Introduction
Diabetes mellitus is a prevalent autoimmune disease
worldwide that can develop at any stage of life, including
adulthood and childhood. Diabetic nephropathy (DN) is a chronic and
severe complication associated with increased risk of end-stage
renal failure and cardiovascular disease (1). Evidence suggests that >30% of all
patients with diabetes develop DN within 10-20 years of the onset
of diabetes (2,3). In patients with diabetes, the
incidence of development of microalbuminuria is 20-40% if DN
remains untreated. Moreover, within 20-25 years, almost 20% of
these patients will develop end-stage renal failure (4) and further require renal
transplantation or chronic hemodialysis (5). Effective medications for DN and
anti-hypertension drugs are still the first-line choice; therefore,
identifying new drug targets and new drug candidates is urgently
required (6,7).
Neutrophil gelatinase-associated lipocalin (NGAL) is
a biomarker of renal tubular injury that is upregulated in distal
tubules and the collecting duct, and its role in acute kidney
injury (AKI) has been extensively evaluated (8,9). NGAL
is a 25-kDa glycoprotein with 178 amino acids that belongs to the
lipocalin superfamily (10). It is
a constituent of specific granules and exists in neutrophils as
part of the NGAL-gelatinase complex (11). It is involved in the antimicrobial
defense mechanism and is upregulated in systemic bacterial
infections (12,13). It also plays a protective role in
epithelial injury due to its antiapoptotic effect (14). As it is not produced by necrotic
nephron, it is a marker of active injury and represents the mass of
salvageable nephrons (15). Its
usefulness as a biomarker in chronic kidney diseases (16,17)
such as DN has been reported by several previous studies, however,
its role and effect in the pathophysiology of DN progression
remains unclear. In the current study both in vitro and
in vivo methods were used. NGAL was knocked down in human
tubular epithelial cells and NGAL knockdown mice were used in order
to investigate the exact role of NGAL in DN.
Materials and methods
Cell culture and NGAL knockdown
Human kidney (HK)2, an immortalized human proximal
tubular cell line, was purchased from the American Type Culture
Collection. HK2 cells were cultured in DMEM (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences) and passaged <10 times.
The cells were supplemented with 2 mmol/l glutamine, 100 U/ml
penicillin and 100 U/ml streptomycin, and maintained in a 37˚C, 5%
CO2 humidified atmosphere. The culture medium was
changed every 2-3 days.
To knock down NGAL expression, a lentivirus
pHBLV-U6-Zsgreen harboring a short hairpin RNA (shRNA) of NGAL
synthesized by Gene Company, Ltd. was used, as previously described
by the supplier. The NGAL shRNA sequence was
5'-GGACTTTTGTTCCAGGTTGTTAACAACCTGGAACAAAAGTCC-3'. A period of one
day before the transfection, HK-2 cells were seeded into a six-well
plate. When ~80% confluency of cells was reached, cells were
transfected. The confluency was calculated as the area of cells/the
whole view under an optical microscope (BM-37XBC, Shanghai BM
optical instruments manufacture Co., Ltd.) at a magnification of
x100. Transfection was performed with 1.2 µg shRNA plasmid
containing NGAL (cat. no. sc-43969-V; Santa Cruz Biotechnology,
Inc.) and 4.5 µl HiPerFect transfection reagent (Qiagen China Co.,
Ltd.) were added into 100 µl DMEM without FBS. A period of 15 min
later, the aforementioned mixture (5 µl) was dropped into wells (2
ml/well), DMEM was replaced with complete culture medium (DMEM+10%
FBS) 24 h later. After incubation at 37˚C for one day, cells were
split 1:5. After another day of culture at 37˚C, stable clones
expression the ShRNA were selected using 10 µg/ml puromucin
dihydrochloride (cat. no. P8230; Beijing Solarbio Science &
Technology Co., Ltd.). The medium containing puromucin selection
was replaced every 3 days and identified the resistant colonies.
Once a cell line was generated, the knockdown efficiency was
determined using reverse transcription-quantitative (RT-q)PCR
(18). A non-targeting shRNA was
also prepared and transfected into HK2 cells as a negative control
(NC). Cells transfected with the NC lentivirus were termed NC-HK2,
while NGAL knockdown cells were called KD-HK2.
mRNA quantification by RT-qPCR
Total RNA was extracted using the TRNzol-A+ reagent
kit (cat. no. DP430; Tiangen Biotech Co., Ltd.). RT-qPCR was
performed with complementary DNA reverse transcribed from total RNA
using a reverse transcription kit (ReverTra Ace® qPCR RT
kit; Toyobo Life Science) and ABI PRISM™ 7000 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The denaturation and annealing
reaction was conducted at 65˚C for 5 min and 4˚C. The reverse
transcription was performed at 45˚C for 20 min, 95˚C for 5 min and
4˚C for 5 min. SYBR green was used as the fluorophore and β-actin
was used as an internal control. The forward and reverse primers
for β-actin were 5'-AAACAGAAGGCAGCTTTACGATG-3' and
5'-AAATGTTCTGATCCAGTAGCG-3', respectively. For NGAL, the forward
primer was 5'-TCCCAGAGCTGAACGG-3' and the reverse primer was
5'-GAAGTCGCGGAGACA-3'. The qPCR thermocycling conditions were 95˚C
for 30 sec, followed by 40 cycles of 95˚C for 15 sec, 60˚C for 30
sec and 72˚C for 30 sec. The 2-∆∆Cq value was normalized
to the signal of the housekeeping gene, β-actin. The fold-change in
expression was calculated as the method described previously
(19).
Stimulation by normal (NG) and high
glucose (HG)
Upon reaching 60-80% confluence, NC-HK2 and KD-HK2
cells were growth-arrested in 0.5% fetal calf serum (HyClone; GE
Healthcare Life Sciences) for 48 h and treated with 5 (as normal
glucose) and 25 mm (as high glucose) D-glucose for ≤48 h.
ELISA for collagen IV (Col IV),
fibronectin (FN) and interleukin (IL)-6 secretion by HK2 cells
under HG stimulation
Exposure of HK2 cells to medium containing high
concentrations of glucose induced the overproduction of FN, Col IV
and IL-6, as described in a previous study (20). To determine the effect of NGAL on
the increased expression of FN, Col IV and IL-6 triggered by HG,
NC-HK2 and KD-HK2 cells were treated with HG for 48 h. The levels
of FN, Col IV and IL-6 in the supernatant were measured with ELISA
kits for FN (cat. no. ab219046; Abcam), IL-6 (cat. no. ab178013;
Abcam) and Col IV (Elabscience). The concentration in the culture
supernatant was normalized to the total protein concentration in
the cells, as quantified by the BCA method.
Analysis of intracellular reactive
oxygen species (ROS) production
The intracellular formation of ROS was detected by
incubating cultured HK2 cells with 1 µm of the
2',7'-dichlorofluorescin diacetate (DCFHA; cat. no. D6883; Merck),
a nonpolar compound that is converted into the nonfluorescent polar
derivative 2',7'-dichlorofluorescin (DCFH) by cellular esterase
upon incorporation into the cells. DCFH is then oxidized to the
highly fluorescent 2',7'-dichlorofluorescein (DCF) in the presence
of oxidants. Briefly, HK2 cells were incubated for 30 min at 37˚C
with 1 µm H2DCFDA in normal glucose (NG) or HG (25 mm) conditions.
The fluorescence intensity was determined immediately with a flow
cytometer [excitation wavelength (λ)=488 nm, emission λ=515 nm; BD
FACSCalibur (BD Biosciences); Cell Quest Pro software (version 5.1;
BD Biosciences)]. The average fluorescence intensity was normalized
to the total cell protein content in each group, as described
previously (21,22).
Generation of NGAL-/-
mice
NGAL-/- C57 BL/6 mice were generated by
Cyagen Biosciences, Inc. The strategy of NGAL knockout was as
follows: A genomic DNA clone containing exons 1-6 of the murine
NGAL gene was isolated from a 129/J mouse genomic library (Cyagen
Biosciences, Inc.). A target vector was designed to replace a
2.5-kb genomic fragment containing NGAL2 exons 1-5 with the PGK-neo
cassette. The diphtheria toxin A gene driven by the pMC1 promoter
was incorporated into the 3' end of the vector for negative
selection. The targeting vector was linearized with NotI and
electroporated into 129/Ola ES cells using a Bio-Rad Gene Pulser at
0.34 kV and 250 µF (Bio-Rad Laboratories, Inc.). According to
manufacturers protocol, 400 µl cells (2x106 cells/ml)
and 20 µg targeting vector in the Opti-MEM (Thermo Fisher
Scientific, Inc.) were mixed in a 0.4 cm cuvette, which was chilled
in ice. The electroporation was conducted at 4˚C and with twice
pulse immediately after the signal, one pulse lasting for 2 sec,
and one pulse lasting for 3 sec. After electroporation, the medium
was replaced with growth medium (DMEM+10% FBS as aforementioned).
The transfected cells were then cultured in growth medium at 20˚C
overnight and then combined with 300 µg/ml Geneticin (G418;
Sigma-Aldrich; Merck KGaA) for 10 days. Homologous recombinants
were identified by PCR and verified by Southern blotting with a
PCR-generated 5' flanking probe (forward and reverse primers,
5'-ATAGCCCTGGCTGTCCTGAA-3' and 5'-TAAGGTCCCCCTCTAAACC-3',
respectively). Correctly targeted clones were injected into
C57BL/6J blastocysts, and the generation of chimeras was performed.
A total of two independently targeted ES cell clones transmitted
the NGAL mutation into the germ line. Male chimeric mice were mated
to C57BL/6 female mice, and the heterozygous F1 progeny was
intercrossed to generate NGAL-deficient mice. The wild-type
littermates from these crosses were used as controls for all
experiments. All mice were maintained in the animal facility of the
Affiliated Hospital of Hebei University of Engineering in
accordance with established ethical regulations of animal care.
Induction and assessment of
diabetes
Adult wild-type male C57 BL/6 mice (18-20 g) were
purchased from Beijing Weitong Lihua Experimental Animal Technology
Co., Ltd. The animals were maintained at 24˚C with a relative
humidity of 45-55% and 12/12 h dark/light cycle, and had free
access to food and water throughout the experimental protocol.
Both wild-type C57 BL/6 (4 male and 4 female mice;
body weight: 24±2 g; age: 6 weeks) and NGAL-/- C57 BL/6
mice (4 male and 4 female mice, body weight: 24±2 g; age: 6 weeks)
were used to establish an experimental model of DN. A single dose
of 60 mg/kg streptozotocin (STZ; cat. no. S0130; Merck KGaA)
prepared in citrate buffer (pH 4.4; 0.1 M) was injected
intraperitoneally to induce diabetes. C57 BL/6 mice who received
PBS injection were considered as the normal control group. Diabetes
was confirmed 4 days after STZ injection. Blood samples were
collected via the retro-orbital plexus using heparinized capillary
glass tubes and plasma glucose levels were estimated with an
enzymatic glucose oxidase-peroxidase diagnostic kit (Span Divergent
Ltd). Animals were anesthetized prior to sample collection. Mice
with plasma glucose levels >250 mg/dl were selected and used in
the present study (for each group there were 8 mice). Wild-type C57
BL/6 mice with DN were named as DN-wild, and NGAL-/- C57
BL/6 mice with DNA were named as DN- NGAL-/-. Healthy
mice were named as the normal control group. Their body weight and
plasma glucose levels were measured before and at the end of the
experiment. The animal health and behavior were monitored every
day, and no animals died during the experiment. All animal welfare
considerations were taken, including efforts to minimize suffering
and distress. Based on literature and a preliminary study, after
three months of STZ-induced diabetes, the mice progress into DN
with proteinuria and glomerulosclerosis; and reaching the humane
endpoint of 20% weight-loss was considered a humane endpoint
(23). After 3 months, all mice
were euthanatized by cervical dislocation, and pupillary response
to light was used to confirm the death of animals. Briefly, a
bright light was shone into the eyes of the animals. A constriction
of the pupil indicated a neurological response. Upon death, the
pupils become dilated and unresponsive to light. Kidney tissue
blocks were collected and fixed in 10% formalin at 4˚C for 24 h,
then the samples were ready for histological examination and
immunohistochemistry (IHC).
The experimental protocol was approved by the
Institutional Animal Ethics Committee of the Affiliated Hospital of
Hebei University of Engineering (Handan, China) and performed in
accordance with the guidelines on animal experimentation of the
Committee for Control and Supervision of Experimentation on
Animals, Government of China (24).
Renal function
Every 4 weeks, blood was sampled through the eyes
under anesthesia with sodium pentobarbital solution (60 mg/kg;
intraperitoneal injection). Blood urea nitrogen (BUN) and serum
creatinine (Scr) was measured using an automated biochemical
analyzer (200 FR; Toshiba Corporation). Urine was collected, the
urine albumin content was measured using the mouse albumin ELISA
kit (cat. no. ab108792; Abcam), and the urine creatinine was
measured using the automated biochemical analyzer. Albuminuria was
calculated as the ratio of urine albumin divided by urine
creatinine.
Histological examination
Fixed renal tissue blocks were embedded in paraffin.
Sections of 2-µm thickness were cut and stained with hematoxylin
for 10 min and then eosin for 5 min at room temperature and
periodic acid-Schiff for 10 min at room temperature. The stained
sections were examined under a light microscope (magnification,
x200). The severity of glomerulosclerosis was graded in a blinded
manner on a scale of 0-4 as previously described (25): Grade 0, normal; grade 1, sclerotic
area ≤25% (minimal); grade 2, sclerotic area >25-50% (moderate);
grade 3, sclerotic area >50-75% (moderate to severe); and grade
4, sclerotic area >75-100% (severe). The scores from each
individual glomerulus examined (100 glomeruli from each animal)
were averaged, and the percentage of glomeruli for each grade was
calculated.
Tubular atrophy, dilation, casts, interstitial
inflammation and fibrosis were the major pathological
characteristics of renal interstitium. A total of 10 microscopic
fields from each animal (magnification, x200) were selected for
quantitation based on a previously established scoring system
(26,27): 0, normal; 1, lesions in <25% of
the area; 2, lesions in 25-50% of the area; 3, lesions in >50%
of the area; and 4, lesions involving the entire area.
Electron microscopy
To further investigate the effect of NGAL on renal
ultrastructure, transmission electron microscopy (TEM) was
conducted. Ultra-thin sections (2 µm) were prepared according to
the method previously described (28). Briefly, a small piece of the left
kidney (1x1 mm) was fixed in 2.5% glutaraldehyde solution buffered
with sodium cacodylate at pH 7.4 for 2 h; post-fixed for 1 h in 1%
osmium tetroxide solution at 25˚C and pH 7.4; and rehydrated in
ethanol. Upon embedding, sections were stained with uranyl acetate
and lead citrate. TEM was performed with a JEM-1400Flash electron
microscope (JEOL, Ltd.) for viewing and image capture. Evaluations
were performed by two independent observers in a blinded manner.
Grading for the presence of changes in the podocyte foot processes
was analysed according to the following scale: 0, no evidence of
changes; I, <25% changes; II, 25-50% changes; III, >50-75%
changes; and IV, >75% changes. Measurements were obtained from
electronographs (magnification, x10,000) (29,30).
IHC analysis
IHC was used to investigate the expression of NGAL
and FN. Rabbit monoclonal antibodies against NGAL (cat. no.
ab216462) and FN (cat. no. ab268020) were purchased from Abcam.
Paraffin-embedded sections were dewaxed by xylene, and endogenous
peroxidase activity was quenched with 3%
H2O2. Sections were digested with proteinase
K for antigen retrieval. 2% BSA (cat. no. A2058; Merck KGaA) was
used as the blocking reagent and incubated with the samples at room
temperature for 30 min. Sections were incubated with primary
antibodies (1:100) overnight at 4˚C and peroxidase conjugated goat
anti-rabbit IgG (1:200, TA140003; OriGene Technologies, Inc.) at
4˚C for 30 min. IHC staining-positive areas were counted in 30
random cortex x400 high power fields (HPFs) under an Olympus
microscope (IXplore; Olympus Corporation). Positive areas were
measured using Image Pro 6.0 software (Media Cybernetics, Inc.) and
expressed as percentage of positive areas per HPF.
Western blot analyses for transforming
growth factor (TGF)-β1-mediated Smad3 and Smad2
phosphorylation
Human recombinant TGF-β1 (cat. no. 240-B; R&D
Systems, Inc.) was dissolved in deionized water supplemented with
0.1% BSA and 4 µm hydrochloric acid. After serum starving for 24 h,
0.5 ng/ml TGF-β1 was added to HK2 cells (5x105 cells/ml)
at 37˚C for 24 h. HK2 cells without TGF-β1 incubation were set as
control. Then, the HK2 cells were harvested and lysed using RIPA
buffer (Applygen Technologies, Inc.). Protein concentration was
determined using the BCA method. A total of 50 µg denatured protein
per lane was separated using 12% SDS-PAGE followed by the transfer
onto PVDF membranes (Bio-Rad Laboratories, Inc.) as described in an
established procedure (31,32). The primary antibodies used (all,
1:100) were as follows: Anti-phosphorylated (p)-Smad2 (cat. no.
3108S); anti-p-Smad3 (cat. no. 9520S); anti-Smad2 (cat. no. 5339S);
and anti-Smad3 (cat. no. 9523S; Cell Signaling Technology, Inc.).
Samples were incubated with the PVDF membranes at 4˚C overnight.
The antibody for the reference β-actin was also purchased from Cell
Signaling Technology, Inc (cat. no. 3108S). The secondary antibody
anti-rabbit IgG (1:1,000; cat. no. 7074S; Cell Signaling
Technology, Inc.) was incubated with the membranes at room
temperature for 1 h. Quantitation analysis of relative protein
expression was performed using ImageJ 1. 53a (National Institutes
of Health).
Western blot analyses for NGAL in
NC-HK2 and KD-HK2 cells
This procedure was similar to that aforementioned.
NC-HK2 and KD-HK2 cells were harvested and lysed using RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd). The primary
antibody was LCN (D4M8L) rabbit monoclonal antibody (1:100; cat.
no. 44058S; Cell Signaling Technology, Inc.), and the secondary
antibody was the same as mentioned above.
MDA, GSH, SOD and catalase assay
The content of MDA, GSH, SOD and catalase was
measured using MDA content assay kit, GSH content assay kit, SOD
content assay kit, and CAT content assay kit (cat. nos. BC0025,
BC1175, BC0170, BC0200; Beijing Solarbio Science & Technology
Co., Ltd.) with the protocols provided from the supplier.
Statistical analysis
All values are expressed as the mean ± standard
deviation. SPSS 25.0 (IBM Corp.) was used for statistical analysis.
The differences between two groups were evaluated using Student's
t-test. Comparison of the same parameters among more than two
groups was performed using one-way ANOVA, followed by post-hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Efficiency of NGAL knockdown in HK2
cells
Both RT-qPCR and western blotting demonstrated that
the mRNA and protein levels of NGAL in KD-HK2 cells were
significantly reduced compared with those in NC-HK2 cells, which
suggested that the knockdown efficiency was satisfactory for
further experiments (Fig. 1).
NGAL knockdown enhances oxidative
stress under hyperglycemia in vitro and in vivo
HG stimulation can induce intracellular oxidative
stress in HK2 cells. Compared with that in NC-HK2 cells, KD-HK2
cells produced higher intracellular ROS levels under HG
stimulation, which was demonstrated by flow cytometry (Fig. 2A and B). In addition, 3 months after the
establishment of diabetes in animals, fresh kidney homogenate from
diabetic mice was used to evaluate its oxidation status. As
represented in Fig. 2C-F, compared
with the findings in normal control animals, 3-months of diabetes
led to higher malonaldehyde (MDA) and lower glutathione (GSH)
levels, and lower activity of superoxide dismutase (SOD) and
catalase (CAT) in kidney tissues. Furthermore, NGAL-/-
mice showed higher oxidative stress in kidney tissues compared with
that of normal control, with higher MDA and lower GSH levels, and
lower activity of SOD and CAT in kidney tissues (Fig. 2C-F).
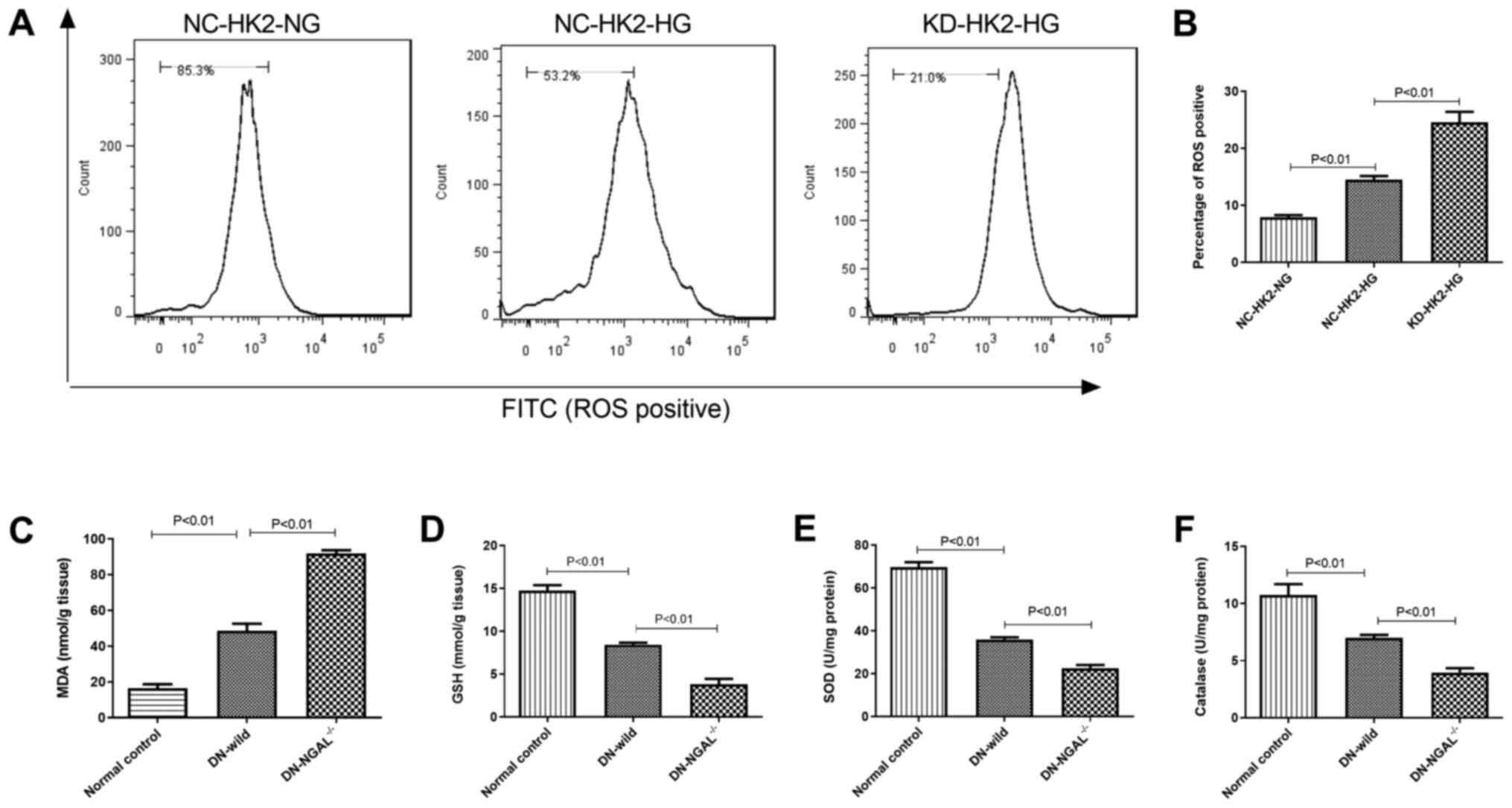 | Figure 2Oxidative stress measurement under
glycemia in vitro and in vivo. (A) ROS test by flow
cytometry in HK2 cells in vitro. (B) Quantitation of ROS
levels in HK2 cells. (C) MDA, (D) GSH, (E) SOD and (F) catalase
concentration or IU in kidney tissues in normal control, DN-wild
and DN NGAL-/- mice in vivo. NGAL, neutrophil
gelatinase-associated lipocalin; HK2, human kidney 2; ROS, reactive
oxygen species; MDA, malonaldehyde; GSH, glutathione; SOD,
superoxide dismutase; DN, diabetic nephropathy; KD, knockdown; NC,
negative control; HG, high glucose; NG, normal glucose. |
NGAL knockdown in HK2 cells reduces
the secretion of FN, Col IV and IL-6 under HG conditions
HG stimulation for 48 h could significantly increase
the secretion of FN, Col IV and IL-6 on HK2 cells in vitro,
as measured by ELISA, compared with that of normal-glucose cultured
cells (NG-NC-HK2 and NG-KD-HK2). This effect was shown in both
HG-NC-HK2 and HG-KD-HK2 cells (Fig.
3). Compared with that of HG-NC-HK2 cells, the concentration of
FN, Col IV and IL-6 in the supernatant of HG-KD-HK2 cell culture
medium was significantly higher.
 | Figure 3Concentration of FN, Col IV and IL-6
in the supernatant of HK2 cells stimulated by HG, as measured by
ELISA. FN, fibronectin; Col IV, collagen IV; IL-, interleukin; HK2,
human kidney 2; HG, high glucose; NG, normal glucose; NC, negative
control; KD, knockdown. |
NGAL knockdown in HK2 cells enhances
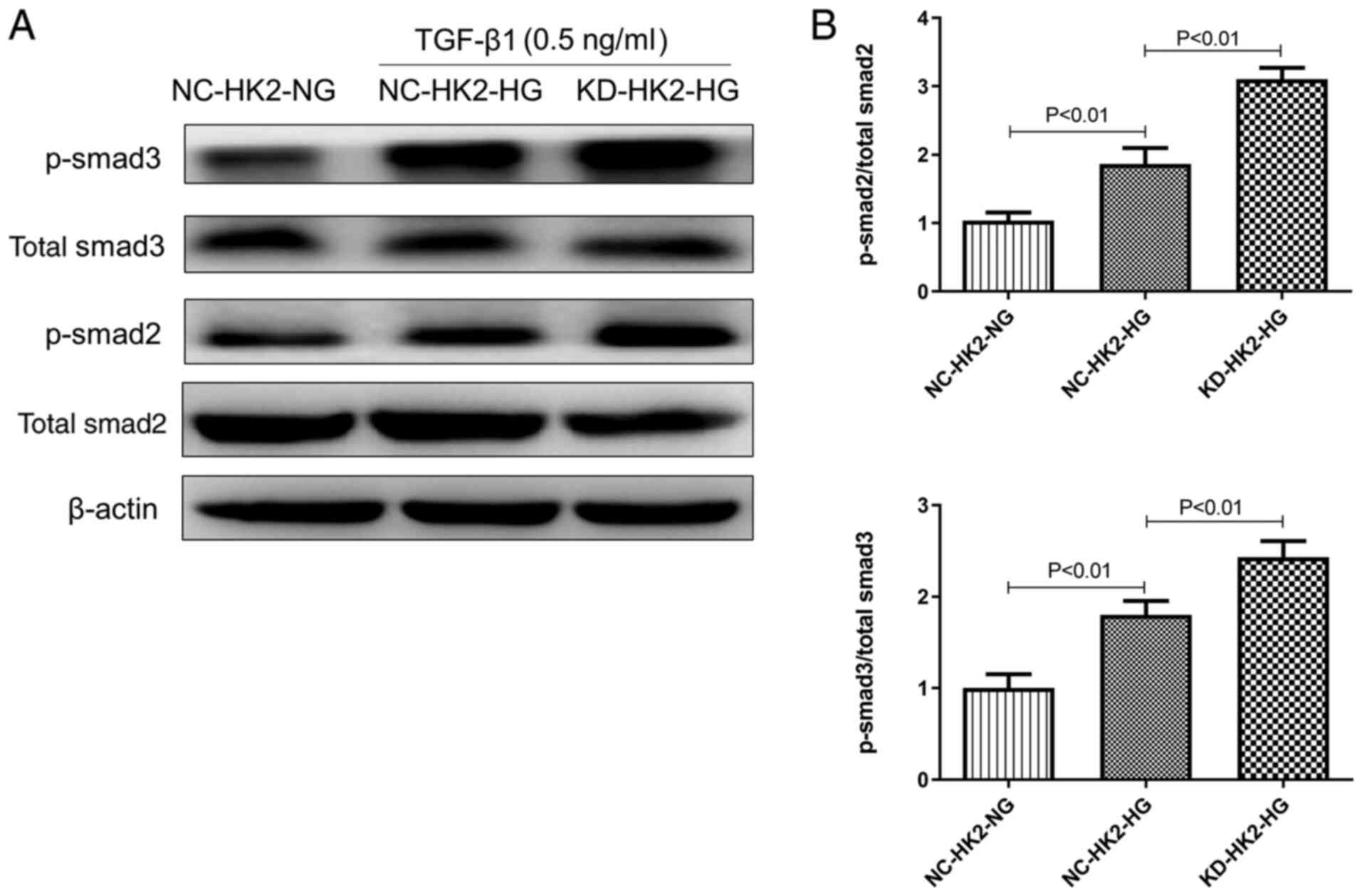
the phosphorylation of Smad2/3 by TGF-β1 stimulation
TGF-β1 is a key pro-fibrotic mediator, mainly via
phosphorylation of Smad2/3. Our preliminary study suggested that
low dosage (0.5 ng/ml) of TGF-β1 stimulation could produce moderate
phosphorylation of Smad2/3. Therefore, 0.5 ng/ml TGF-β1 was used in
the current study. As shown in Fig.
4A and 4B, under the same
concentration of TGF-β1, the protein level of p-Smad2/3 in high
glucose treated KD-HK2 cells was significantly higher than that in
NC-HK2 cells treated with either normal or high glucose. However,
the total protein content of Smad2/3 did not change markedly among
the three groups.
NGAL-/- mice show rapid
deterioration of renal function and serious glomerular and tubular
injury upon the establishment of diabetes
At 4 months, the BUN and Scr levels were measured.
Compared with that exhibited by normal-control and DN-wild mice,
DN-NGAL-/- mice showed deterioration of renal function
with higher BUN and Scr levels, and albuminuria (Fig. 5A-C), but no significant difference
in fasting blood glucose was found between wild-type and
NGAL-/- mice. By histopathological analysis, the tubular
injuries were noted to be more serious in NGAL-/- mice
compared with those in wild-type mice, which had severe vacuolar
degeneration in proximal tubules. By analyzing the glomerular
morphology, NGAL-/- mice were also observed to have
serious glomerular sclerosis, glomerular hypertrophy, segmental and
diffuse mesangial expansion compared with the glomerular morphology
of wild-type mice (Fig. 5E-G). In
order to further understand the ultrastructural alterations, TEM
was performed to focus on the morphological changes of podocytes.
As shown in Fig. 5H and I, the glomeruli from normal control
animals had clear and orderly arranged foot processes; however,
animals with DN exhibited severe podocyte foot process effacement.
Compared with that of wild-type control mice, NGAL-/-
mice showed more serious foot process effacement, which partially
explained their higher albuminuria.
As shown in Fig. 6A,
IHC confirmed that knockout NGAL-/- mice did not express
NGAL in kidney tissues, while normal mice showed light staining of
NGAL and diabetic animals showed strong NGAL staining in the kidney
cortex, mainly around tubular epithelial cells. FN staining was
consistent with the in vitro results, and glycemia induced
higher FN levels in the glomeruli, and DN-NGAL-/- mice
exhibited higher FN deposit compared with that in DN-wild group
(Fig. 6B).
 | Figure 6NGAL knockout exacerbates FN
deposition in glomeruli in vivo, as analyzed by IHC. (A)
NGAL expression in renal cortex, as detected by IHC, which
confirmed that NGAL was not expressed in NGAL-/- mice,
scale bar, 200 µm. (B) FN deposit in glomeruli, as detected by IHC
in the three groups, scale bar, 50 µm. IHC, immunohistochemistry;
FN, fibronectin; NGAL, neutrophil gelatinase-associated lipocalin;
DN, diabetic nephropathy. |
Discussion
The majority of studies on NGAL have focused on its
role in AKI, and NGAL seems to be a potential biomarker to predict
kidney injury (33). However, its
role in the pathophysiology and disease progression of chronic
kidney dysfunctions, particularly DN, remains unclear. Since NGAL
is mostly produced by proximal tubular epithelial cells, in the
present study, the human tubular cell line HK2 was selected to
investigate the effects of NGAL knockdown by RNA interference.
Oxidation plays a detrimental role in the
progression of DN (34). In the
current study, HG incubation caused higher ROS production in HK2
cells, and diabetic mice also showed higher oxidative stress in
kidney tissues compared with that in normal mice. Flow cytometry
suggested that NGAL significantly reduced ROS production under HG
stimulation. Higher oxidative stress was also found in the kidney
of NGAL-/- mice compared with that of wild-type mice,
evidence by the ROS, MDA, GSH, SOD and catalase results. Therefore,
it is hypothesized that this is one of the mechanisms by which NGAL
slows down the progression and deterioration of DN.
Chronic inflammation is another detrimental factor
that can accelerate DN progression (35). NGAL is regarded an inflammatory
mediator secreted by neutrophils to play a role in the early stage
of acute inflammation (36).
However, DN is associated with chronic inflammation; thus, the role
of NGAL in the chronic inflammation of DN requires further
investigation. In the current study, HG stimulation produced higher
IL-6 levels in HK2 cells, and IL-6 has been demonstrated to be
positively associated with DN progression and severity in a
previous study (31). In addition,
NGAL increased the production of IL-6 under HG stimulation. IL-6 is
an important enhancer of chronic inflammation and its expression
has been considered an important mediator of DN progression
(37).
Previous studies have demonstrated that NGAL has a
protective effect on experimental AKI, including LPS-induced AKI
and cisplatin-induced AKI (38) by
suppressing apoptosis via inhibition of caspase 3 activation
(39). However, its role in DN
remains unclear. The major characteristics of DN pathology include
glomerulosclerosis, tubular epithelial cell necrosis and apoptosis,
and the present study suggests that NGAL knockout may enhance these
pathological injuries, and facilitate the progression of DN.
IHC of NGAL confirmed that diabetes could induce
NGAL expression in the tubular area of kidney tissues, and also
confirmed that NGAL was not expressed in knockout mice. Fibrosis is
an important driving factor for glomerulosclerosis. In
vitro, under HG and TGF-β1 stimulation, HK2 cells with NGAL
knockdown produced higher levels of extracellular matrix proteins,
such as FN and Col IV, which are major components of the ECM. In
vivo results also showed that knockout NGAL caused increased
secretion of matrix proteins. Regarding the underlying mechanism of
inhibiting fibrosis, the present study demonstrated that
NGAL-/- could promote Smad2/3 phosphorylation, which is
considered a key process in the TGF-β1 stimulation signaling
pathway (40). The detailed
mechanism by which NGAL cross talks with the TGF-Smad signaling
pathway remains unclear and needs further study.
The limitation of the present study is that the
mouse NGAL and the human NGAL sequences do not share close
homogeneity; therefore, the bioactivities observed in mice may be
different from those of humans.
In conclusion, by NGAL knockdown in vitro and
by knockout in vivo, the renal protective effect of NGAL was
confirmed under glycemia conditions. However, its detailed
mechanism requires further investigation.
Acknowledgements
Not applicable.
Funding
Funding: Financial support was received from the Hebei Medical
Research Key Subject Program for 2017 (grant no. 20160627).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and XZ designed all the experiments and carried
out the experiments. XD performed the statistical analysis, and
wrote the manuscript. XW and TW created the figures and helped with
designing the experiments. SF, HZ, and CC assisted in experiments
and manuscript writing. GL contributed to the conception of the
study and manuscript revision. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Institutional Animal Ethics Committee of the Affiliated Hospital of
Hebei University of Engineering (Handan, China) and performed in
accordance with the guidelines on animal experimentation of the
Committee for Control and Supervision of Experimentation on
Animals, Government of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papadopoulou-Marketou N, Margeli A,
Papassotiriou I, Chrousos GP, Kanaka-Gantenbein C and Wahlberg J:
NGAL as an early predictive marker of diabetic nephropathy in
children and young adults with type 1 diabetes mellitus. J Diabetes
Res. 2017(7526919)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sadar S, Kaspate D and Vyawahare N:
Protective effect of L-glutamine against diabetes-induced
nephropathy in experimental animal: Role of KIM-1, NGAL, TGF-β1,
and collagen-1. Ren Fail. 38:1483–1495. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Scelo G and Larose TL: Epidemiology and
risk factors for kidney cancer. J Clin Oncol.
36(JCO2018791905)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tarchini R, Bottini E, Botti P, Talassi E,
Baraldi O, Lambertini D, Gaetti L and Bellomi A: Type 2 diabetic
nephropathy: Clinical course and prevention proposals 2004. G Ital
Nefrol. 22 (Suppl 31):S15–S19. 2005.PubMed/NCBI(In Italian).
|
|
5
|
Leehey DJ, Singh AK, Alavi N and Singh R:
Role of angiotensin II in diabetic nephropathy. Kidney Int. (Suppl
77):S93–S98. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang S, Wang D, Xue N, Lai F, Ji M, Jin J
and Chen X: Nicousamide protects kidney podocyte by inhibiting the
TGFβ receptor II phosphorylation and AGE-RAGE signaling. Am J
Transl Res. 9:115–125. 2017.PubMed/NCBI
|
|
7
|
Zhang S, Li Y, Li H, Zheng X and Chen X:
Renal-protective effect of nicousamide on hypertensive nephropathy
in spontaneously hypertensive rats. Biomed Rep. 1:34–40.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oh SM, Park G, Lee SH, Seo CS, Shin HK and
Oh DS: Assessing the recovery from prerenal and renal acute kidney
injury after treatment with single herbal medicine via activity of
the biomarkers HMGB1, NGAL and KIM-1 in kidney proximal tubular
cells treated by cisplatin with different doses and exposure times.
BMC Complement Altern Med. 17(544)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaul A, Behera MR, Rai MK, Mishra P,
Bhaduaria DS, Yadav S, Agarwal V, Karoli R, Prasad N, Gupta A and
Sharma RK: Neutrophil gelatinase-associated lipocalin: As a
predictor of early diabetic nephropathy in type 2 diabetes
mellitus. Indian J Nephrol. 28:53–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cowland JB and Borregaard N: Molecular
characterization and pattern of tissue expression of the gene for
neutrophil gelatinase-associated lipocalin from humans. Genomics.
45:17–23. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Borregaard N, Sehested M, Nielsen BS,
Sengeløv H and Kjeldsen L: Biosynthesis of granule proteins in
normal human bone marrow cells. Gelatinase is a marker of terminal
neutrophil differentiation. Blood. 85:812–817. 1995.PubMed/NCBI
|
|
12
|
Flo TH, Smith KD, Sato S, Rodriguez DJ,
Holmes MA, Strong RK, Akira S and Aderem A: Lipocalin 2 mediates an
innate immune response to bacterial infection by sequestrating
iron. Nature. 432:917–921. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Venge P, Douhan-Håkansson L, Garwicz D,
Peterson C, Xu S and Pauksen K: Human neutrophil lipocalin as a
superior diagnostic means to distinguish between acute bacterial
and viral infections. Clin Vaccine Immunol. 22:1025–1032.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mishra J, Mori K, Ma Q, Kelly C, Yang J,
Mitsnefes M, Barasch J and Devarajan P: Amelioration of ischemic
acute renal injury by neutrophil gelatinase-associated lipocalin. J
Am Soc Nephrol. 15:3073–3082. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mori K and Nakao K: Neutrophil
gelatinase-associated lipocalin as the real-time indicator of
active kidney damage. Kidney Int. 71:967–970. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bolignano D, Coppolino G, Campo S, Aloisi
C, Nicocia G, Frisina N and Buemi M: Urinary neutrophil
gelatinase-associated lipocalin (NGAL) is associated with severity
of renal disease in proteinuric patients. Nephrol Dial Transplant.
23:414–416. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang W, Li Z, Chen Y, Wu H, Zhang S and
Chen X: Prediction value of serum NGAL in the diagnosis and
prognosis of experimental acute and chronic kidney injuries.
Biomolecules. 10(981)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mauro C, Pacifico F, Lavorgna A, Mellone
S, Iannetti A, Acquaviva R, Formisano S, Vito P and Leonardi A:
ABIN-1 binds to NEMO/IKKgamma and co-operates with A20 in
inhibiting NF-kappaB. J Biol Chem. 281:18482–18488. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yan YM, Ai J, Zhou LL, Chung ACK, Li R,
Nie J, Fang P, Wang XL, Luo J, Hu Q, et al: Lingzhiols,
unprecedented rotary door-shaped meroterpenoids as potent and
selective inhibitors of p-Smad3 from ganoderma lucidum. Org Lett.
15:5488–5491. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang S, Ma J, Sheng L, Zhang D, Chen X,
Yang J and Wang D: Total coumarins from Hydrangea paniculata show
renal protective effects in lipopolysaccharide-induced acute kidney
injury via anti-inflammatory and antioxidant activities. Front
Pharmacol. 8(872)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sen Z, Weida W, Jie M, Li S, Dongming Z
and Xiaoguang C: Coumarin glycosides from Hydrangea
paniculata slow down the progression of diabetic nephropathy by
targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis.
Phytomedicine. 57:385–395. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nørgaard SA, Sand FW, Sørensen DB, Abelson
KS and Søndergaard H: Softened food reduces weight loss in the
streptozotocin-induced male mouse model of diabetic nephropathy.
Lab Anim. 52:373–383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
National Technical Committee for
Standardization of Laboratory Animals.
|
|
25
|
Naito T, Ma LJ, Yang H, Zuo Y, Tang Y, Han
JY, Kon V and Fogo AB: Angiotensin type 2 receptor actions
contribute to angiotensin type 1 receptor blocker effects on kidney
fibrosis. Am J Physiol Renal Physiol. 298:F683–F691.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pörsti I, Fan M, Kööbi P, Jolma P,
Kalliovalkama J, Vehmas TI, Helin H, Holthöfer H, Mervaala E, Nyman
T and Tikkanen I: High calcium diet down-regulates kidney
angiotensin-converting enzyme in experimental renal failure. Kidney
Int. 66:2155–2166. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li P, Ma LL, Xie RJ, Xie YS, Wei RB, Yin
M, Wang JZ and Chen XM: Treatment of 5/6 nephrectomy rats with
sulodexide: A novel therapy for chronic renal failure. Acta
Pharmacol Sin. 33:644–651. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aunapuu M, Pechter U, Arend A, Suuroja T
and Ots M: Ultrastructural changes in the remnant kidney (after 5/6
nephrectomy) glomerulus after losartan and atenolol treatment.
Medicina (Kaunas). 39:975–979. 2003.PubMed/NCBI
|
|
29
|
Fernandes SM, Cordeiro PM, da Fonseca CD
and Vattimo MF: The role of oxidative stress in
streptozotocin-induced diabetic nephropathy in rats. Arch
Endocrinol Metab. 60:443–449. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Han H, Cao A, Wang L, Guo H, Zang Y, Li Z,
Zhang X and Peng W: Huangqi decoction ameliorates
streptozotocin-induced rat diabetic nephropathy through antioxidant
and regulation of the TGF-β/MAPK/PPAR-γ signaling. Cell Physiol
Biochem. 42:1934–1944. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang S, Yang J, Li H, Li Y, Liu Y, Zhang
D, Zhang F, Zhou W and Chen X: Skimmin, a coumarin, suppresses the
streptozotocin-induced diabetic nephropathy in wistar rats. Eur J
Pharmacol. 692:78–83. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sen Z, Weida W, Li Y, Zhaojun L, Nina X
and Xiaoguang C: Nicousamide attenuates renal dysfunction and
glomerular injury in remnant kidneys by inhibiting TGF-β1
internalisation and renin activity. Eur J Pharmacol. 845:74–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han M, Li Y, Liu M, Li Y and Cong B: Renal
neutrophil gelatinase associated lipocalin expression in
lipopolysaccharide-induced acute kidney injury in the rat. BMC
Nephrol. 13(25)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Papadopoulou-Marketou N, Paschou SA,
Marketos N, Adamidi S, Adamidis S and Kannaka-Gantenbein C:
Diabetic nephropathy in type 1 diabetes. Minerva Med. 109:218–228.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pérez-Morales RE, Del Pino MD, Valdivielso
JM, Ortiz A, Mora-Fernández C and Navarro-González :
Inflammation in diabetic kidney diseases. Nephron. 143:12–16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shang W and Wang Z: The update of NGAL in
acute kidney injury. Curr Protein Pept Sci. 18:1211–1217.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Feigerlová E and Battaglia-Hsu SF: IL-6
signaling in diabetic nephropathy: From pathophysiology to
therapeutic perspectives. Cytokine Growth Factor Rev. 37:57–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ma Q, Devarajan SR and Devarajan P:
Amelioration of cisplatin-induced acute kidney injury by
recombinant neutrophil gelatinase-associated lipocalin. Ren Fail.
38:1476–1482. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Han M, Li Y, Wen D, Liu M, Ma Y and Cong
B: NGAL protects against endotoxin-induced renal tubular cell
damage by suppressing apoptosis. BMC Nephrol.
19(168)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Higgins SP, Tang Y, Higgins CE, Mian B,
Zhang W, Czekay RP, Samarakoon R, Conti DJ and Higgins PJ:
TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal. 43:1–10.
2018.PubMed/NCBI View Article : Google Scholar
|