–304. 1994.PubMed/NCBI
|
|
32
|
Altman RD and Moskowitz R: Hyalgan Study
Group. Intraarticular sodium hyaluronate (Hyalgan) in the treatment
of patients with osteoarthritis of the knee: A randomized clinical
trial. J Rheumatol. 25:2203–2212. 1998.PubMed/NCBI
|
|
33
|
Amiel D, Toyoguchi T, Kobayashi K, Bowden
K, Amiel ME and Healey RM: Long-term effect of sodium hyaluronate
(Hyalgan) on osteoarthritis progression in a rabbit model.
Osteoarthritis Cartilage. 11:636–643. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tamoto K, Tada M, Shimada S, Nochi H and
Mori Y: Effects of high-molecular-weight hyaluronates on the
functions of guinea pig polymorphonuclear leukocytes. Semin
Arthritis Rheum. 22 (Suppl 1):4–8. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sheehan KM, DeLott LB, Day SM and DeHeer
DH: Hyalgan has a dose-dependent differential effect on macrophage
proliferation and cell death. J Orthop Res. 21:744–751.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ghosh P, Read R, Numata Y, Smith S,
Armstrong S and Wilson D: The effects of intraarticular
administration of hyaluronan in a model of early osteoarthritis in
sheep. II. Cartilage composition and proteoglycan metabolism. Semin
Arthritis Rheum. 22 (Suppl 1):31–42. 1993.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang PY, Tang CC, Chang YC, Huang SY,
Hsieh SP, Fan SS, Lee HP, Lin SC, Chen WF, Wen ZH, et al: Effects
of tibolone on osteoarthritis in ovariectomized rats: Association
with nociceptive pain behaviour. Eur J Pain. 18:680–690.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wen ZH, Tang CC, Chang YC, Huang SY, Hsieh
SP, Lee CH, Huang GS, Ng HF, Neoh CA, Hsieh CS, et al: Glucosamine
sulfate reduces experimental osteoarthritis and nociception in
rats: Association with changes of mitogen-activated protein kinase
in chondrocytes. Osteoarthritis Cartilage. 18:1192–1202.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fernihough J, Gentry C, Malcangio M, Fox
A, Rediske J, Pellas T, Kidd B, Bevan S and Winter J: Pain related
behaviour in two models of osteoarthritis in the rat knee. Pain.
112:83–93. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lee CH, Wen ZH, Chang YC, Huang SY, Tang
CC, Chen WF, Hsieh SP, Hsieh CS and Jean YH: Intra-articular
magnesium sulfate (MgSO4) reduces experimental
osteoarthritis and nociception: Association with attenuation of
N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and
apoptosis in rat chondrocytes. Osteoarthritis Cartilage.
17:1485–1493. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bartley EJ, Palit S and Staud R:
Predictors of osteoarthritis pain: The importance of resilience.
Curr Rheumatol Rep. 19(57)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kao JH, Lin SH, Lai CF, Lin YC, Kong ZL
and Wong CS: Shea nut oil triterpene concentrate attenuates knee
osteoarthritis development in rats: Evidence from knee joint
histology. PLoS One. 11(e0162022)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Monticone M, Frizziero A, Rovere G,
Vittadini F, Uliano D, LA Bruna S, Gatto R, Nava C, Leggero V and
Masiero S: Hyaluronic acid intra-articular injection and exercise
therapy: Effects on pain and disability in subjects affected by
lower limb joints osteoarthritis. A systematic review by the
Italian Society of Physical and Rehabilitation Medicine (SIMFER).
Eur J Phys Rehabil Med. 52:389–399. 2016.PubMed/NCBI
|
|
46
|
Hashizume M, Koike N, Yoshida H, Suzuki M
and Mihara M: High molecular weight hyaluronic acid relieved joint
pain and prevented the progression of cartilage degeneration in a
rabbit osteoarthritis model after onset of arthritis. Mod
Rheumatol. 20:432–438. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Stoop R, Buma P, van der Kraan PM,
Hollander AP, Billinghurst RC, Meijers TH, Poole AR and van den
Berg WB: Type II collagen degradation in articular cartilage
fibrillation after anterior cruciate ligament transection in rats.
Osteoarthritis Cartilage. 9:08–315. 2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bakilan F, Armagan O, Ozgen M, Tascioglu
F, Bolluk O and Alatas O: Effects of native type II collagen
treatment on knee osteoarthritis: A randomized controlled trial.
Eurasian J Med. 48:95–101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang Z, Wei X, Gao J, Zhao Y, Zhao Y, Guo
L, Chen C, Duan Z, Li P and Wei L: Intra-articular injection of
cross-linked Hyaluronic acid-dexamethasone hydrogel attenuates
osteoarthritis: An experimental study in a rat model of
osteoarthritis. Int J Mol Sci. 17(411)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dejica VM, Mort JS, Laverty S, Percival
MD, Antoniou J, Zukor DJ and Poole AR: Cleavage of type II collagen
by cathepsin K in human osteoarthritic cartilage. Am J Pathol.
173:161–169. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Aigner T and Kim HA: Apoptosis and
cellular vitality: Issues in osteoarthritic cartilage degeneration.
Arthritis Rheum. 46:1986–1996. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lotz M, Hashimoto S and Kühn K: Mechanisms
of chondrocyte apoptosis. Osteoarthritis Cartilage. 7:389–391.
1999.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Barreto RB, Sadigursky D, de Rezende MU
and Hernandez AJ: Effect of hyaluronic acid on chondrocyte
apoptosis. Acta Ortop Bras. 23:90–93. 2015.PubMed/NCBI View Article : Google Scholar
|