Introduction
Demodex (class Arachnida, superorder
Acariformes), a microscopic and elongated mite, is one of the most
common ectoparasites in humans and may be present on the face and
most other parts of the body (1,2). The
facial T-zone (forehead, cheeks and nose), meibomian glands (MGs)
and follicles of eyelashes are the most common habitats of
Demodex. Two parasitic species have been detected in humans,
namely Demodex folliculorum and Demodex brevis.
Eyelash follicles are the habitat of Demodex folliculorum,
while Demodex brevis resides in the MGs and the sebaceous
glands of the eyelids (3-5).
Demodex infection gradually develops to Demodex
blepharitis, which is characterized as chronic inflammation of the
eyelid and MG and ultimately leads to MG dysfunction (MGD). MGD is
the major cause of evaporated dry eye disease, characterized as
tear film instability and chronic ocular surface inflammation
(6,7). Tea tree oil (TTO) may forcefully
eradicate Demodex mites and alleviate ocular surface
inflammation associated with Demodex blepharitis (8). Hence, TTO treatment is currently
accepted as the most effective therapy for Demodex
blepharitis. Although TTO has a certain therapeutic effect on
Demodex blepharitis, the pungent odor of TTO may cause mild
to moderate ocular irritation and discomfort at higher
concentrations or with prolonged periods of exposure, especially to
the elderly and children (6).
Abelmoschus esculentus L., also known as
okra, is a well-known tropical vegetable that is widely grown
worldwide. In addition to serving as a food source, okra may serve
as a traditional medicine to cure numerous diseases, such as
dysentery and diarrhea (9). Due to
abundant bioactive compounds, such as polysaccharides, flavonoids,
polyphenols, caffeine and pectin in okra, the antibacterial,
anti-inflammatory and immune regulatory effects of okra have been
gradually proven (10-12).
Okra has been applied widely in the cosmetics industry, where the
safety and efficacy of okra have been gradually confirmed (13). Okra has been reported to exert
anti-oxidative and anti-inflammatory effects by suppressing the
Akt-mediated NF-κB pathway in a murine BV2 microglial cell line
(14). Akt-mediated NF-κB pathway
has been demonstrated to serve an important role in dry eye and
blepharitis (15,16). Due to the curative and medicinal
effects of okra, it could be speculated that okra may confer
therapeutic effects in patients with blepharitis and dry eye.
The present study was designed to determine the
potential anti-demodectic and therapeutic effects of an okra eyelid
patch on Demodex blepharitis in vivo and in
vitro.
Materials and methods
Subjects
The present study was performed in accordance with
the tenets of the Declaration of Helsinki and was approved by the
Institutional Review Board of the Eye, Ear, Nose and Throat (EENT)
Hospital of Fudan University. The study was registered as a
clinical trial in the Chinese Clinical Trial Registry (ChiCTR) in
November 2018 (registration no. ChiCTR-1,800,019,466). A total of
52 patients who experienced Demodex blepharitis were
recruited from the EENT hospital between December 2018 and May
2019. Demodex blepharitis was confirmed by the total number
of Demodex on epilated lashes under light microscopic
examination. In brief, the procedure was as follows: A total of two
lashes with cylindrical dandruff (CD) each from the upper and lower
eyelids were epilated and mounted on glass slides; one drop of
cedar oil was dripped on the bottom of each eyelash to dissolve the
CD and to allow the embedded Demodex to migrate out, which
occurs almost immediately; the total number of Demodex mites
was counted by the same inspector (WL) under a light microscope
(CX23; Olympus Corp.). If the number of Demodex mites was
≥3, the patient was considered as Demodex-positive (17,18).
Furthermore, ‘absolute Demodex eradication’ was defined as
complete Demodex eradication with the Demodex count
reduced to zero after treatment (19,20).
The inclusion criteria for the present study were
Demodex blepharitis patients with Demodex-positive
eyelashes aged 18-70 years who voluntarily participated in the
experiment. As patients with certain ocular diseases (recurrent
herpes simplex, ocular impairment associated with immune diseases)
or receiving physiotherapy for blepharitis (intense pulsed light,
baby shampoo, LipiFlow® and other Demodex
treatments) in the last 6 months may have confounded the results,
they were excluded from the study. Patients were also excluded if
they had a related ocular surgery, including cataract surgery,
trichiasis surgery or refractive surgery in the past 3 months.
After the procedure and potential consequences of the treatments
had been elaborately explained, informed consent was obtained from
each participant prior to participation.
Treatments
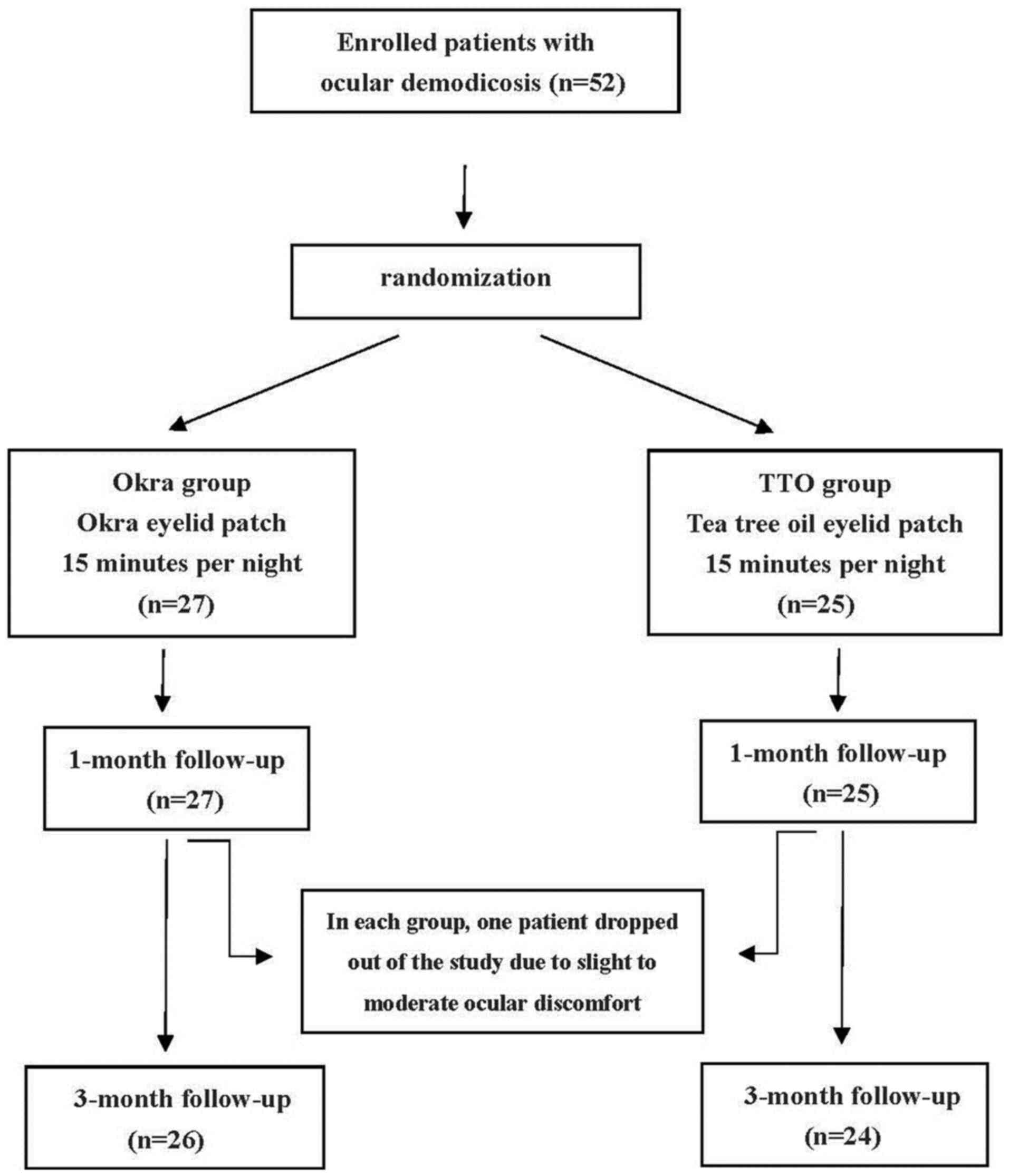
A total of 52 participants were randomly divided
into two groups using a random number table. The okra group (n=27)
received okra eyelid patch treatment (YourGa®; Shanghai
YourGa Co., Ltd.; http://www.ganyanzheng.com/?_l=zh_CN). and the TTO
group (n=25) received TTO eye care patch treatment (YourGa;
Shanghai YourGa Co., Ltd.; Fig. 1).
The treatment was performed by participants at home every night
before sleep for three months. The procedure was as follows: After
the eyelid margin was cleaned by eye rinse (YourGa), the eyelid was
heated to 42˚C from the outside by a Moisture Chamber (YourGa) for
20 min. Finally, eyelid patches (okra or TTO) were applied to both
eyes for 15 min and then removed.
The order of examinations performed was as follows:
Ocular surface disease index (OSDI), slit-lamp biomicroscopic
examination, Schirmer I test (SIT), tear film break-up time (TBUT),
MG assessment and corneal fluorescein staining (CFS). Each
participant was subjected to the examinations three times, namely
on the day prior to treatment and 1 and 3 months after treatment.
All examinations were performed by a skilled researcher (WL) to
reduce operational error. In addition, both eyes were examined and
treated, but only the data of the right eye were analyzed.
OSDI
The OSDI questionnaire, a 12-item questionnaire with
a scale of 0-100, has been designed to rapidly evaluate ocular
discomfort symptoms (e.g. soreness, light sensitiveness, blurred
vision). The OSDI provides an assessment of vision-related
dyspraxia (difficulty reading, driving, operating a computer and
watching TV). There is a positive correlation between OSDI scores
and the severity of ocular discomfort, with higher scores
representing greater ocular discomfort (21). Prior to treatment, the OSDI
questionnaire was completed by participants to set the
baseline.
SIT
After a sterile dry strip (Jingming®) was
inserted into the lateral canthus of the lower eyelid away from the
cornea for 5 min, the wetted length of the strip absorbed with
tears was then measured to assess tear production. Potential SIT
scores ranged from 0 to 30 mm.
TBUT
A fluorescein strip (Jingming) moistened with
preservative-free saline solution gently touched the central lower
lid margin the patient was then requested to blink several times to
ensure adequate coating of the complete corneal dye. After several
natural blinks, the patient was required to rapidly open the eye
and the time of eye opening was recorded as the starting point
(time=0 sec). TBUT was defined as the interval between the starting
point and the first black spot appearing in the stained team film
with a cobalt blue filter and slit lamp microscope. The test was
repeated three times on each patient and the average TBUT was
calculated (22).
MG expressibility (MGE)
A total of 5 consecutive MGE measurements of the
central lower eyelid were assessed with an MG evaluator (MGE-1000;
TearScience). The patient was instructed to look upwards and the
lower eyelid gland orifices were gently cleaned with a cotton swab.
The MG evaluator was held in position and pressure was maintained
for 10-15 sec to evaluate whether each gland secreted or not. The
MGE score was recorded according to the number of secretory glands:
0, all five glands; 1, three to four glands; 2, one to two glands;
and 3, none of the glands (7).
Meibum quality
The meibum quality of eight central MGs of the upper
eyelid was also assessed with the MG evaluator. The assessment
procedure was the same as above. According to the secretion
characteristics of each gland, the grade was ranked on a scale of 0
to 3 for each gland: 0, clear liquid; 1, colored/cloudy liquid; 2,
cloudy with debris (granular); and 3, thick like toothpaste (total
grade range, 0-24 scores) (7).
CFS
The inspection method of CFS was similar to that for
the assessment of the TBUT. The cornea was divided into five zones
(central, superior, temporal, nasal and inferior). Corneal
epithelial injury was graded on a scale from 0 to 3: 0, no
epithelial injury; 1, <30 corneal punctate stains; 2, >30
corneal punctate stains but not fusion; and 3, fusion of corneal
staining or ulcer. The total CFS score ranged from 0 to 15(23).
Tolerance
During the treatment, any symptoms regardless of the
cause were promptly recorded by the researcher (WL). Common
symptoms may include eye redness, pain, allergy, irritation and
foreign body sensation. Rare symptoms may involve blurred vision
and acute ocular infection. If the participant requested withdrawal
from the study due to discomfort, the study will be terminated
immediately.
Survival time (ST) of Demodex
Written informed consents have been obtained from
all patients before eyelashes have been extracted. After eyelashes
have been extracted from the participants’ eyelid in each group at
room temperature, these eyelashes with demodex mites were instantly
placed on a glass slide. A total of 60 mites were then divided
randomly into three groups (20 mites in each group). Okra and TTO
eyelid patch extractions were then dripped onto the glass with a
micropipette respectively. Saline was used as the blank group. As
Demodex is more vulnerable at an earlier stage of life, only
adult Demodex with four pairs of well-developed legs and a
stumpy body were tested (8). After
the eyelash had been extracted from eyelid, the movements of the
Demodex body and legs were observed immediately and
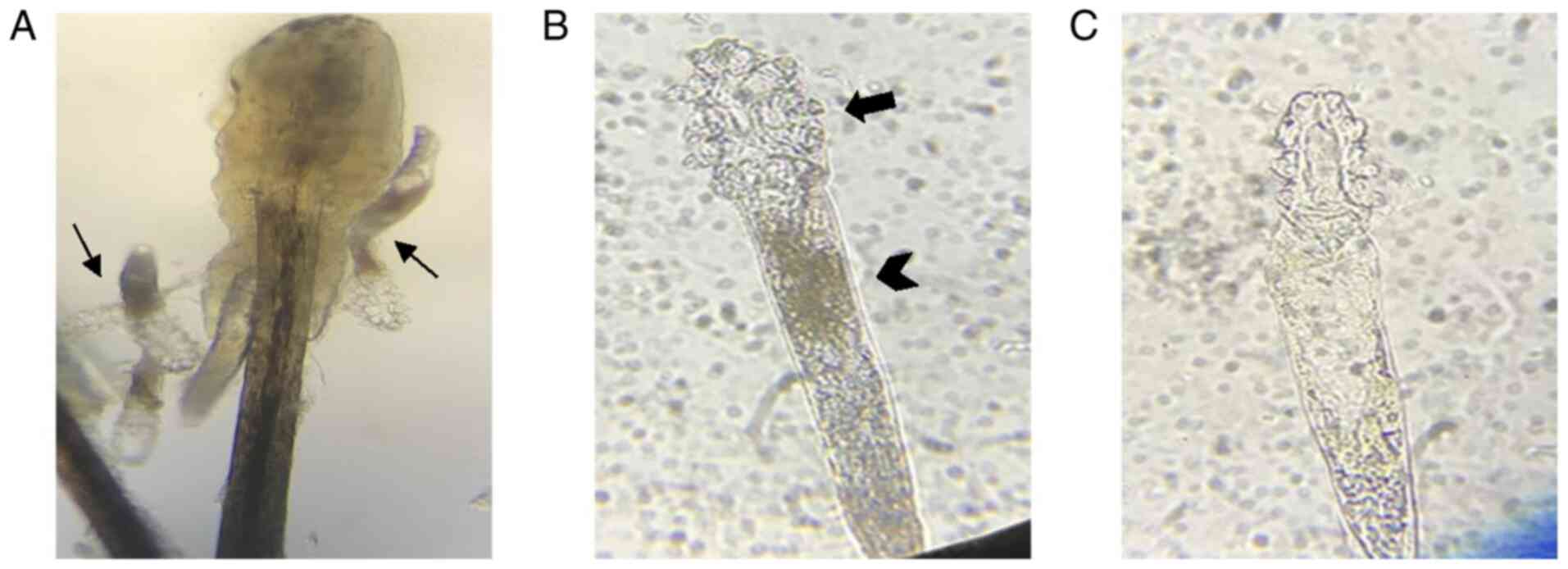
continuously under a microscope at a magnification of x40. The ST
was defined as the duration from the time-point of eyelid patch
extraction dripped on the body to the cessation of movement
(Fig. 2). The average ST of
Demodex mites was compared among the control (n=20 mites),
okra (n=20 mites) and TTO (n=20 mites) groups.
Statistical analysis
Randomization method was used to designate
participants into Okra and TTO groups. Data were analyzed using
SPSS v.17.0 software (SPSS Inc.). Categorical data (sex) between
two groups were evaluated for statistical significance using the
chi-square test. Continuous variables were presented as the mean ±
standard deviation. The normal distribution test
(Kolmogorov-Smirnov) was performed to check whether the numerical
variables were normally distributed. Data on age and ocular
parameters between the two groups were evaluated for statistical
significance using Student's t-test. The data at the baseline and
at 1 and 3 months after treatment in the Okra group were
homoscedastic and normally distributed and the differences between
various time-points were analyzed by repeated-measures ANOVA
followed by a least-significant differences test. The results are
indicated as P-values, where P<0.05 was considered to indicate a
statistically significant difference. According to a previous
study, the Demodex eradication rate of TTO therapy is ~90%
(20). In the present study, it was
previously estimated that there would be a 25% relative difference
between the Okra and the TTO groups (20), which meant that a sample size of 23
patients in each group was required to achieve a statistical power
of 80% for a significance level of 0.25% with a two-tailed
test.
Results
Patient baseline characteristics
A total of 27 participants underwent okra eyelid
patch treatment (13 males and 14 females, aged 46.21±13.03 years),
while 25 participants received TTO eyelid patch treatment (13 males
and 12 females, aged 40.54±10.39 years). No significant differences
in terms of sex (c2=0.103, P=0.749) and age (P=0.160)
were determined between the two groups. There were no differences
in the Demodex count, OSDI, meibum quality, MGE, SIT, TBUT
and CFS between the okra and control groups prior to treatment
(P>0.05; Table I).
 | Table IComparison of demographic data,
baseline data between the okra and TTO treatment groups. |
Table I
Comparison of demographic data,
baseline data between the okra and TTO treatment groups.
| Item | Okra (n=27) | TTO (n=25) | P-value |
|---|
| Age (years) | 46.21±13.03 | 40.54±10.39 | 0.160 |
| Females/males | 14/13 | 12/13 | 0.749 |
| Demodex
mites | 10.15±4.53 | 11.24±5.88 | 0.455 |
| OSDI score | 40.51±10.85 | 35.86±12.77 | 0.162 |
| Meibum quality
score | 8.56±4.26 | 6.68±4.72 | 0.138 |
| MGE | 1.15±0.66 | 1.44±0.51 | 0.082 |
| SIT (mm/5 min) | 9.63±5.13 | 7.20±6.70 | 0.147 |
| TBUT (sec) | 4.59±1.74 | 5.12±1.88 | 0.298 |
| CFS | 2.22±1.60 | 1.40±1.68 | 0.077 |
Demodex eradication and ocular
parameters
The Demodex counts and ocular parameters at 1
and 3 months in the okra group were compared with those at baseline
(Table II). Okra eyelid patch
treatment can significantly eradicate Demodex mites from
10.15±4.53 to 1.31±1.41 on patients with ocular demodicosis after 3
months (P<0.01). The average OSDI score in the okra group
decreased from baseline (40.51±10.85) to the end of treatment
(23.67±10.71; P<0.01). Other ocular parameters, including SIT,
TBUT, CFS, meibum quality and MGE, were also compared before and
after 1 and 3 months of treatment, respectively. Statistically
significant improvements in TBUT (P=0.007), CFS (P<0.01) and
meibum quality (P<0.01) have been observed in Okra group by 3
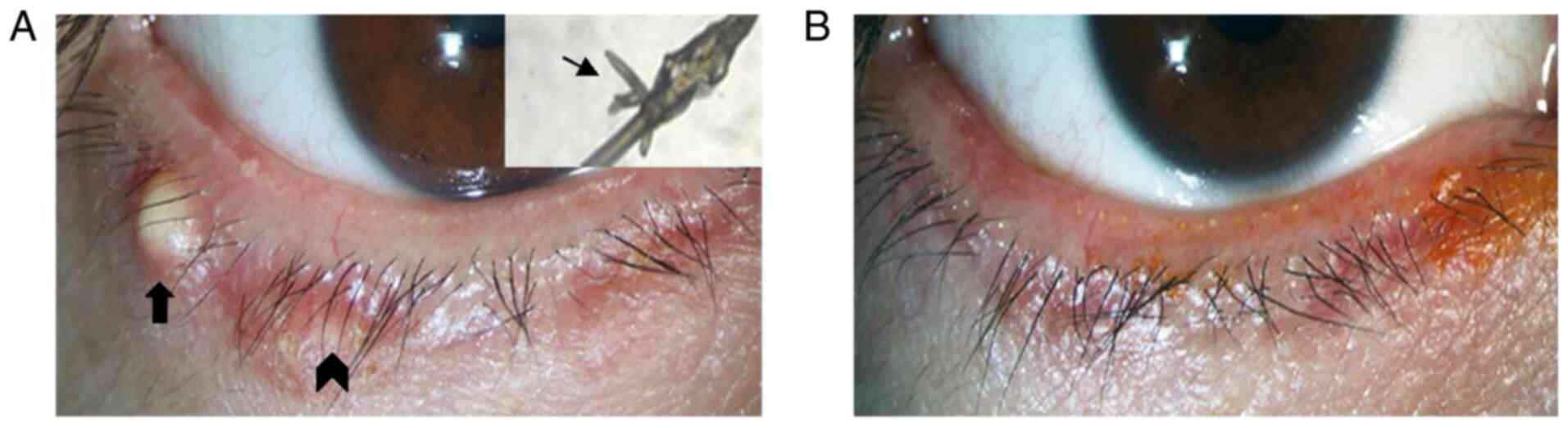
months. Blepharitis of 13 patients was observed to be improved from
slight to obvious degrees after Okra eyelid treatment. Among these
patients, the sign of eyelid margin in one patient who was improved
markedly (Fig. 3).
 | Table IIVariation in the parameters from
baseline to 1 and 3 months after treatment in the Okra group. |
Table II
Variation in the parameters from
baseline to 1 and 3 months after treatment in the Okra group.
| Item | Prior to
treatment | 1 month | 3 months | F | P1 | P2 |
|---|
| Demodex
mites | 10.15±4.53 | 3.26±2.03 | 1.30±1.41 | 125.55 | <0.01 | <0.01 |
| OSDI score | 40.51±10.85 | 29.55±12.15 | 23.67±10.71 | 29.74 | <0.01 | <0.01 |
| Meibum quality
score | 8.56±4.26 | 5.85±4.29 | 5.48±4.08 | 16.39 | <0.01 | <0.01 |
| MGE | 1.15±0.66 | 0.93±0.62 | 1.04±0.71 | 1.44 | 0.056 | 0.449 |
| SIT | 9.63±5.13 | 10.74±4.71 | 11.22±4.53 | 3.03 | 0.119 | 0.014 |
| TBUT (sec) | 4.59±1.74 | 5.56±1.50 | 5.96±1.58 | 12.74 | 0.006 | <0.01 |
| CFS | 2.22±1.60 | 1.11±1.12 | 0.78±0.93 | 43.54 | <0.01 | <0.01 |
The Demodex counts and ocular parameters at 1
and 3 months of treatment were also compared between the okra and
TTO groups (Table III). The
average Demodex count in the okra group had decreased by
-8.85±3.84 after 3 months of treatment, whilst the Demodex
count in the TTO group decreased -9.36±6.03 after 3 months of
treatment (P=0.716). There was no significant difference in the
anti-demodectic effects between the two groups at 3 months
(P=0.716). Compared with that in the TTO group, the
anti-demodectic effect of okra was slightly milder at 1 month, but
no significant difference was observed between the two treatments
at 1 and 3 months. The absolute Demodex eradication rate in
the okra group (11/27, 40.74%) was slightly lower than that in the
TTO group (12/25, 48%). The improvements in each of ocular
parameters between okra and TTO groups did not exhibit significant
difference, except for SIT (P=0.035) and CFS (P=0.023) at 3
months.
 | Table IIIVariation in the parameters from
baseline to 1 and 3 months after treatment compared between the two
groups. |
Table III
Variation in the parameters from
baseline to 1 and 3 months after treatment compared between the two
groups.
| | 1 month | 3 months |
|---|
| Item | Okra | TTO | P1 | Okra | TTO | P2 |
|---|
| Demodex mite
eradication | -6.89±3.24 | -7.84±6.24 | 0.489 | -8.85±3.84 | -9.36±6.03 | 0.716 |
| OSDI score | -8.65±11.49 | -12.40±9.53 | 0.627 | -16.84±10.17 | -17.44±16.05 | 0.873 |
| Meibum quality
score | -2.70±2.30 | -1.56±2.45 | 0.089 | -3.07±3.15 | -2.48±3.42 | 0.517 |
| MGE | -0.22±0.58 | -0.04±0.20 | 0.141 | -0.11±0.75 | -0.32±0.63 | 0.284 |
| SIT (mm/5 min) | 1.11±3.58 | -0.36±1.68 | 0.067 | 1.59±3.13 | 0.04±1.81 | 0.035 |
| TBUT (sec) | 0.96±1.68 | 0.16±1.72 | 0.095 | 1.37±1.47 | 0.76±1.83 | 0.190 |
| CFS | -1.11±0.89 | -0.44±0.87 | 0.008 | -1.44±0.93 | -0.72±1.28 | 0.023 |
Tolerance
Any ocular discomfort, including allergic reaction,
irritation and pruritus, acute ocular infection and visual acuity
loss, was recorded during treatment. No allergic reactions or acute
ocular infections were reported during treatment in either group.
Of the 27 participants in the okra group, 26 (96.3%) did not report
any adverse events or tolerability issues during the treatment
period. However, one participant (3.7%) reported transient ocular
pruritus and discomfort following application of the okra eyelid
patch close to the eyelash margin and surrounding skin. This
participant was then dropped out of the study and lost to
follow-up. Of the 25 patients in the TTO group, four (16%) reported
slight to moderate irritation with conjunctival congestion. Among
these four participants, one was subsequently lost to follow-up due
to dropping out.
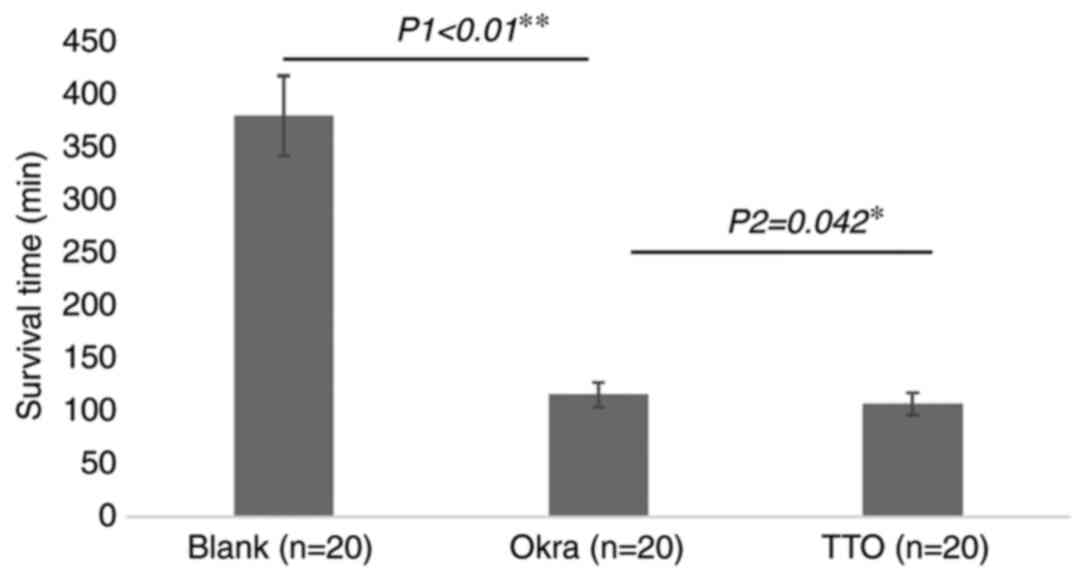
ST of Demodex
The average ST of Demodex in the different
groups is provided in Fig. 4. The
average ST in the okra group was 115.25 min, which was
significantly lower compared with the average ST of 378.75 min in
the blank group (P<0.01). Compared with that in the TTO group
(106.7 min), the ST in the okra group was slightly but
significantly longer (P=0.042).
Discussion
Increased attention has been paid to Demodex
blepharitis by ophthalmologists in the past two decades. Several
pathogenic mechanisms of Demodex blepharitis have been
postulated in previous studies. First, Demodex mites may
significantly damage the habitat where they live by continuous
movement and invasion. Furthermore, Demodex mites may block
the hair follicles and sebaceous ducts mechanically to induce
epithelial hyperplasia and hyperkeratinization, while debris or
waste from Demodex mites may elicit inflammatory responses
or an innate immune response (24).
In addition to the mechanisms described above, the pathogenic role
of other microbial infections, including Streptococci,
Staphylococci, Propionibacterium acnes associated with
Demodex infestation that leads to MGD and dry eye has also
gained increasing attention.
Different TTO products are now widely applied in
Demodex blepharitis treatments. A weekly eyelid scrub with
50% TTO proved successful in eradicating ocular Demodex
infestation and Demodex counts as low as zero have been
confirmed in the majority of patients after 4 weeks of treatment
(25-27).
The present study indicated that okra had a similar effect to that
of TTO in terms of Demodex eradication on the eyelids of
patients, and furthermore, it significantly shortened the survival
time of Demodex in vitro. Therefore, okra is expected to be
developed as a potential treatment for Demodex
blepharitis.
As TTO may exert antibacterial, antifungal and
anti-inflammatory actions, its therapeutic effects may not be
attributed to killing Demodex mites only; TTO also possesses
anti-inflammatory and bacterial colonization reduction properties.
In previous studies, TTO demonstrated a strong effect on
Demodex eradication and among a total of 15 major active
components in TTO, terpinen-4-ol has been identified as the most
potent ingredient (8,26).
Regarding bacterial infection, okra fruit has a high
tannin content that may abolish several common types of bacteria,
including several Gram-positive and Gram-negative bacteria
(28). Furthermore, Lengsfeld et
al (29) reported that H.
pylori adhesion on human stomach sections was almost completely
inhibited by fresh okra fruit juice. In addition to its
antibacterial effects, a number of studies suggested that crude
okra acts as an immunomodulator with both immune-stimulatory and
immunosuppressive activities (30).
Okra is also able to act as an immune modulator by activating
phagocytes to produce more proinflammatory cytokines (e.g. TNF-α
and IL-17) to improve the host defense against various bacteria.
Among the medicinal herbs, okra is an important plant that is
widely distributed worldwide; furthermore, okra is a Chinese
medicine and is less pungent than TTO, resulting in minor toxicity
and few side effects (31).
Among antiparasitic remedies, polysaccharides from
different sources have become a prime research topic. Volatile oils
from certain Chinese crude medicines with abundant polysaccharides
exerted the most potent effect among various Demodex
blepharitis treatments (32). Apart
from this, sulfated polysaccharides from algae were reported to
serve as an alternative to heparin in the treatment of
leishmaniasis to significantly reduce the count of the
promastigotes of L. amazonensis in a dose-dependent manner
(33). The possible anti-demodectic
mechanism of polysaccharides is to regulate the host's immune
system by activating immune cells such as lymphocytes, macrophages
and natural killer cells. Furthermore, the attachment between host
and parasite may be attenuated (34). Okra contains an abundance of
polysaccharides, which are considered to be responsible for
effective Demodex eradication.
In addition, okra, which contains an abundance of
flavonoids, polyphenol and vitamin C, may act as an antioxidant and
anti-inflammatory agent with low toxicity and few side effects
(35,36). The antioxidant activity of
methanolic seed extracts of okra has been reported in several
studies, revealing antioxidant activity of okra seed extracts under
different conditions (37,38). Furthermore, the reactive oxygen
species (ROS) oxidation pathway has been confirmed to involve in
the pathogenesis of dry eye disease, whereas okra may interfere
with dry eye by inhibiting the ROS oxidation pathway to alleviate
ocular discomfort (39,40).
Similar to Demodex blepharitis, rosacea is a
characteristic cutaneous dermatosis due to the presence of multiple
small, dome-shaped erythematous papules and papulopustules and
Demodex mites may also have a vital role in its pathogenesis
(41,42). Certain studies have suggested that
rosacea remedies, including antibacterial, anti-inflammatory and
immune regulation treatments, have proven curative effects
(43). A number of previous studies
suggested that antibiotic treatments, such as oral antibiotics,
including tetracycline or topical metronidazole, may obviously
alleviate skin inflammation and symptoms of rosacea. Superantigens
produced by Streptococci and Staphylococci may have a
role in the induction of rosacea (43). Hence, researchers should also pay
more attention to the role of microorganisms in the treatment of
Demodex infestation (44,45).
Due to the reported anti-inflammatory and antioxidant effects of
okra (28,37), it could be speculated that the
symptoms of Demodex blepharitis may be improved by these
compounds. In addition, it has been proposed that Demodex
may be eradicated by changing the living environment of
Demodex and reducing the colonization of related bacteria in
MG in view of the antibacterial, anti-inflammatory and
immunoregulatory effects of okrae30,36). In the present
study, okra caused less irritation than TTO, was more comfortable
and resulted in good compliance. In conclusion, okra may be a
potential remedy for the treatment of Demodex blepharitis
and MGD.
Due to the limitation of the small number of
participants in the present study, the efficacy of okra eyelid
patches for Demodex blepharitis requires further
verification in a larger cohort. Although the anti-demodectic
effects of the okra eyelid patch in Demodex blepharitis are
explicit, the mechanisms of the anti-demodectic effects of okra's
remain to be fully elucidated. Future studies are required to
clarify the anti-demodectic mechanism of okra.
In conclusion, the okra eyelid patch effectively
eradicated Demodex mites both in patients and in
vitro and its application was associated with reduced ocular
discomfort. The okra eyelid patch presented superior ocular
tolerance and may be more comfortable to use than the TTO eyelid
patch.
Acknowledgements
Not applicable.
Funding
Funding: The present study was sponsored by Shanghai Sailing
Program (grant. no. 19YF1405800).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG participated in the project design, sample size
calculation and revision of the manuscript. WL was responsible for
the enrolment and follow-up of patients and participated in
performing the statistical analysis. WL also drafted the
manuscript. Both authors confirm the authenticity of the raw data
and read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was strictly performed on the
basis of the Declaration of Helsinki for research involving human
participants and was approved by the Ethics Committee of the EENT
Hospital of Fudan University (Shanghai, China). After the
experimental details and potential benefits and risks were
explained, written informed consent was obtained from all
participants prior to the examination and treatment.
Patient consent for publication
Oral consent from the patient whose lid margin and
Demodex photographs are displayed in Fig. 3 was obtained prior to
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basta-Juzbasić A, Subić JS and Ljubojević
S: Demodex folliculorum in development of dermatitis
rosaceiformis steroidica and rosacea-related diseases. Clin
Dermatol. 20:135–140. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wesolowska M, Knysz B, Reich A,
Blazejewska D, Czarnecki M, Gladysz A, Pozowski A and Misiuk-Hojlo
M: Prevalence of Demodex spp. in eyelash follicles in
different populations. Arch Med Sci. 10:319–324. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang XB, Ding YH and He W: The
association between Demodex infestation and ocular surface
manifestations in meibomian gland dysfunction. Int J Ophthalmol.
11:589–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fromstein SR, Harthan JS, Patel J and
Opitz DL: Demodex blepharitis: Clinical perspectives. Clin
Optom (Auckl). 10:57–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luo X, Li J, Chen C, Tseng S and Liang L:
Ocular demodicosis as a potential cause of ocular surface
inflammation. Cornea. 36 (Suppl 1):S9–S14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bron AJ, de Paiva CS, Chauhan SK, Bonini
S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, et
al: TFOS DEWS II pathophysiology report. Ocul Surf. 15:438–510.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tomlinson A, Bron AJ, Korb DR, Amano S,
Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R and Dogru M: The
international workshop on meibomian gland dysfunction: Report of
the diagnosis subcommittee. Invest Ophthalmol Vis Sci.
52:2006–2049. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao YY, Di Pascuale MA, Li W,
Baradaran-Rafii A, Elizondo A, Kuo CL, Raju VK and Tseng SC: In
vitro and in vivo killing of ocular Demodex by tea tree oil.
Br J Ophthalmol. 89:1468–1473. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Doreddula SK, Bonam SR, Gaddam DP, Desu
BS, Ramarao N and Pandy V: Phytochemical analysis, antioxidant,
antistress, and nootropic activities of aqueous and methanolic seed
extracts of ladies finger (Abelmoschus esculentus L.) in
mice. ScientificWorldJournal. 2014(519848)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Adelakun OE, Oyelade OJ, Ade-Omowaye BI,
Adeyemi IA and Van de Venter M: Chemical composition and the
antioxidative properties of nigerian okra seed (Abelmoschus
esculentus Moench) flour. Food Chem Toxicol. 47:1123–1126.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liao H, Dong W, Shi X, Liu H and Yuan K:
Analysis and comparison of the active components and antioxidant
activities of extracts from Abelmoschus esculentus L.
Pharmacogn Mag. 8:156–161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cho CW, Han CJ, Rhee YK, Lee YC, Shin KS,
Shin JS, Lee KT and Hong HD: Cheonggukjang polysaccharides enhance
immune activities and prevent cyclophosphamide-induced
immunosuppression. Int J Biol Macromol. 72:519–525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Durazzo A, Lucarini M, Novellino E, Souto
EB, Daliu P and Santini A: Abelmoschus esculentus (L.):
Bioactive components' beneficial properties-focused on antidiabetic
role-for sustainable health applications. Molecules.
24(38)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mairuae N, Cheepsunthorn P, Cheepsunthorn
CL and Tongjaroenbuangam W: Okra (Abelmoschus esculentus
Linn) inhibits lipopolysaccharide-induced inflammatory mediators in
BV2 microglial cells. Trop J Pharm Res. 16(1285)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang X, Yin Y, Yue L and Gong L:
Selective serotonin reuptake inhibitors aggravate
depression-associated dry eye via activating the NF-κB pathway.
Invest Ophthalmol Vis Sci. 60:407–419. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang FM, Fan D, Yang XQ, Zhu FH, Shao MJ,
Li Q, Liu YT, Lin ZM, Cao SQ, Tang W, et al: The artemisinin analog
SM934 alleviates dry eye disease in rodent models by regulating
TLR4/NF-κB/NLRP3 signaling. Acta Pharmacol Sin: Aug 3, 2020 (Epub
ahead of print).
|
|
17
|
Gao YY, Di Pascuale MA, Li W, Liu DT,
Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK and Tseng SC:
High prevalence of Demodex in eyelashes with cylindrical
dandruff. Invest Ophthalmol Vis Sci. 46:3089–3094. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kheirkhah A, Blanco G, Casas V and Tseng
SC: Fluorescein dye improves microscopic evaluation and counting of
demodex in blepharitis with cylindrical dandruff. Cornea.
26:697–700. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Salem DA, El-Shazly A, Nabih N, El-Bayoumy
Y and Saleh S: Evaluation of the efficacy of oral ivermectin in
comparison with ivermectin-metronidazole combined therapy in the
treatment of ocular and skin lesions of Demodex
folliculorum. Int J Infect Dis. 17:e343–e347. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang X, Song N and Gong L: Therapeutic
effect of intense pulsed light on ocular demodicosis. Curr Eye Res.
44:250–256. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schiffman RM, Christianson MD, Jacobsen G,
Hirsch JD and Reis BL: Reliability and validity of the ocular
surface disease index. Arch Ophthalmol. 118:615–621.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yokoi N, Georgiev GA, Kato H, Komuro A,
Sonomura Y, Sotozono C, Tsubota K and Kinoshita S: Classification
of fluorescein breakup patterns: A novel method of differential
diagnosis for dry eye. Am J Ophthalmol. 180:72–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lemp MA: Report of the national eye
institute/industry workshop on clinical trials in dry eyes. CLAO J.
21:221–232. 1995.PubMed/NCBI
|
|
24
|
Bevins CL and Liu FT: Rosacea: Skin innate
immunity gone awry? Nat Med. 13:904–906. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao YY, Di Pascuale MA, Elizondo A and
Tseng SC: Clinical treatment of ocular demodecosis by lid scrub
with tea tree oil. Cornea. 26:136–143. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tighe S, Gao YY and Tseng SC:
Terpinen-4-ol is the most active ingredient of tea tree oil to kill
Demodex mites. Transl Vis Sci Technol. 2(2)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Koo H, Kim TH, Kim KW, Wee SW, Chun YS and
Kim JC: Ocular surface discomfort and Demodex: Effect of tea
tree oil eyelid scrub in Demodex blepharitis. J Korean Med
Sci. 27:1574–1579. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Messing J, Thöle C, Niehues M, Shevtsova
A, Glocker E, Borén T and Hensel A: Antiadhesive properties of
Abelmoschus esculentus (Okra) immature fruit extract against
Helicobacter pylori adhesion. PLoS One. 9(e84836)2014.
|
|
29
|
Lengsfeld C, Titgemeyer F, Faller G and
Hensel A: Glycosylated compounds from okra inhibit adhesion of
Helicobacter pylori to human gastric mucosa. J Agric Food
Chem. 52:1495–1503. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wahyuningsih SPA, Pramudya M, Putri IP,
Winarni D, Savira NII and Darmanto W: Crude polysaccharides from
okra pods (Abelmoschus esculentus) grown in indonesia
enhance the immune response due to bacterial infection. Adv
Pharmacol Sci. 2018(8505383)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Polito L, Bortolotti M, Maiello S,
Battelli MG and Bolognesi A: Plants producing ribosome-inactivating
proteins in traditional medicine. Molecules.
21(1560)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu JX, Sun YH and Li CP: Volatile oils of
Chinese crude medicines exhibit antiparasitic activity against
human Demodex with no adverse effects in vivo. Exp
Ther Med. 9:1304–1308. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lehnhardt Pires C, Rodrigues SD, Bristot
D, Gaeta HH, de Oliveira Toyama D, Lobo Farias WR and Toyama MH:
Evaluation of macroalgae sulfated polysaccharides on the leishmania
(L.) amazonensis promastigote. Mar Drugs. 11:934–943.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang MH, Zhu L and Jiang JG:
Immunoregulatory actions of polysaccharides from Chinese herbal
medicine. Expert Opin Ther Targets. 14:1367–1402. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shui G and Peng LL: An improved method for
the analysis of major antioxidants of hibiscus esculentus Linn. J
Chromatogr A. 1048:17–24. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen H, Jiao H, Cheng Y, Xu K, Jia X, Shi
Q, Guo S, Wang M, Du L and Wang F: In vitro and in vivo
immunomodulatory activity of okra (Abelmoschus esculentus
L.) polysaccharides. J Med Food. 19:253–265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hu L, Yu W, Li Y, Prasad N and Tang Z:
Antioxidant activity of extract and its major constituents from
okra seed on rat hepatocytes injured by carbon tetrachloride.
Biomed Res Int. 2014(341291)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Khomsug P, Thongjaroe W, Pakdeenaro N,
Suttajit M and Chantirati P: Antioxidative activities and phenolic
content of extracts from okra (Abelmoschus esculentus L.).
Res J Biol Sci. 5:310–313. 2010.
|
|
39
|
Chi W, Hua X, Chen X, Bian F, Yuan X,
Zhang L, Wang X, Chen D, Deng R, Li Z, et al: Mitochondrial DNA
oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes
by activation of caspase-8 and BRCC36 in dry eye. J Autoimmun.
80:65–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Saravanan S, Pandikumar P, Pazhanivel N,
Paulraj MG and Ignacimuthu S: Hepatoprotective role of
Abelmoschus esculentus (Linn.) moench., on carbon
tetrachloride-induced liver injury. Toxicol Mech Methods.
23:528–536. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Powell FC: Clinical practice. Rosacea. N
Engl J Med. 352:793–803. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li J, O'Reilly N, Sheha H, Katz R, Raju
VK, Kavanagh K and Tseng SC: Correlation between ocular
Demodex infestation and serum immunoreactivity to bacillus
proteins in patients with facial rosacea. Ophthalmology.
117:870–877.e1. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Baldwin HE: Diagnosis and treatment of
rosacea: State of the art. J Drugs Dermatol. 11:725–730.
2012.PubMed/NCBI
|
|
44
|
Huang CN, Wang CJ, Lee YJ and Peng CH:
Active subfractions of Abelmoschus esculentus substantially
prevent free fatty acid-induced β cell apoptosis via inhibiting
dipeptidyl peptidase-4. PLoS One. 12(e0180285)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mollick MMR, Bhowmick B, Mondal D, Maity
D, Rana D, Dash SK, Chattopadhyay S, Roy S, Sarkar J, Acharya K, et
al: Anticancer (in vitro) and antimicrobial effect of gold
nanoparticles synthesized using Abelmoschus esculentus (L.)
pulp extract via a green route. RSC Adv. 4:37838–37848. 2014.
|