Introduction
Cholelithiasis is a prevalent gastrointestinal
disease that has a prevalence of ~10-15% in developed countries and
~13% in China (1,2). However, this rate is increasing on an
annual basis mainly due to dietary and lifestyle changes (3). Cholelithiasis is caused by multiple
factors, including cholesterol supersaturation in the bile,
abnormal gallbladder function and impairments in enterohepatic bile
acid circulation, where cholesterol supersaturation is a necessary
condition for the onset of disease (4). Under physiological conditions,
relative concentrations of cholesterol, phospholipids and bile
acids in bile are regulated in balance. Supersaturation occurs when
the levels of cholesterol are too high or when there is an
insufficient solubility of phospholipids and bile acids, which in
turn affect this dynamic balance (5). Excessive cholesterol accumulation can
then form crystals, eventually forming gallstones (6).
Cholesterol metabolism serves a critical role in
cholelithiasis. In total, ~50% cholesterol is directly secreted
into the bile by the coordinated functions of cholesterol
transporters ATP-binding cassette subfamily G member (ABCG) 5/8
(ABCG5/8) and scavenger receptor class B type I (SRBI) (7). ABCG5/8 is a heterodimer that is
localized in the canalicular membrane of hepatocytes and is
responsible for transporting cholesterol out of hepatocytes, whilst
SRBI can transport cholesterol bi-directionally and convert it into
bile acid (8,9). The secretion of cholesterol also
requires the assistance of bile acids and phospholipids, which are
separately mediated by the bile salt export pump ABCB11 and the
phospholipid transporter ABCB4(10). Cholesterol absorption is
multifaceted processes that is regulated by a number of factors on
the enterocyte level. Previous studies have shown that ABCG5 and
ABCG8, which are also highly expressed on the apical brush border
membranes of enterocytes, can reduce cholesterol absorption and
promote the active efflux of cholesterol from the enterocyte into
the intestinal lumen for excretion (11,12).
Niemann-Pick C1 Like 1 (NPC1L1) is an essential transporter that
mediates exogenous cholesterol uptake by the enterocyte (13). The capacity of the small intestine
tissue to absorb cholesterol can also significantly affect the
concentration of cholesterol in the human blood circulation
(14).
Although patients with cholelithiasis are generally
asymptomatic, progression of this disease can result in serious
consequences. Laparoscopic cholecystectomy is considered to be the
gold-standard therapy for patients with symptomatic cholelithiasis,
which can reduce pain and produce low rates of recurrence (15). However, this type of surgery is
associated with a variety of adverse events, including functional
dyspepsia, gastroesophageal reflux disease, postcholecystectomy
syndrome and bile duct injury (16). Therefore, alternative treatment
approaches remain in demand for patients with cholelithiasis.
Traditional Chinese Medicine (TCM) have been
considered for the treatment of cholelithiasis, since it has been
reported to effectively discharge gallstones and prevent the
recurrence of this condition (17).
Yinchenhao decoction (YCHD, Inchin-ko-to or TJ-135 in Japan) is a
well-known TCM formulation that was first recorded in the Treatise
on Febrile Diseases (Shanghan Lun) by Zhongjing Zhang in 150-215
A.D. and has been widely used for the treatment of hepatic and
biliary disorders. This prescription is comprised of three Chinese
medicinal herbs: Artemisiae scopariae herba (Yinchen),
Gardeniae Fructus (Zhizi) and Radix et Rhizoma Rhei
(Dahuang) (18,19). These ingredients exhibit synergistic
properties that intensify the therapeutic efficacy of YCHD towards
hepatic injury according to a previous biochemical analysis
(20). Pharmacological studies have
previously indicated that YCHD can inhibit hepatic steatosis,
apoptosis, necrosis, anti-inflammation and regulate immunization,
in addition to exhibiting choleretic effects (21-24).
Previous in vivo experiments have also demonstrated that
YCHD exerts beneficial preventative and therapeutic effects against
lipid disturbance in rats (25,26).
In addition, YCHD has also been revealed to confer potent
ameliorative effects on rats with cholestasis, which was associated
with its regulatory actions on the expression of metabolic enzymes
and transporters including UDP-glucuronosyltransferase 1-A1,
multidrug resistance-associated protein 2, bile salt export pump
and organic anion-transporting polypeptide 1a4 in the cholestatic
liver (27).
Although YCHD has been applied for centuries for
treating cholelithiasis in clinical practice in China, the possible
underlying mechanism remains unclear. Therefore, the aim of the
present study was to investigate the effects and potential
mechanism of YCHD on mice with lithogenic diet (LD)-induced
cholelithiasis in vivo, to provide a scientific and
objective theoretical basis for clinical treatment using YCHD.
Materials and methods
Drug preparation
YCHD consists of Artemisiae scopariae herba
(27 g), Gardeniae Fructus (6 g), and Radix et Rhizoma
Rhei (9 g) (28). The medicinal
herbs were obtained from the Chinese Pharmacy at Shuguang Hospital
Affiliated to Shanghai University of Traditional Chinese Medicine.
The process of extraction was performed according to a previous
published patent (patent no. ZL201010501560.7) and article
(29). Briefly, herbs were soaked
in distilled water (1:10, w/v) for 2 h at 20˚C and then boiled
twice for 30 min at 100˚C. The twice-boiled solutions were blended
and filtered through a 0.2 µm syringe filter (EMD Millipore).
Finally, the filtered liquids were concentrated under pressure at
60˚C and stored at 4˚C. tauroursodeoxycholic acid (TUDCA) is a
water-soluble bile acid that is commonly used in clinical settings
(30) and was provided by
Bruschettini S.R.L. TUDCA was used as the positive control drug in
the present study.
Animals and treatments
A total of 100 male specific-pathogen-free grade
C57BL/6 mice (age, 8 weeks; weight, 22.5±2.5 g) were purchased from
The Shanghai Model Organisms Center, Inc. All animals were housed
in a controlled environment with the temperature at 22±1˚C,
relative humidity of 60±10% and a 12-h light/dark cycle. Mice had
free access to food and water throughout experimentation. All
animal studies, including the mice euthanasia procedure, were
approved and performed in compliance with the regulations and
guidelines of the Ethics Committee of Shanghai University of
Traditional Chinese Medicine and conducted according to the
Association for Assessment and Accreditation of Laboratory Animal
Care and the institutional animal care and use committee guidelines
(approval no. PZSHUTCM191108007) (31,32).
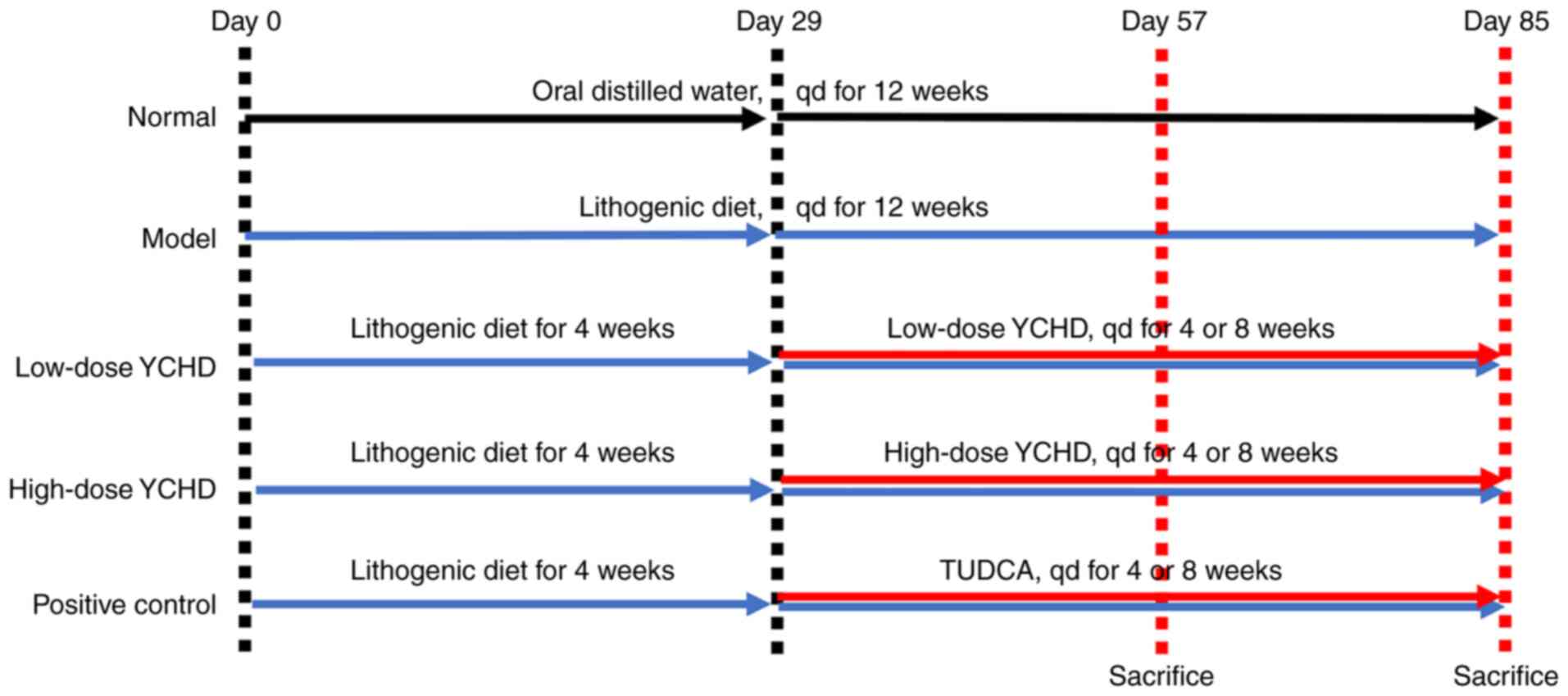
After 1 week of acclimatization, mice were randomly
divided into five groups (n=20 mice per group; Fig. 1): i) Normal (N); ii) model (M); iii)
low dose YCHD (YCHD-L); iv) high dose YCHD (YCHD-H); and v)
positive control (TUDCA). Throughout the experiment, mice in the
normal group were fed with standard mice chow, whilst all other
mice were fed on a lithogenic diet, which consists of standard chow
diet containing 1.25% cholesterol and 0.5% cholic acid (33). In addition, mice in the normal and
model groups were provided with distilled water via oral gavage
once a day from weeks 5 to 12. Mice in the YCHD-L group were given
a daily dose of 7 g/kg body weight YCHD, whilst those in the YCHD-H
group were given a daily dose of 14 g/kg body weight YCHD. All YCHD
treatments were administered once a day, for 8 consecutive weeks
from weeks 5 to 12. Mice in the TUDCA group were given a daily dose
of 100 mg/kg TUDCA via oral gavage once a day for 8 consecutive
weeks from weeks 5 to 12. The amount of the TUDCA drug is
equivalent to the clinical dose of 60 kg/day body weight in adults
(34).
Animal health and behavior were monitored every day.
In total, 10 mice in each group were randomly sacrificed at the end
of week 8, whilst the rest of the mice were sacrificed at the end
of week 12. The humane endpoints established in the present study
for animal euthanasia are as follows: i) Lack of responsiveness to
manual stimulation; ii) immobility; and/or iii) an inability to eat
or drink freely. Mice were anesthetized with 1% pentobarbital
sodium (40 mg/kg) by intraperitoneal injection prior to
exsanguination from the retroorbital vein. Mice were sacrificed
with 150 mg/kg pentobarbital sodium by intraperitoneal injection.
100 µl serum, as well as liver, gallbladder and intestine tissue
samples, were then collected and stored at -80˚C for subsequent
analysis.
Biochemical assays
Serum was obtained by centrifuging the blood at
3,000 x g for 15 min at 4˚C. The kits used for the determination of
serum alanine aminotransferase (ALT; cat. no. HC41T02) and alkaline
phosphatase (ALP) activities (cat. no. HC02T03), in addition to the
levels of total cholesterol (TC; cat. no. HC12S03) and low-density
lipoprotein cholesterol (LDL-C; cat. no. HC73T03) were purchased
from Shanghai Huachen biological reagent Co. Ltd. Serum ALT and ALP
activities and the levels of TC and LDL-C were measured using a
TBA-40FR Biochemical Analyzer (Toshiba Corporation) according to
the manufacturer's experimental protocol.
Analysis of cholelithiasis and
bile
The presence of cholesterol crystals or stones in
the bile was clearly visible to the naked eye and was graded
according to the six-level judgment criteria previously described
by Akiyoshi et al (35) in a
double-blinded manner at the end of week 8 and 12. These criteria
were as follows: i) Grade 0, gallbladder is filled with clear bile;
ii) grade I, a few fine crystals are found; iii) grade II, ~10 fine
crystals are found; iv) grade III: Fine crystals occupy ~50% of the
gallbladder; v) grade IV, crystals occupy >50% of the
gallbladder; and vi) grade V, round gallstones are found. Bile was
collected through common bile duct drainage. The levels of TC,
phospholipids (PL) and total bile acid (TBA) in the bile were
measured by enzyme colorimetry according to the manufacturer's
experimental protocol, which were then used to calculate the
cholesterol saturation index [CSI, moles percent (TC) x
100/(TC+PL+TBA)] (36). The TC
detection kit was purchased from Roche Diagnostics (cat. no.
11491458216), the PL kit was from Wako Pure Chemical Industries
Ltd. (cat. no. 296-63801) and the TBA kit was obtained from Randox
Laboratories, Ltd. (cat. no. BI2672). All kits were used in
accordance with the manufacturer's protocol.
Histopathological analysis
Liver tissues were fixed in 10% formalin for 24 h at
room temperature, dehydrated in an ascending ethanol gradient,
embedded in paraffin and cut into 5-µm-thick sections. The sections
were then stained with hematoxylin and eosin (H&E, hematoxylin
for 5 min and eosin for 20 sec at room temperature) and observed
under a light microscope (IX70; Olympus Corporation; magnification,
x100).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the liver and proximal
small intestine tissues using TRIzol® reagent according
to manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA concentration was determined by measuring the absorbance
at wavelengths of 260 and 280 nm using the Nanodrop™ 2000 ultra
microspectrophotometer (Thermo Fisher Scientific, Inc.). High
Capacity cDNA Reverse Transcription Kit (cat. no. 4368814; Thermo
Fisher Scientific, Inc.) was used for reverse transcribing total
RNA into cDNA, according to the manufacturer's protocol. Relative
mRNA expression was then measured in triplicate in the QuantStudio™
6 Flex Real-time PCR Instrument, using PowerUp™ SYBR™ Green Master
Mix kit (cat. no. A25742; Thermo Fisher Scientific Inc.). The
thermocycling conditions for the PCR assay cycles were as follows:
Initial denaturation at 95˚C for 1 min, followed by 45 cycles of
95˚C for 15 sec and 60˚C for 1 min. GAPDH was used as the
normalizing control gene, where the mRNA levels of target genes
were measured using the 2-ΔΔCq method (37). The forward and reverse primer
sequences that were used in the present study are shown in Table I.
 | Table IPCR primer sequences. |
Table I
PCR primer sequences.
| Gene | Primers | Sequence
(5'-3') |
|---|
| GAPDH | Forward |
TGTGTCCGTCGTGGATCTGA |
| | Reverse |
CCTGCTTCACCACCTTCTTGAT |
| ABCG5 | Forward |
AATGCTGTGAATCTGTTTCCCA |
| | Reverse |
CCACTTATGATACAGGCCATCCT |
| ABCG8 | Forward |
CTGTGGAATGGGACTGTACTTC |
| | Reverse |
GTTGGACTGACCACTGTAGGT |
| SRBI | Forward |
CGGGAGCGTGGACCCTATGT |
| | Reverse |
ACACGGTGTCGTTGTCATTGA |
| ABCB11 | Forward |
CAATAGACAGGCAACCCGTCA |
| | Reverse |
GTGGAACTCAATTTCGCCCTT |
| ABCB4 | Forward |
GCAGCGAGAAACGGAACAG |
| | Reverse |
GGTTGCTGATGCTGCCTAGTT |
| NP1CL1 | Forward |
ATCCTCATCCTGGGCTTTGC |
| | Reverse |
GCAAGGTGATCAGGAGGTTGA |
Statistical analysis
All quantitative data were presented as the mean ±
SD and analyzed using the SPSS software (version 22.0; SPSS, Inc.).
One-way ANOVA was used to analyze the differences between the
groups, followed by Tukey's test. Grade data were analyzed using
the Kruskal-Wallis test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of YCHD on the general parameters of
mice. Throughout the experiment, the vitality and stools of
mice were basically normal in appearance. Following prolonged
feeding time of LD, the body weights of mice in the model group
were significantly decreased compared with those in the normal
group, whilst the liver weights and liver /body weight ratio of
were also significantly increased (P<0.01; Table II). Compared with those in the
model group, mice in the YCHD-treated groups exhibited
significantly reduced the liver weights (P<0.01) and liver/body
weight ratios (P<0.05; Table
II), where high dose YCHD for 8 weeks demonstrated greater
potency. Although treatment with TUDCA significantly reduced the
liver weights and liver/body weight ratios in mice compared with
those in the model group (P<0.01), no significant difference
between the model and TUDCA groups was observed in body weight.
 | Table IIBiochemical parameters of serum and
liver. |
Table II
Biochemical parameters of serum and
liver.
| A, After treatment
for 4 weeks |
|---|
| Parameters | N | M | YCHD-L | YCHD-H | TUDCA |
|---|
| Number of mice | 10 | 10 | 9 | 10 | 10 |
| Body weight
(g) | 27.85±3.31 |
24.65±1.92a | 24.05±1.15 | 24.09±1.19 | 23.53±1.29 |
| Liver weight
(g) | 1.13±0.16 |
1.99±0.23a | 2.02±0.18 | 1.79±0.12 |
1.46±0.28c,e |
| Liver/body weight
ratio (%) | 4.06±0.32 |
8.07±0.45a | 8.4±0.61 |
7.47±0.61d |
6.18±0.94c,e |
| ALT (U/l) | 40.57±14.21 |
412.45±176.27a | 355.43±77.11 |
201.44±78.72c |
98.3±68.59c,d |
| ALP (U/l) | 51.71±12.45 |
220.4±73.43a | 201.14±65.03 | 159.38±40.65 |
124.4±49.99c |
| TC (mmol/l) | 2.5±0.62 |
5.12±1.25a | 5.43±0.7 | 4.69±1.19 | 3.74±1.61 |
| LDL-C (mmol/l) | 0.23±0.07 |
0.98±0.29a | 0.94±0.06 | 0.9±0.24 | 0.74±0.34 |
| B, After treatment
for 8 weeks |
| Parameters | N | M | YCHD-L | YCHD-H | TUDCA |
| Number of mice | 10 | 9 | 9 | 10 | 10 |
| Body weight
(g) | 32.69±3.29 |
22.81±1.92a | 22.38±2.24 | 23.06±2.4 | 23.42±1.18 |
| Liver weight
(g) | 1.21±0.13 |
2.83±0.37a |
2.4±0.35b |
2.2±0.24c |
1.45±0.27c,e |
| Liver/body weight
ratio (%) | 3.69±0.15 |
12.4±1.22a | 10.86±2.26 |
9.57±0.86c |
6.18±0.85c,e |
| ALT (U/l) | 46.29±5.31 |
595.63±234.26a |
350.22±84.47c |
289.14±71.99c |
76.6±89.45c,e |
| ALP (U/l) | 63.57±11.36 |
333.38±68.32a | 247.56±99.45 |
170.43±52.28c |
132.5±50.32c,e |
| TC (mmol/l) | 1.92±0.61 |
5.76±0.86a | 4.36±1 |
3.72±1.68c |
2.76±0.91c,d |
| LDL-C (mmol/l) | 0.17±0.03 |
1.03±0.27a | 0.81±0.28 |
0.66±0.29b |
0.49±0.23c |
In comparison with those in the normal group, the
serum levels of ALT, ALP, TC and LDL-C were found to be
significantly increased in the model group (P<0.01) but reversed
by YCHD treatment. When compared with the model group, low dose
YCHD only significantly restored the serum level of ALT after
treatment for 8 weeks (P<0.01), whereas high dose YCHD
significantly restored the serum levels of ALT, ALP, TC and LDL-C
in mice (P<0.01). Treatment with TUDCA also decreased the serum
ALT, ALP, TC and LDL-C levels compared with the model group
(P<0.01) and those in the YCHD-L group (P<0.05; Table II).
Effect of YCHD on cholelithiasis in
mice
The estimated extent of cholelithiasis in mice was
visually graded according to the six-level judgment criteria
(Fig. 2). Compared with that in the
normal group, there was a statistically significant increased
difference in the grade of gallstones in the model group
(P<0.01). LD-fed C57BL/6 mice exhibited a 100% cholelithiasis
formation rate, where the severity of cholelithiasis was directly
associated with feeding time. There was also a decreased tendency
of cholelithiasis formation for each treatment group in comparison
to the model group (Fig. 2).
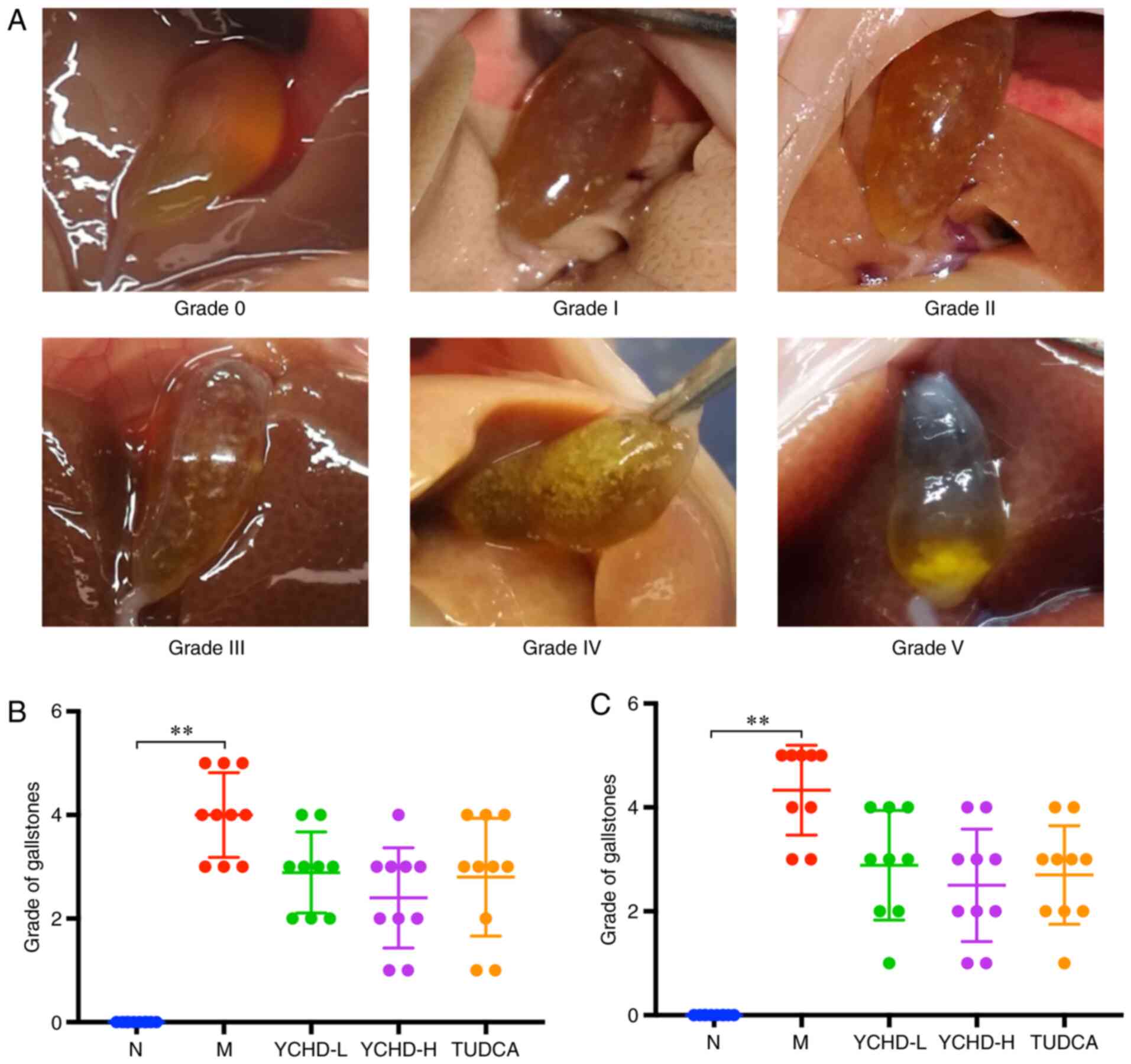 | Figure 2Conditions of mice with
cholelithiasis in the five different groups. (A) Representative
images of gallbladders with each category of cholelithiasis, as
graded using the six-level judgment criteria: Grade 0, gallbladder
is filled with clear bile; grade I: A few fine crystals are found;
grade II, ~10 fine crystals are found; grade III, fine crystals
occupy ~50% of the gallbladder; grade IV: Crystals occupy >50%
of the gallbladder; grade V: Round gallstones are found. The grade
of cholelithiasis in mice after (B) treatment for 4 weeks (C) and
for 8 weeks. **P<0.01. N, normal; M, model; L, low
dose; H, high dose; YCHD, Yinchenhao Decoction; TUDCA,
tauroursodeoxycholic acid. |
Effect of YCHD on biliary lipid
composition and CSI in mice
The biliary lipid composition and CSI of mice in the
five treatment groups are shown in Fig.
3. For feeding LD at 8 weeks, the concentration of TC in the
model group was significantly increased (P<0.01) compared with
the normal group, while the TBA content was decreased (P<0.05).
Feeding LD for 12 weeks resulted in significantly increased
concentrations of TC and PL in the model group (P<0.01) compared
with the normal group. Additionally, TBA content was significantly
decreased (P<0.05). In addition, the severity of CSI in the
model group was also found to be significantly increased compared
with that in the control group (P<0.01), suggesting that the
levels of cholesterol in the bile was supersaturated and the
relative dynamic balance between TC, PL and TBA was disrupted.
After treatment with high dose YCHD for 4 weeks, the concentration
of TC and CSI in bile was reduced (P<0.05), whilst TBA content
was increased (P<0.05) compared with the model group. After
treatment with YCHD-L or YCHD-H for 8 weeks, the concentration of
TC, PL and CSI in the bile was significantly reduced (P<0.01),
whilst TBA content was significantly increased (P<0.01) compared
with the model group, indicating that YCHD restored the imbalance
in the bile, thereby ameliorating cholelithiasis in mice. The
difference between the high dose and low dose groups at 8-weeks of
treatment was also found to be significant (P<0.05). The same
results were found for the TUDCA group compared with the model
group (P<0.01) and the YCHD-L group (P<0.05).
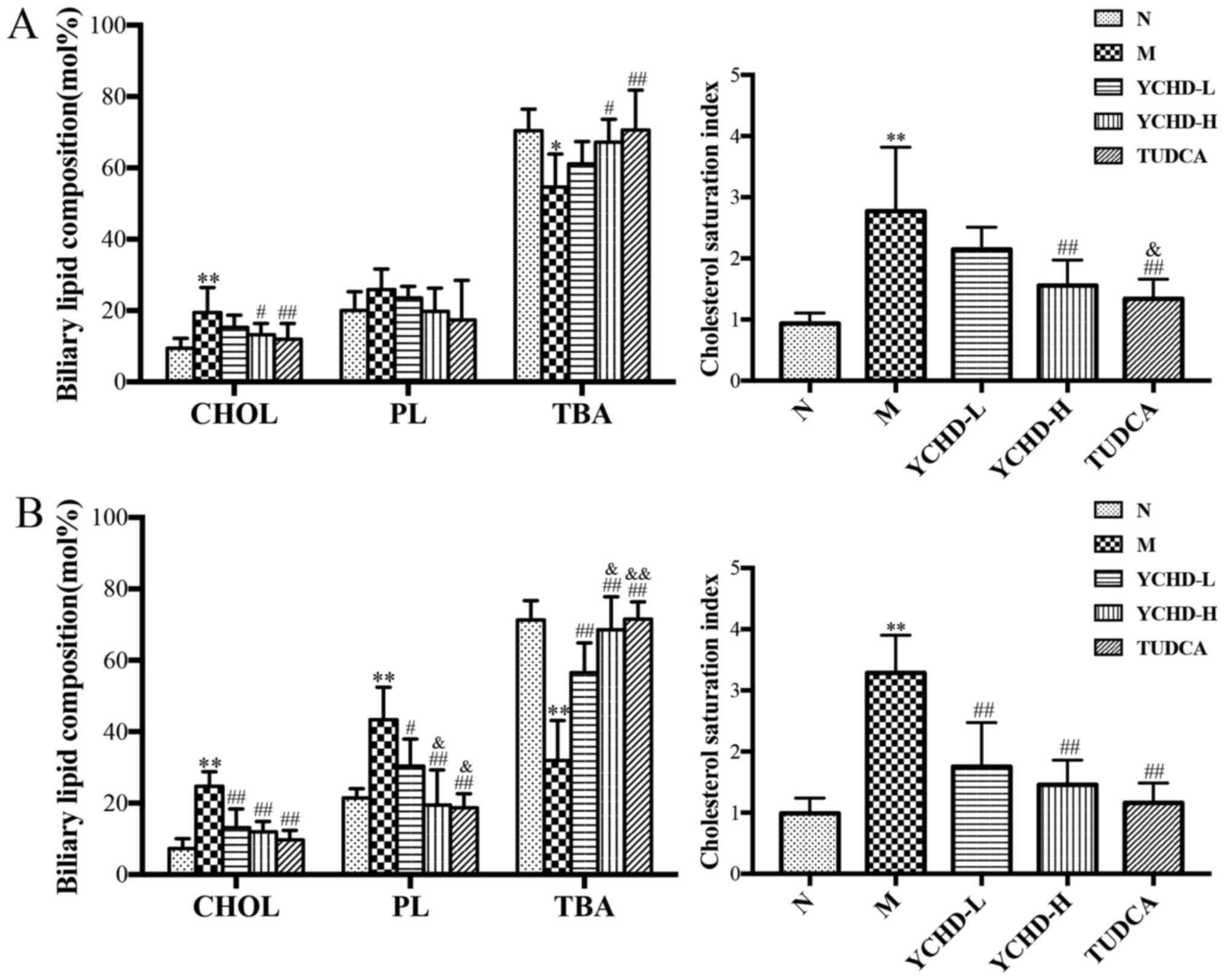 | Figure 3Biliary lipid composition and
cholesterol saturation index in mice from the five treatment
groups. Measurements of cholesterol, phospholipids and total bile
acid after treatment for (A) 4 weeks and (B) 8 weeks.
*P<0.05, **P<0.01 vs. N;
#P<0.05, ##P<0.01 vs. M;
&P<0.05, &&P<0.01 vs.
YCHD-L. N, normal; M, model; L, low dose; H, high dose; YCHD,
Yinchenhao Decoction; TUDCA, tauroursodeoxycholic acid; TC, total
cholesterol; PL, phospholipid; TBA, total bile acid. |
Histopathological effect of YCHD in
mice
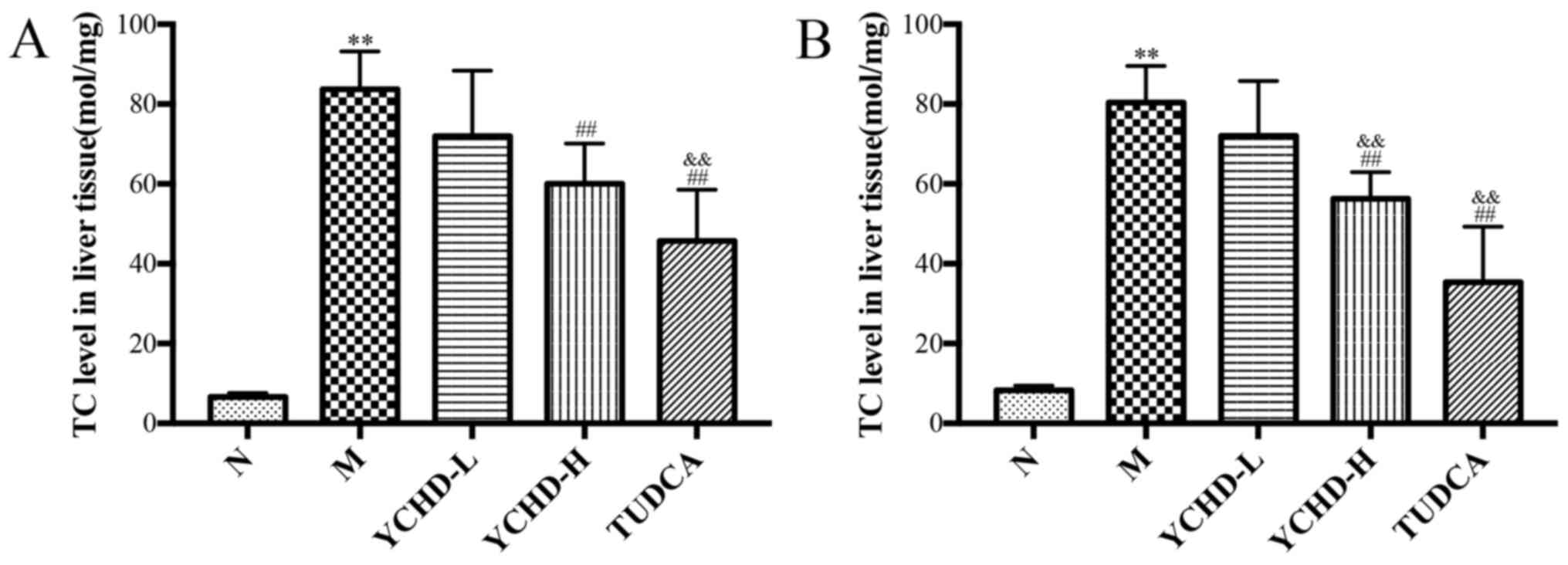
As shown in Fig. 4,
with prolonged feeding on LD, TC levels in the liver tissue of the
model group were revealed to be significantly increased compared
with those in the normal group (P<0.01). Compared with the model
group, TC levels in the liver tissue of the YCHD-H group were
significantly reduced (P<0.01). The potency of high dose
treatment for 8 weeks was significantly higher compared with the
low dose group (P<0.01). Treatment with TUDCA also significantly
reduced hepatic TC levels compared with those in the model group
(P<0.01) and the YCHD-L group (P<0.01).
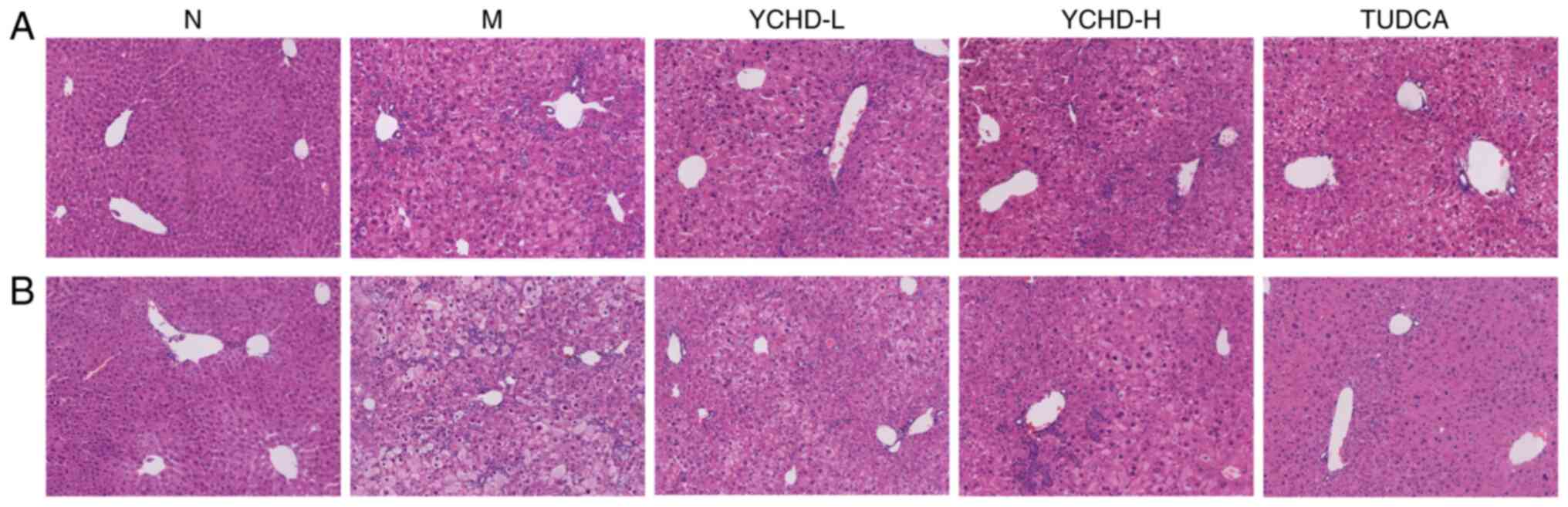
Representative images of liver specimens stained
with H&E are shown in Fig. 5.
H&E staining showed that all the mice in the normal group
appeared to have normal structures. However, the cytoplasm of
hepatocytes isolated from mice in the model group was loose, along
with inflammatory cell infiltration and capillary bile duct
hyperplasia. In addition, the degree of hepatocyte injury was
positively associated the length of the modeling time. Local
capillary bile duct hyperplasia occurred in the mice fed on LD for
8 weeks, whilst capillary bile duct hyperplasia in the liver
parenchyma was evident in mice fed on LD for 12 weeks. In the
YCHD-treated groups, the extents of cytoplasmic loosening in
hepatocytes, inflammatory cell infiltration and damage to
hepatocytes were all alleviated with the preferable changes
observed in the high dose group. These findings suggest that YCHD
treatment reduced cholesterol deposition in liver tissues and
attenuated the pathological changes of hepatocyte injury.
Effect of YCHD on the expression of
key components of cholesterol metabolisms in the liver and small
intestine tissues of mice
As shown in Fig. 6,
compared with those in the normal group, the expression levels of
liver ABCG5, ABCG8, SRBI and ABCB11 mRNA in the 8-weeks model group
were significantly increased (P<0.01). Additionally, the
expression levels of liver ABCG5, ABCG8 and SRBI mRNA in the
12-week model group were significantly increased (P<0.01). In
addition, the relative expression of ABCG5, ABCG8 and NPC1L1 in the
proximal small intestine were also significantly increased compared
with that in the normal group (P<0.01). After treatment with
YCHD for 4 weeks, compared with those in the model group, the
aforementioned increased expression of ABCG5, ABCG8, SRBI, ABCB11
and ABCB4 in the liver and ABCG5, ABCG8 and NPC1L1 in the small
intestine were all significantly reversed in the YCHD-L (P<0.01)
and YCHD-H (P<0.01) groups. Similar to YCHD, treatment with
TUDCA also significantly reversed the increased ABCG5, ABCG8,
NPC1L1 and SRBI expression in the liver and/or small intestine
compared with those in the model group (P<0.01).
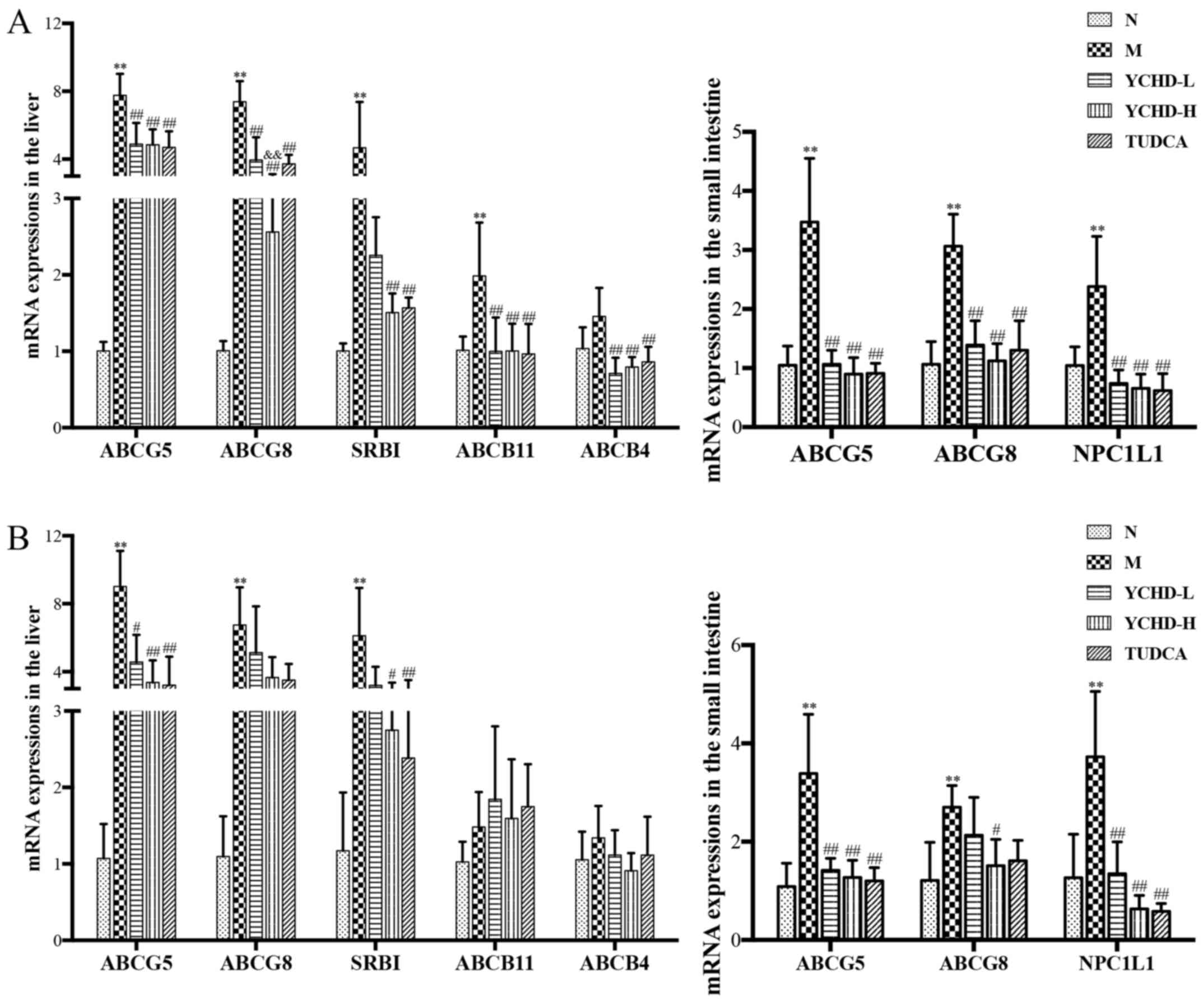 | Figure 6Expression of key components of the
cholesterol metabolism pathway in the liver and small intestine
tissues of mice from the five treatment groups. Measurements of
ABCG5, ABCG8, NPC1L1, SRBI, ABCB11 and ABCB4 mRNA expression in
liver and small intestine tissues of mice from the five treatment
groups after treatment for (A) 4 weeks and (B) 8 weeks.
**P<0.01 vs. N; #P<0.05,
##P<0.01 vs. M; &&P<0.01 vs.
YCHD-L. N, normal; M, model; L, low dose; H, high dose; YCHD,
Yinchenhao Decoction; TUDCA, tauroursodeoxycholic acid; ABCG,
ATP-binding cassette subfamily G; SRBI, scavenger receptor class B
type I; ABCB, ATP-binding cassette subfamily B; NPC1L1,
Niemann-Pick C1 Like 1. |
Discussion
Gallstone disease is highly prevalent among the
general population, with incidence ranging from 10-15% in Europe,
the USA and other developed countries (38). The majority of symptomatic gallstone
diseases are caused by cholesterol gallstones (39). From a therapeutic point of view,
effective treatment options for cholesterol gallstones are limited
and the cost of therapeutic interventions are high. Surgery can
result in potential side effects, whilst oral litholysis with
hydrophilic bile salts has demonstrated limited effectiveness
(40). Therefore, it remains
necessary to explore promising novel agents to confront the problem
of this increasing prevalence of cholelithiasis to aid the progress
of therapeutic and drug development.
The etiology of gallstone formation is
multifactorial, which includes gender, genetic, lifestyle and
comorbidity-associated factors (41). Cholesterol gallstones tend to occur
more frequently in women compared with men due to elevated estrogen
levels (42). The pathogenesis of
gallstone includes hepatic hypersecretion of cholesterol,
supersaturated bile and rapid phase transition of cholesterol,
gallbladder hypomotility and intestinal factors (43,44). A
previous study has shown that excessive intake of cholesterol leads
to increased cholesterol levels in the blood and increased
cholesterol saturation in the bile in the human body, eventually
leading to the formation of gallstones (45). In the present study, LD was used to
induce cholelithiasis in male mice. Since the role of estrogen in
regulating cholesterol and bile acid metabolism in the liver has
been demonstrated in a previous study (46), male mice were used to rule out the
impact of estrogen on the present study of drug efficacy. The
results showed that compared with the that in the normal group,
cholelithiasis model was successfully established in LD-fed C57BL/6
mice. The presence of cholesterol crystals or stones was clearly
visible in the gallbladder of these mice, where the severity of
cholelithiasis was positively associated with the modeling time.
Treatment with YCHD or TUDCA improved the condition of
cholelithiasis in mice. Based on these findings, the mechanism of
YCHD-mediated cholelithiasis formation inhibition was further
examined by measuring biochemical indices in the serum in addition
to analyzing liver tissue pathology, gallbladder bile composition
and expression levels of proteins involved in cholesterol
metabolism.
Positive associations between high serum lipid
levels, liver enzymes and the development of cholesterol
gallstones, have been previously reported (47). Compared with healthy individuals,
patients with gallstones showed higher TC and LDL-C levels
(48). High serum cholesterol and
LDL levels may increase cholesterol excretion with bile and cause
cholesterol gallstone disease (49,50).
In liver enzyme profiles, patients with gallstones presented with
higher serum ALT and ALP activities (51). The results of the present study were
consistent with previous reports, where levels of serum ALT, ALP,
TC and LDL-C levels in the mice in the model group were
significantly increased compared with those in the normal group,
reflective of hepatic injury and the pathological status. It has
been previously documented that hepatic cholesterol accumulation
causes pathological liver damage and inflammation (52). In the present study, TC levels in
the liver tissues of mice in the model group were also found to be
significantly increased. H&E staining of liver tissues showed
that the cytoplasm of hepatocytes in mice in the model group was
loose with inflammatory cell infiltration and capillary bile duct
hyperplasia. In addition, the degree of hepatocyte injury was
associated with modeling time. These pathological results were
consistent with the results of serum biochemical indicators,
confirming that there was hepatocyte injury in mice in the model
group. The intervention of YCHD or TUDCA significantly reversed the
increased serum lipid and liver injury indicator levels and
alleviated the damage to the hepatocytes. The extent of restoration
of damaged hepatocytes was found to be proportional to the dose of
treatment of YCHD. These results are in line with earlier reports,
which demonstrated that YCHD treatment could repair liver injury
and reverse steatosis (53,54).
Lithogenicity of the bile is determined by the
relative concentration of cholesterol, phospholipids and bile acids
that are synthesized in the hepatocytes (55). Cholesterol and phospholipids are
insoluble in water and must be combined with bile acids to form
mixed micelles under normal circumstances (56). When cholesterol is excessively
secreted or the excretion of bile salts or phospholipids are
reduced, impaired lipid absorption leads to the sustained
supersaturation of cholesterol, increasing the susceptibility to
cholesterol crystallization and gallstone formation (57,58).
Cholesterol supersaturation or deficiency of bile acids in the bile
results in a high CSI value, which is a semi-quantitative measure
of the biliary cholesterol content (59). A significantly higher CSI value was
previously found in patients with cholelithiasis compared to that
in healthy individuals (60). These
observations are supported by the present study, where mice in the
model group showed significantly increased biliary CHOL
concentrations, higher CSI values and reduced TBA concentrations,
suggesting failure of biliary cholesterol homeostasis. Treatment
with YCHD or TUDCA reversed this alteration in biliary lipid and
cholesterol contents and restored the dynamic imbalance, thereby
inhibiting the formation and growth of cholesterol gallstones.
Cholesterol is absorbed in the small intestine,
transported in the blood and taken up by the liver (61). Hepatic secretion of bile salts and
cholesterol into the bile forms the basis for eliminating excess
cholesterol from the body (62).
Cholesterol homeostasis in the body is mainly maintained by
intestinal cholesterol absorption, cholesterol uptake and de
novo biosynthesis in the liver, biliary excretion and its
conversion to various products such as bile acids (63). ABCG5/G8 and SRBI are the principal
mediators of biliary cholesterol efflux that prevent intestinal
cholesterol absorption and facilitate cholesterol efflux from the
liver (42,43). This has the effect of simultaneously
lower plasma cholesterol levels and increase biliary cholesterol
excretion (64,65). ABCB4 and ABCB11 transporter also
have been reported to regulate the composition of primary bile
(66,67). The process of intestinal cholesterol
absorption is a complex process, but it is clear that the small
intestine serves a vital role in both dietary and biliary
cholesterol absorption (68).
Cholesterol uptake can also be regulated by variants of NPC1L1, a
transmembrane protein that is highly expressed in the intestinal
epithelial cells of the small intestine in mammals (69). Increased expression of these
transporters has been previously demonstrated to increase biliary
sterol secretion whilst reducing intestinal cholesterol absorption
during cholelithiasis (70,71). The relative expression levels of
these critical components involved in lipid metabolism were
measured in the present study to further clarify the mechanism of
YCHD on cholelithiasis. YCHD effectively inhibited the
overexpression of ABCG5/8 and SRBI transporters in the liver and
ABCG5/8 and NPC1L1 in the small intestine, which promote
cholesterol secretion back into the intestinal lumen. This
potentially served to reduce intestinal cholesterol absorption and
hepatic cholesterol transportation, in turn protecting against the
formation and growth of cholesterol gallstones.
According to rules of TCM theory, classic formulae
consist of the following four elements: i) The monarch drug, which
is the core in this formula and directly targets the disease; ii)
the minister drug, which promotes the therapeutic effect of the
monarch drug or target the accompanying symptoms; iii) the
assistant drug, which reduces the adverse effects and/or increase
the potency of the whole formula; and iv) the servant drug, which
guides the monarch and minister drugs to reach the target organs or
to harmonize their actions (72).
The therapeutic efficacy of YCHD can be attributed to the combined
action of a mixture of the three herbs, Artemisiae scopariae
herba, Gardeniae Fructus and Radix et Rhizoma Rhei. They
harmoniously interacted with each other to achieve the therapeutic
outcome and diminished the possible adverse reactions among the
multiple herbs. Previous pharmacological studies indicated that
synergism between three components tend to exert a more robust
effect compared with either one or two of the three individual
compounds, by hitting multiple targets (73).
YCHD has been previously used in clinical practice
for jaundice and liver disorders. Previous studies have verified
the efficacy and mechanism of YCHD for the treatment of diseases,
including cholestatic liver disease, liver fibrosis and
non-alcoholic steatohepatitis (74-76).
However, insufficient studies have examined its effect and possible
mechanism on cholelithiasis. The occurrence of cholelithiasis is
closely associated with abnormal cholesterol metabolism, which
involves the synthesis, absorption and transportation of
cholesterol in the liver, gallbladder and intestine. The possible
mechanism of YCHD was initially confirmed in vivo in mice
with cholelithiasis based on the cholesterol metabolism pathway in
the liver and intestine in the present study. The present study is
a useful attempt on the study of this TCM formula for the treatment
of cholelithiasis. For future studies, the specific mechanisms of
YCHD on cholelithiasis from multiple perspectives will be further
explored through additional in vitro and in vivo
experiments, including its effects on inflammation and intestinal
flora through metabolomics. In addition, the dose of YCHD used in
the present study was the same or twice of the original
prescription, which is far less than the maximum available dose
(77). In future studies, a higher
dose and a longer course of treatment will be applied.
In conclusion, the present study demonstrated that
YCHD ameliorates serum and liver biochemical abnormalities in mice
with LD-induced cholelithiasis and improves biliary cholesterol
supersaturation to adjust biliary cholesterol homeostasis, which
may involve the regulation of cholesterol metabolism.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation for Youth Scholars of China (grant no.
81603560), the Discipline Leader Program of Pudong New Area (grant
no. PWRd2019-04), the Peak Discipline Project of Preventive
Treatment of Disease in Traditional Chinese Medicine of Pudong New
Area (grant no. PDZY-2018-0603) and Science and Technology
Innovation Action Plan of Science and Technology Commission of
Shanghai Municipality (grant no. 19401972700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ performed the animal study, collected samples,
acquired the data for analyses and drafted the manuscript. HH
performed the animal study and analyzed the data. GZ acquired the
data for analyses and analyzed the data. PL designed the study and
revised the manuscript. YW designed the study and drafted the
manuscript. HZ designed the study and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Animal Ethics Committee of Shanghai University of Traditional
Chinese Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stinton LM and Shaffer EA: Epidemiology of
gallbladder disease: Cholelithiasis and cancer. Gut Liver.
6:172–187. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu L, Aili A, Zhang C, Saiding A and
Abudureyimu K: Prevalence of and risk factors for gallstones in
Uighur and Han Chinese. World J Gastroenterol. 20:14942–14949.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Littlefield A and Lenahan C:
Cholelithiasis: Presentation and management. J Midwifery Womens
Health. 64:289–297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reshetnyak VI: Concept of the pathogenesis
and treatment of cholelithiasis. World J Hepatol. 4:18–34.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cremer A and Arvanitakis M: Diagnosis and
management of bile stone disease and its complications. Minerva
Gastroenterol Dietol. 62:103–129. 2016.PubMed/NCBI
|
|
6
|
Venneman NG and van Erpecum KJ:
Pathogenesis of gallstones. Gastroenterol Clin North Am.
39:171–183, vii. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dikkers A, Freak de Boer J, Annema W,
Groen AK and Tietge UJ: Scavenger receptor BI and ABCG5/G8
differentially impact biliary sterol secretion and reverse
cholesterol transport in mice. Hepatology. 58:293–303.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Van Erpecum KJ: Pathogenesis of
cholesterol and pigment gallstones: An update. Clin Res Hepatol
Gastroenterol. 35:281–287. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hoekstra M and Sorci-Thomas M:
Rediscovering scavenger receptor type BI: Surprising new roles for
the HDL receptor. Curr Opin Lipidol. 28:255–260. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chan J and Vandeberg JL: Hepatobiliary
transport in health and disease. Clin Lipidol. 7:189–202.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patel SB, Graf GA and Temel RE: ABCG5 and
ABCG8: More than a defense against xenosterols. J Lipid Res.
59:1103–1113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Krawczyk M, Lütjohann D, Schirin-Sokhan R,
Villarroel L, Nervi F, Pimentel F, Lammert F and Miquel JF:
Phytosterol and cholesterol precursor levels indicate increased
cholesterol excretion and biosynthesis in gallstone disease.
Hepatology. 55:1507–1517. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Reboul E, Soayfane Z, Goncalves A,
Cantiello M, Bott R, Nauze M, Tercé F, Collet X and Coméra C:
Respective contributions of intestinal Niemann-Pick C1-like 1 and
scavenger receptor class B type I to cholesterol and tocopherol
uptake: In vivo v. in vitro studies. Br J Nutr. 107:1296–1304.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vinarova L, Vinarov Z, Tcholakova S,
Denkov ND, Stoyanov S and Lips A: The mechanism of lowering
cholesterol absorption by calcium studied by using an in vitro
digestion model. Food Funct. 7:151–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Portincasa P, Ciaula AD, Bonfrate L and
Wang DQ: Therapy of gallstone disease: What it was, what it is,
what it will be. World J Gastrointest Pharmacol Ther. 3:7–20.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu.
EASL Clinical Practice Guidelines on the prevention, diagnosis and
treatment of gallstones. J Hepatol. 65:146–181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Q, Zhang Y, Li S, Chen S, Lin X, Li C
and Asakawa T: Mechanisms underlying the prevention and treatment
of cholelithiasis using traditional chinese medicine. Evid Based
Complement Alternat Med. 2019(2536452)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang A, Sun H, Yuan Y, Sun W, Jiao G and
Wang X: An in vivo analysis of the therapeutic and synergistic
properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on
its active constituents. Fitoterapia. 82:1160–1168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang J, Cheung F, Tan HY, Hong M, Wang N,
Yang J, Feng Y and Zheng Q: Identification of the active compounds
and significant pathways of yinchenhao decoction based on network
pharmacology. Mol Med Rep. 16:4583–4592. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang X, Zhang A, Wang P, Sun H, Wu G, Sun
W, Lv H, Jiao G, Xu H, Yuan Y, et al: Metabolomics coupled with
proteomics advancing drug discovery toward more agile development
of targeted combination therapies. Mol Cell Proteomics.
12:1226–1238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Z, Ma X, Zhao Y, Wang J, Zhang Y, Li
J, Wang R, Zhu Y, Wang L and Xiao X: Yinchenhao decoction in the
treatment of cholestasis: A systematic review and meta-analysis. J
Ethnopharmacol. 168:208–216. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li JY, Cao HY, Sun L, Sun RF, Wu C, Bian
YQ, Dong S, Liu P and Sun MY: Therapeutic mechanism of Yin-Chen-Hao
decoction in hepatic diseases. World J Gastroenterol. 23:1125–1138.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Asakawa T, Yagi M, Tanaka Y, Asagiri K,
Kobayashi H, Egami H, Tanikawa K and Kage M: The herbal medicine
Inchinko-to reduces hepatic fibrosis in cholestatic rats. Pediatr
Surg Int. 28:379–384. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang B, Sun MY, Long AH, Cao HY, Ren S,
Bian YQ, Lu X, Gu HT, Liu CH and Liu P: Yin-Chen-Hao-Tang
alleviates biliary obstructive cirrhosis in rats by inhibiting
biliary epithelial cell proliferation and activation. Pharmacogn
Mag. 11:417–425. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Man-ting L, Ying F and Shao-Dong C: Effect
and mechanism of Yinchenhao Decoction in preventing lipid metabolic
disturbance for rats fed on high fat diet. China J Tradit Chin Med
Pharm. 26:2428–2430. 2011.(In Chinese).
|
|
26
|
Lv J, Jin S, Yuan H, Han J, Fu S, Jin S,
Guo JJ and Xiao X: Rational daily administration times of
yinchenhao decoction in rats with jaundice based on PD/PK. Chin
Herb Med. 4:150–156. 2012.
|
|
27
|
Yi YX, Ding Y, Zhang Y, Ma NH, Shi F, Kang
P, Cai ZZ and Zhang T: Yinchenhao decoction ameliorates
alpha-naphthylisothiocyanate induced intrahepatic cholestasis in
rats by regulating phase II metabolic enzymes and transporters.
Front Pharmacol. 9(510)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang X, Sun H, Zhang A, Jiao G, Sun W and
Yuan Y: Pharmacokinetics screening for multi-components absorbed in
the rat plasma after oral administration traditional Chinese
medicine formula Yin-Chen-Hao-Tang by ultra performance liquid
chromatography-electrospray ionization/quadrupole-time-of-flight
mass spectrometry combined with pattern recognition methods.
Analyst. 136:5068–5076. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yan J, Xie G, Liang C, Hu Y, Zhao A, Huang
F, Hu P, Liu P, Jia W and Wang X: Herbal medicine Yinchenhaotang
protects against α-naphthylisothiocyanate-induced cholestasis in
rats. Sci Rep. 7(4211)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cheng L, Huang C and Chen Z:
Tauroursodeoxycholic acid ameliorates lipopolysaccharide-induced
depression like behavior in mice via the inhibition of
neuroinflammation and oxido-nitrosative stress. Pharmacology.
103:93–100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
National Research Council: Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
Association for Assessment and Accreditation of Laboratory Animal
Care. In: Guide for the Care and Use of Laboratory Animals.
Washington DC, 2011.
|
|
32
|
Committee IACaU: Institutional animal care
and use committee guidebook. In: Office of Laboratory Animal
Welfare. National Institutes of Health, Bethesda, MD, 2002.
|
|
33
|
Wang Q, Jiao L, He C, Sun H, Cai Q, Han T
and Hu H: Alteration of gut microbiota in association with
cholesterol gallstone formation in mice. BMC Gastroenterol.
17(74)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Akiyoshi T, Uchida K, Takase H, Nomura Y
and Takeuchi N: Cholesterol gallstones in alloxan-diabetic mice. J
Lipid Res. 27:915–924. 1986.PubMed/NCBI
|
|
36
|
Carey MC: Critical tables for calculating
the cholesterol saturation of native bile. J Lipid Res. 19:945–955.
1986.PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Portincasa P, Di Ciaula A, de Bari O,
Garruti G, Palmieri VO and Wang DQ: Management of gallstones and
its related complications. Expert Rev Gastroenterol Hepatol.
10:93–112. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shabanzadeh DM: Incidence of gallstone
disease and complications. Curr Opin Gastroenterol. 34:81–89.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Di Ciaula A, Wang DQ, Wang HH, Bonfrate L
and Portincasa P: Targets for current pharmacologic therapy in
cholesterol gallstone disease. Gastroenterol Clin North Am.
39:245–264, viii-ix. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Di Ciaula A, Wang DQ, Bonfrate L and
Portincasa P: Current views on genetics and epigenetics of
cholesterol gallstone disease. Cholesterol.
2013(298421)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Simonsen MH, Erichsen R, Froslev T, Rungby
J and Sorensen HT: Postmenopausal estrogen therapy and risk of
gallstone disease: A population-based case-control study. Drug Saf.
36:1189–1197. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lammert F, Gurusamy K, Ko CW, Miquel JF,
Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ
and Wang DQ: Gallstones. Nat Rev Dis Primers.
2(16024)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Di Ciaula A, Wang DQ and Portincasa P: An
update on the pathogenesis of cholesterol gallstone disease. Curr
Opin Gastroenterol. 34:71–80. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Di Ciaula A, Garruti G, Frühbeck G, De
Angelis M, de Bari O, Wang DQ, Lammert F and Portincasa P: The role
of diet in the pathogenesis of cholesterol gallstones. Curr Med
Chem. 26:3620–3638. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lavoie JM: Dynamics of hepatic and
intestinal cholesterol and bile acid pathways: The impact of the
animal model of estrogen deficiency and exercise training. World J
Hepatol. 8:961–975. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen LY, Qiao QH, Zhang SC, Chen YH, Chao
GQ and Fang LZ: Metabolic syndrome and gallstone disease. World J
Gastroenterol. 18:4215–4220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Han T, Zhang D, Fu Z, Sun Y, Yang W and
Yuan C: Retinol-binding protein 4 as a risk factor for cholesterol
gallstone formation. Mol Cell Biochem. 377:219–227. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Andreotti G, Chen J, Gao YT, Rashid A,
Chang SC, Shen MC, Wang BS, Han TQ, Zhang BH, Danforth KN, et al:
Serum lipid levels and the risk of biliary tract cancers and
biliary stones: A population-based study in China. Int J Cancer.
122:2322–2329. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Atamanalp SS, Keles MS, Atamanalp RS,
Acemoglu H and Laloglu E: The effects of serum cholesterol, LDL,
and HDL levels on gallstone cholesterol concentration. Pak J Med
Sci. 29:187–190. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang S, Zhang W, Shi L, Xie A, Shao Y, Ye
Y, Pan X, Lin Z, Li X and Zhang Y: Hepatic CXCL16 is increased in
gallstone accompanied with liver injury. Eur J Clin Invest.
47:667–674. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Makino A, Hullin-Matsuda F, Murate M, Abe
M, Tomishige N, Fukuda M, Yamashita S, Fujimoto T, Vidal H, Lagarde
M, et al: Acute accumulation of free cholesterol induces the
degradation of perilipin 2 and Rab18-dependent fusion of ER and
lipid droplets in cultured human hepatocytes. Mol Biol Cell.
27:3293–3304. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liu C, Sun M, Wang L, Wang G, Chen G, Liu
C and Liu P: Effects of Yinchenhao Tang and related decoctions on
DMN-induced cirrhosis/fibrosis in rats. Chin Med.
3(1)2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jiang SL, Hu XD and Liu P:
Immunomodulation and liver protection of Yinchenhao decoction
against concanavalin A-induced chronic liver injury in mice. J
Integr Med. 13:262–268. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yago MD, Gonzalez V, Serrano P, Calpena R,
Martínez MA, Martínez-Victoria E and Mañas M: Effect of the type of
dietary fat on biliary lipid composition and bile lithogenicity in
humans with cholesterol gallstone disease. Nutrition. 21:339–347.
2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Morita SY, Ikeda Y, Tsuji T and Terada T:
Molecular mechanisms for protection of hepatocytes against bile
salt cytotoxicity. Chem Pharm Bull (Tokyo). 67:333–340.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tazuma S, Kanno K, Sugiyama A and
Kishikawa N: Nutritional factors (nutritional aspects) in biliary
disorders: Bile acid and lipid metabolism in gallstone diseases and
pancreaticobiliary maljunction. J Gastroenterol Hepatol. 28 (Suppl
4):S103–S107. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Vazquez MC, Rigotti A and Zanlungo S:
Molecular mechanisms underlying the link between nuclear receptor
function and cholesterol gallstone formation. J Lipids.
2012(547643)2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fracchia M, Pellegrino S, Secreto P, Gallo
L, Masoero G, Pera A and Galatola G: Biliary lipid composition in
cholesterol microlithiasis. Gut. 48:702–706. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pasternak A, Matyja A, Gil K, Gajda M,
Tomaszewski KA, Gajda M, Tomaszewski KA, Matyja M, Walocha JA and
Kulig J: Interstitial cajal-like cells and bile lithogenicity in
the pathogenesis of gall-stone disease. Pol Przegl Chir.
85:311–316. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ioannou GN, Landis CS, Jin GY, Haigh WG,
Farrell GC, Kuver R, Lee SP and Savard C: cholesterol crystals in
hepatocyte lipid droplets are strongly associated with human
nonalcoholic steatohepatitis. Hepatol Commun. 3:776–791.
2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhao B, Natarajan R and Ghosh S: Human
liver cholesteryl ester hydrolase: Cloning, molecular
characterization, and role in cellular cholesterol homeostasis.
Physiol Genomics. 23:304–310. 2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Di Ciaula A, Wang DQ, Garruti G, Wang HH,
Grattagliano I, de Bari O and Portincasa P: Therapeutic reflections
in cholesterol homeostasis and gallstone disease: A review. Curr
Med Chem. 21:1435–1447. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hoekstra M, Van Berkel TJ and Van Eck M:
Scavenger receptor BI: A multi-purpose player in cholesterol and
steroid metabolism. World J Gastroenterol. 16:5916–5924.
2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Stender S, Frikke-Schmidt R, Nordestgaard
BG and Tybjaerg-Hansen A: The ABCG5/8 cholesterol transporter and
myocardial infarction versus gallstone disease. J Am Coll Cardiol.
63:2121–2128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Dikkers A and Tietge UJ: Biliary
cholesterol secretion: More than a simple ABC. World J
Gastroenterol. 16:5936–5945. 2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Stokes CS and Lammert F: Transporters in
cholelithiasis. Biol Chem. 393:3–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Xie M, Kotecha VR, Andrade JDP, Fox JG and
Carey MC: Augmented cholesterol absorption and sarcolemmal sterol
enrichment slow small intestinal transit in mice, contributing to
cholesterol cholelithogenesis. J Physiol. 590:1811–1824.
2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang LJ and Song BL: Niemann-Pick C1-Like
1 and cholesterol uptake. Biochim Biophys Acta. 1821:964–972.
2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yoon JH, Choi HS, Jun DW, Yoo KS, Lee J,
Yang SY and Kuver R: ATP-binding cassette sterol transporters are
differentially expressed in normal and diseased human gallbladder.
Dig Dis Sci. 58:431–439. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang LJ, Wang J, Li N, Ge L, Li BL and
Song BL: Molecular characterization of the NPC1L1 variants
identified from cholesterol low absorbers. J Biol Chem.
286:7397–7408. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhou X, Seto SW, Chang D, Kiat H,
Razmovski-Naumovski V, Chan K and Bensoussan A: Synergistic effects
of Chinese herbal medicine: A comprehensive review of methodology
and current research. Front Pharmacol. 7(201)2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Zhang A, Sun H, Qiu S and Wang X:
Advancing drug discovery and development from active constituents
of yinchenhao tang, a famous traditional Chinese medicine formula.
Evid Based Complement Alternat Med. 2013(257909)2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Cai FF, Wu R, Song YN, Xiong AZ, Chen XL,
Yang MD, Yang L, Hu Y, Sun MY and Su SB: Yinchenhao decoction
alleviates liver fibrosis by regulating bile acid metabolism and
TGF-β/Smad/ERK signalling pathway. Sci Rep. 8(15367)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mase A, Makino B, Tsuchiya N, Yamamoto M,
Kase Y, Takeda S and Hasegawa T: Active ingredients of traditional
Japanese (kampo) medicine, inchinkoto, in murine concanavalin
A-induced hepatitis. J Ethnopharmacol. 127:742–749. 2010.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Chen SD, Fan Y and Xu WJ: Effects of
yinchenhao decoction for non-alcoholic steatohepatitis in rats and
study of the mechanism. J Tradit Chin Med. 31:220–223.
2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Li Y, Pan H, Li X, Jiang N, Huang L, Lu Y
and Shi F: Role of intestinal microbiota-mediated genipin
dialdehyde intermediate formation in geniposide-induced
hepatotoxicity in rats. Toxicol Appl Pharmacol.
377(114624)2019.PubMed/NCBI View Article : Google Scholar
|