Introduction
Osteoporosis is one of the most common diseases
among the elderly and post-menopausal women (1). It is characterized by low bone mineral
density and a high risk of fractures (2), which are frequently associated with
high mortality and significant morbidity rates, in addition to high
risks of pain and disability (3).
It was reported osteoporotic fracture is high and lies within the
range of 40-50% in women and 13-22% in men aged >50 years
(4). Therefore, investigating the
cause of this disease is of importance. As previous studies have
shown, osteoporosisis a disease caused by the imbalance between the
activities of osteoblasts (OBs) and osteoclasts (OCs) (5,6),
specifically the reduced number of OBs and increased number of OCs
(7). OCs are giant multinucleated
bone-resorbing cells that are differentiated from mononuclear
macrophages (8). Throughout an
individual's life, bones continuous undergo remodeling through bone
matrix formation and mineralization (anabolic process) by OBs
(9) and mineralized bone matrix
degradation (catabolic process) by OCs (10). During osteoclastogenesis, macrophage
colony-stimulating factor (M-CSF) and receptor activator of NF-κB
ligand (RANKL) are essential for OC differentiation, which also
support the function and survival of mature osteoclasts (11,12).
Disruptionsin the interaction between RANKL and RANK using the
human monoclonal antibody denosumab was found to prevent OC
formation, activity and survival (13-15).
However, novel therapeutic strategies for the intervention of
osteoclastogenesis have not been extensively studied. Potential
anti resorptive drugs include estrogens (with or without
progesterone), bisphosphonates (alendronate, risedronate,
ibandronate and zoledronic acid), the estrogen agonist/antagonis
traloxifene and salmon calcitonin (16). However, thus far teriparatide and
recombinant human parathyroid hormone are the only approved
anabolic agents (17), Denosumab, a
human monoclonal antibody for RANKL, has been found to inhibit
osteoclastogenesis and was approved for treating osteoporosis
(18).
4-hexylresorcinol (4HR) is a small organic compound
that has been previously used as an additive antiseptic and
antioxidant (19). 4HR was first
used as an anti-parasitic and antiseptic agent (20). Due to the development of superior
antiparasitic agents, the use of 4HR as an anti-parasitic agent has
now diminished (21). However, 4HR
remains to be used widely as an anti-microbial in cosmetics and
antiseptics (22). 4HR was shown to
accelerate orthodontic tooth movement and increase the expression
levels of bone turnover markers, including osteocalcin, osteotonpin
and RUNX2, in ovariectomized rats, indicating a role for 4HR in
bone remodeling (23). However, the
role of 4HR in osteoclastogenesis remain to be fully
elucidated.
Since it was reported that 4HR can inhibit the NF-κB
pathway in human nasopharyngeal carcinoma cells (21), it was therefore hypothesized that
4HR could inhibit osteoclastogenesis by suppressing the NF-κB
signaling pathway. In the present study, the role of 4HR in
osteoclastogenesis in both BMM cells and in a OVX mouse model was
examined. Results from the present study may provide a novel
therapeutic strategy for the treatment of osteoporosis.
Materials and methods
Chemicals and reagents
An anti-β-actin mouse monoclonal antibody (cat. no.
A5441; 1:6,000), 4HR (cat. no. 209465), tartrate resistant acid
phosphatase (TRAP) staining kit (cat. no. 387A) were purchased from
Sigma-Aldrich; Merck KGaA. Rabbit polyclonal anti-TRAP (cat. no.
11594-1-AP; 1:1,000), Mouse monoclonal anti-nuclear factor of
activated T-cells cytoplasmic 1 (NFATc1; cat. no. 66963-1-Ig;
1:1,000) and rabbit polyclonal anti-Cathepsin K (cat. no.
11239-1-AP; 1:1,000) antibodies were from ProteinTech Group, Inc.
Rabbit monoclonal anti-phosphorylated (p)-IκB kinase β (pIKKβ; cat.
no. 2697S, 1:1,000) and total anti-IKKβ rabbit monoclonal (cat. no.
8943S; cat. no. 1:1,000) antibodies were obtained from Cell
Signaling Technology, Inc. Recombinant soluble M-CSF and RANKL were
obtained from PeproTech, Inc. Cell Counting Kit-8 (CCK-8) kit was
purchased from Wuhan Boster Biological Technology, Ltd. Serum
cross-linked procollagen type 1amino-terminal propeptide (P1NP,
cat. no. SEA570Mu) and C-terminal telopeptide of type1 collagen
(CTX1, cat. no. CEA665Mu) ELISA kits were purchased from
Cloud-Clone Corp.
In vitro osteoclastogenesis
The tibia and femur were dissected from 6-8-week-old
male C57BL/6J mice (six mice were used; weight, 22.1±2.3 g), mice
were obtained from Experimental Animal Center of Tongji Medical
College, then the mice were kept in animal center at 22˚C, humidity
at 55%, 12 h light/dark cycle with food and water ad
libitum. After euthanasia, bone marrow-derived macrophages
(BMMs) were flushed from tibia and femur of the mice. The cells
were then centrifuged at 340 x g for 5 min at room temperature and
plated onto a 100-mm tissue culture dish containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 10 ng/ml M-CSF. Because BMMs
adhere slower than mesenchymal stem cells, the following day,
non-adherent BMMs were collected according to a previous study by
Zhao et al (24). The BMMs
were cultured in α-minimum essential medium (α-MEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and 10 ng/ml
M-CSF. For osteoclastogenesis, BMMs were cultured at 37˚C with 5%
CO2 in α-MEM containing 10% FBS in the presence of 10
ng/ml M-CSF and 100 ng/ml RANKL for 5 days (25). BMMs at passage two were used. BMMs
were treated with 0, 5, 10 or 20 µg/ml 4HR for 5 days at 37˚C with
5% CO2 in the presence of 10 ng/ml M-CSF and 100 ng/ml
RANKL. TRAP staining was performed to visualize large mature OCs.
The images were taken using a light microscope (Nikon Corporation)
at x100 magnification. The number and area of mature OCs in each
well were quantified using ImageJ 1.52v (National Institutes of
Health). Briefly, the number of OCs was counted and the area of OCs
was contoured and measured in each well, where the area was shown
as % osteoclastogenesis area/total area. For reverse
transcription-quantitative PCR (RT-qPCR) and western blotting BMMs
at passage two were treated with 20 µg/ml 4HR for 3 days or at
different time points (0, 15, 30, 60 min) at 37˚C with 5%
CO2 in the presence of 10 ng/ml M-CSF and 100 ng/ml
RANKL.
RT-qPCR
BMMs were cultured at 37˚C in α-MEM containing 10%
FBS in the presence of 10 ng/ml M-CSF and 100 ng/ml RANKL for 3
days at 37˚C and 5% CO2 atmosphere, RNA was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) (26). Total RNA was reverse transcribed
into cDNA for RT-qPCR using the ReverAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), briefly, the
template RNA, primers, RiboLock RNase inhibitor and 10 mM dNTP were
mixed, incubated for 60 min at 42˚C and terminated by heating at
70˚C for 5 min. qPCR was then performed using
SsoAdvaced™ Universal SYBR Green Supermix (Bio-Rd
Laboratories, Inc.) in CFX96 Real-time PCR Detection System
(Bio-Rad Laboratories, Inc.). The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of 95˚C for 5 sec, 60˚C for 30 sec and 72˚C for 30 sec. The
primers were designed as follows: NFATc1 forward,
5'-TCTTCCGAGTTCACATCCC-3' and reverse, 5'-GACAGCACCATCTTCTTCC-3';
TRAP forward, 5'-CAGCAGCCAAGGAGGACTAC-3' and reverse,
5'-ACATAGCCCACACCGTTCTC-3' and Cathepsin K forward,
5'-CCAGTGGGAGCTATGGAAGA-3' and reverse, 5'-TGGTTCATGGCCAGTTCATA-3'.
mRNA expression levels were normalized to those of mouse β-actin
(forward, 5'-TGTTACCAACTGGGACGACA-3' and reverse,
5'-GGGGTGTTGAAGGTCTCAAA-3'). The relative mRNA levels of target
genes were calculated using the 2-∆∆Cq method (27,28).
Western blot analysis
BMMs were cultured at 37˚C in α-MEM containing 10%
FBS in the presence of 10 ng/ml M-CSF and 100 ng/ml RANKL for 5
days. Protein extracts were prepared in RIPA buffer (Wuhan Boster
Biological Technology, Ltd.) at 4˚C supplemented with 1% protease
inhibitor and phosphatase inhibitors. Proteins were quantified
using bicinchoninic acid protein assay (Wuhan Boster Biological
Technology, Ltd.). In total, 10 µg total protein per lane were
separated using 10% SDS-PAGE and then transferred onto PVDF
membranes (EMD Millipore). Membranes were blocked with 5% BSA
(Wuhan Boster Biological Technology, Ltd.) in 0.1% TBS-Tween 20 for
60 min at room temperature and then incubated with the indicated
primary antibodies overnight. The membranes were incubated with the
appropriate horseradish peroxidase-conjugated secondary antibodies
(1:10,000; cat. no. 31450 for anti-mouse, cat. no. 31460 for
anti-rabbit; Thermo Fisher Scientific, Inc.) at room temperature
for 2 h. Finally, the membranes were visualized using Immun-Star
HRP Chemiluminescent Substrate Kit (cat. no. 1705040; Bio-Rad
Laboratories, Inc.) and the band densities were quantified using
the Image Lab 5.1 software (Bio-Rad Laboratories, Inc.) and
normalized to β-actin (29).
Luciferase assay
293T cells (a gift from Dr Jun Xiao, Department of
Orthopedics, Tongji Hospital, Huazhong University of Science and
Technology; Wuhan, China) was cultured under a humidified 5%
CO2 atmosphere at 37˚C in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS. In total, 1x104
293T cells were seeded onto each well in 96-well plate and were
transfected with 200 ng NF-κB-luciferase reporter plasmid (pGL3),
together with 50 ng phRG-TK Renilla-luciferase reporter
plasmid (Promega Corporation) as a reference control, using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, cells were incubated with or without
20 µg/ml 4HR for 30 min. The luciferase activity measurement was
performed according to the manufacturer's protocols
(Dual-luciferase® reporter assay kit; Promega
Corporation) (30), the luciferase
signals were normalized to that of Renilla luciferase
signals.
Animals and ovariectomy (OVX)
All animal experiments were approved by the Medical
Ethics Committee of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China). The 4-month-old female
(28.3±1.8 g) C57BL/6J mice used in the study were purchased from
the Experimental Animal Center of Tongji Medical College. A total
of 18 mice were used (n=6 per group). All mice were housed under
standard laboratory conditions at 22˚C, humidity at 55%, 12-h
light/dark cycle with ad libitum access to water and
food.
For OVX and drug treatment, 1% isoflurane was used
for anesthesia prior to surgery. The ovaries were bilaterally
removed from all mice, except for those in the sham-operated group,
where the ovaries were exposed but not removed, as previously
described (31). Sham and one group
of OVX mice received an equal volume of DMSO through an
intraperitoneal injection daily for 60 days after surgery. The
second OVX group received 1 mg/kg 4HR via an intraperitoneal
injection daily for 60 days after surgery, which was designated as
the OVX + 4HR group (32). The mice
were anaesthetized in 1% isoflurane and then the serum was
collected from 0.5 ml blood before euthanasia. To obtain the serum,
the blood samples were then centrifuged at 300 x g at room
temperature, then the supernatant was collected. All mice were
sacrificed by cervical dislocation at 60 days after surgery
following which the tibias were collected and scanned using
microCT.
Bone specimen collection and microCT
scanning
The tibias were dissected and fixed in 4%
neutrally-buffered formalin for 48 h. Following fixation, the
tibias were scanned using vivaCT 40 (Scanco Medical AG) at a
resolution of 15 µm and 70 kVP, withan X-ray energy of 112 µA
(33). The parameters of bone
volume per tissue volume (BV/TV), trabecular number (Tb.N),
trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were
analyzed for each sample using SCANCO Medical's software 1.0
(Scanco Medical AG).
Histology
Following microCT scanning, the tibias were
decalcified in 10% EDTA for 4 weeks at room temperature. Before
sectioning, the tissues were embedded in paraffin. Sections were
cut to a 5 µm thickness, hydrated using xylene followed by a
descending ethanol gradient and then stained with hematoxylin for 5
min at room temperature and eosin (H&E) for 5 min at room
temperature. TRAP staining was performed using a 387A kit (Merck
KGaA), Briefly, a mixed solution of citrate solution, Fast Gamet
GBC Base solution, naphthol AS-BI phosphonic acid solution and
sodium nitrite solution were preheated at 37˚C before tartrate
solution was added to the mixture, the slides were then submerged
into the mixed solution for 1 h at 37˚C as previously described
(34). All the pictures were taken
under a light microscope at x200 magnification.
Analysis of serum biomarkers
Serum P1NP, an indicator of bone formation (35) and CTXI levels, an indicator of bone
resorption (36), were measured
using ELISA kits (Cloud-Clone Corp.) as previously described
(37).
Statistical analysis
Data are presented as the mean ± SD. For in
vitro study, each experiment was repeated for ≥ three times,
for in vivo study, each group contained six mice. For the
cytotoxicity assays, RT-qPCR and western blotting analysis, data
are presented as a percentage of the control or relative
expression. For other experiments, real values are shown for each
group. All data were analyzed using the SPSS 22 software (IBM
Corp.). One-way ANOVAs followed by least significant difference
(for three groups) or Tukey's post-hoc (for four groups) test were
used to test the differences among the groups. Student's t-test was
used to compare differences between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
4HR reverses RANKL-induced
osteoclastogenesis
Osteoclastogenesis is a multistep process that is
mediated by OC proliferation, commitment, fusion and activation
triggered by RANKL (38) and the
formation of giant multi-nucleated cells. To test the role of 4HR
in osteoclastogenesis, BMMs were first isolated from the long bones
of mice before cell viability was measured using CCK-8 assay. No
significant difference could be observed when BMMs were exposed to
different concentrations of 4HR (0, 5, 10 and 20 µg/ml; Fig. 1A), doses used was according to a
study by Kim et al previously (21), indicating the lack of cytotoxicity
by 4HR. To mimic osteoclastogenesis in vitro, BMMs were then
treated with RANKL together with M-CSF to induce
osteoclastogenesis, with or without the pretreatment with 4HR. 4HR
significantly reduced the number of multinucleated TRAP-positive
cells and TRAP-positive area in a dose-dependent manner (0, 5, 10
and 20 µg/ml; Fig. 1B-D). The mRNA
level of osteoclastogenesis-related genes, including TRAP,
Cathepsin K, NFATc1 and c-fos, were significantly downregulated by
preincubation with 4HR in a dose-dependent manner, suggesting an
inhibitory effect of 4HR against osteogenesis (Fig. 1E-H). To further verify the effects
of 4HR in osteoclastogenesis, the protein expression levels of
those genes were also determined, where 20 µg/ml 4HR exposure
significantly reduced the protein expression of TRAP, NFATc1 and
Cathepsin Kin BMMs in the presence of RANKL (Fig. 2A and B).
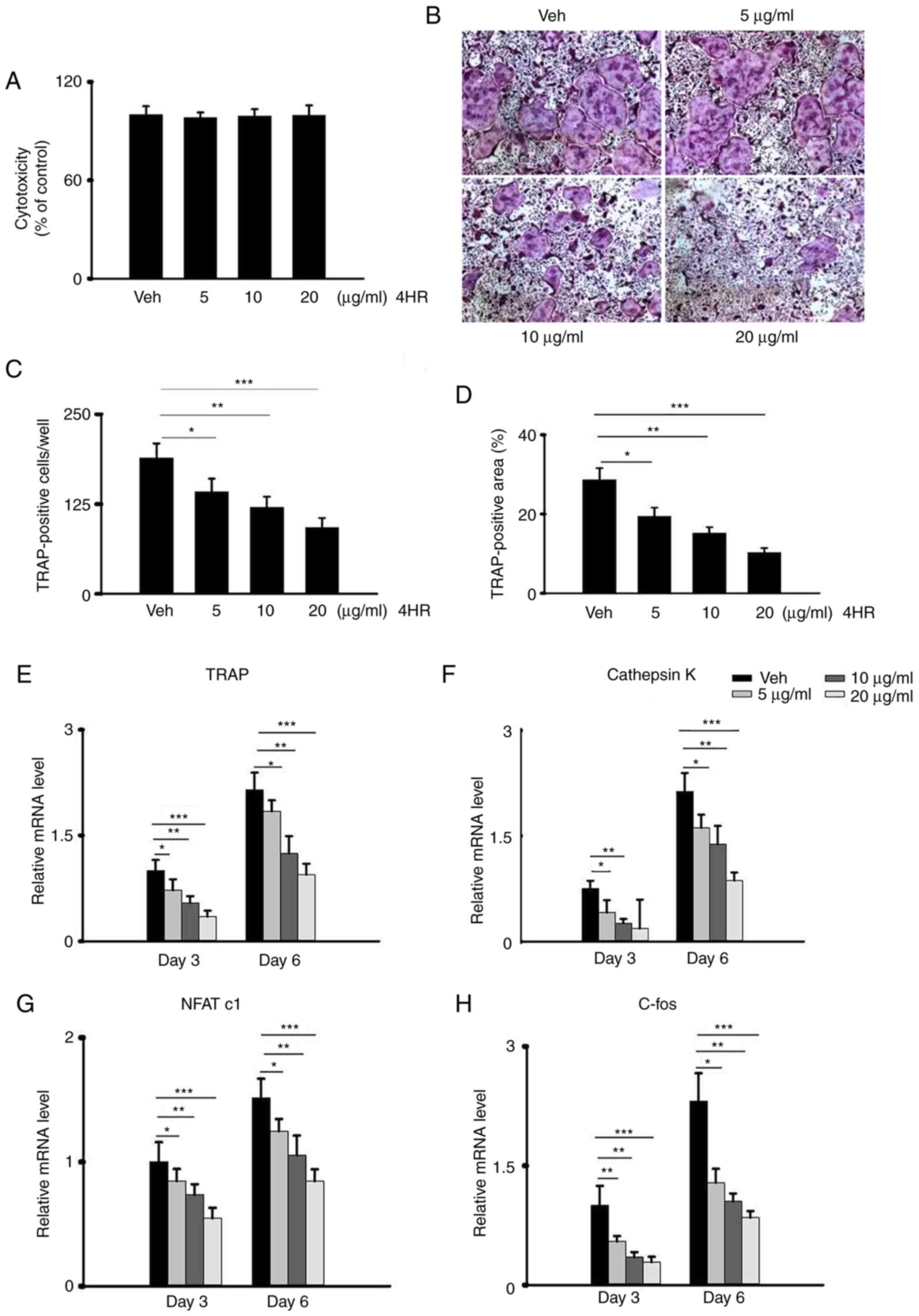 | Figure 14HR inhibits RANKL-induced
osteoclastogenesis in vitro. (A-D) BMMs were seeded into
96-well plates and cultured at 37˚C with complete medium
supplemented with 10 ng/ml M-CSF and 100 ng/ml RANKL. (A) BMMs were
treated with different concentrations of 4HR (0, 5, 10 and 20
µg/ml) for 3 days in the presence of M-CSF. Cell viability was
assessed using a Cell Counting Kit-8. No significant difference was
observed among the groups. (B) Representative TRAP staining images
on day 5 of OC differentiation following incubation with different
concentrations of 4HR (0, 5, 10 and 20 µg/ml). Magnification, x100.
(C) The number of TRAP-positive cells with ≥ three nuclei were
quantified in each well in (B). *P<0.05,
**P<0.01 and ***P<0.001. (D) The
percentage of the TRAP-positive cell area in each well was measured
in (B). *P<0.05, **P<0.01 and
***P<0.001. (E-H) BMMs were treated with different
concentrations of 4HR (0, 5, 10 and 20 µg/ml) in the presence of 10
ng/ml M-CSF and 100 ng/ml RANKL. mRNA expression levels of
osteoclastogenesis markers (E) TRAP, (F) Cathepsin K, (G) NFATc1
and (H) c-Fos on day 3 of OC differentiation are shown for each
group. *P<0.05, **P<0.01 and
***P<0.001. 4HR, 4-hexylresorcinol; RANKL, receptor
activator of nuclear factor kappa B ligand; M-CSF, macrophage
colony-stimulating factor; BMMs, bone marrow-derived macrophages;
TRAP, tartrate resistant acid phosphatase; Veh, Vehicle; NFATc1,
nuclear factor of activated T-cells c1. |
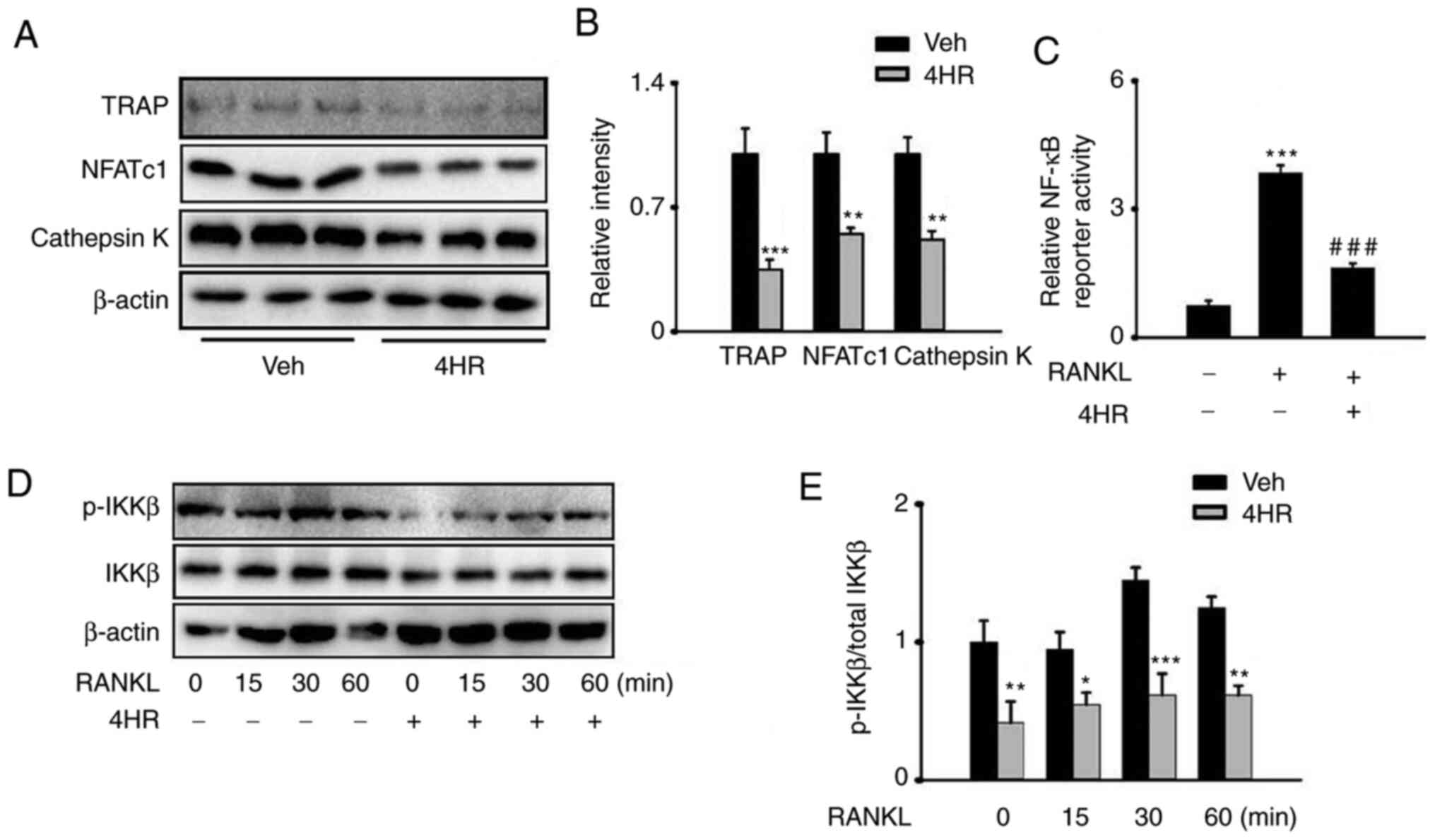 | Figure 24HR suppresses RANKL-induced
activation of the NF-κB pathway. (A-E) BMMs cultured at 37˚C with
complete medium supplemented with 10 ng/ml M-CSF and 100 ng/ml
RANKL. (A) Representative images of western blot analysis showing
that 4HR inhibited the RANKL-induced protein expression of TRAP,
NFATc1 and Cathepsin K in BMMs after 20 µg/ml 4HR treatment for 3
days in the presence of 10 ng/ml M-CSF and 100 ng/ml RANKL. (B)
Quantification of (A). *P<0.05,
**P<0.01, ***P<0.001 vs. Veh. (C)
Luciferase assay of the NF-κB luciferase reporter plasmid in BMMs
pretreated with or without 4HR (20 µg/ml) for 30 min in the
presence or absence of 10 ng/ml M-CSF and 100 ng/ml RANKL.
***P<0.001 vs. Veh without 4HR treatment and RANKL
stimulation, ###P<0.001 vs. BMMs without 4HR
treatment but with RANKL stimulation. (D) Western blot analysis of
the lysate following 100 ng/ml RANKL incubation for the indicated
time points (0, 15, 30 and 60 min) in BMMs with or without 4HR
preincubation for 30 min (20 µg/ml) in the presence of 10 ng/ml
M-CSF. (E) Quantification of (D). *P<0.05,
**P<0.01 and ***P<0.001 vs. Veh without
4HR exposure at the corresponding time points. 4HR,
4-hexylresorcinol; RANKL, receptor activator of NF-κB ligand; TRAP,
tartrate resistant acid phosphatase; NFATc1, nuclear factor of
activated T-cells cytoplasmic 1; BMMs, bone marrow-derived
macrophages; M-CSF, macrophage colony-stimulating factor; Veh,
Vehicle; p-IKKβ, phosphorylated-IκB kinase β. |
4HR suppresses the RANKL-induced
activation of the NF-κB pathway
NF-κB serves an important role in OC-induced
osteoporosis and osteoclastogenesis (39,40).
To explore the molecular mechanism underlying 4HR in regulating the
activity of the NF-κB pathway, the effect of 4HR treatment on the
NF-κB luciferase reporter was first examined in the 293T cell line.
Following transfection, the cells were treated with RANKL, with or
without 4HR preincubation, which resulted in a significant
inhibition of NF-κB transactivation following preincubation with 20
µg/ml 4HR (Fig. 2C).
Subsequently, BMMs were further treated with M-CSF
and RANKL in the presence of 20 µg/ml 4HR for 0, 15, 30 and 60 min,
which lead to the phosphorylation of IKKβ being significantly
reduced following pretreatment with 20 µg/ml 4HR (Fig. 2D and E). These results suggest that 4HR
treatment suppressed osteoclastogenesis by inhibiting the NF-κB
pathway.
4HR treatment reverses
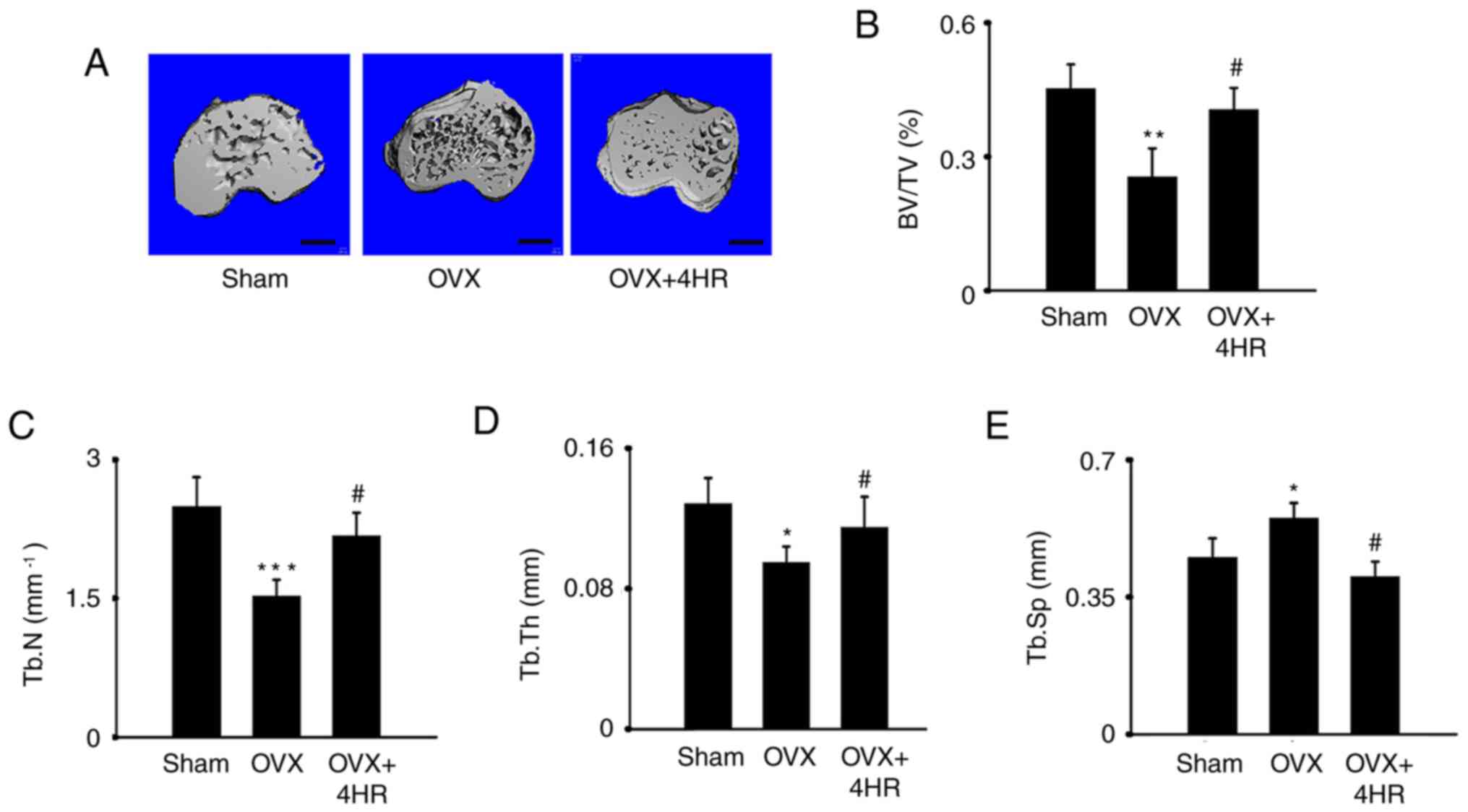
ovariectomy-induced bone loss in vivo
To test the role of 4HRin osteoporosis in
vivo, an OVX model was constructed in female 4-month-old mice.
MicroCT data showed that OVX significantly decreased bone mass in
the proximal tibia, as evidenced by the significantly reduced
BV/TV, Tb.N and Tb.Th values but significantly increased Tb.Sp
values, in the OVX group compared with those in the sham group
(Fig. 2). However, in mice that
received 1 mg/kg 4HR administration, a marked reversal of
OVX-induced bone loss was indicated by the significantly increased
BV/TV, Tb.N and Tb.Th values but significantly decreased Tb.Sp
values as compared with those in the OVX group. These results
suggested a protective role for 4HR in osteoporosis in vivo
(Fig. 3).
Bone mass was next examined using H&E staining
in paraffin-embedded bone slides. It was found that OVX reduced
trabecular bone mass in the proximal tibia, since less trabecular
bone was seen in the image. 4HR application, however, markedly
rescued the bone loss, compared with that in OVX mice (Fig. 4A). To measure the OC number in
vivo, TRAP staining was performed. OVX significantly increased
the TRAP-positive cell number in the proximal tibia area, but 4HR
treatment significantly reduced the TRAP-positive cell number
compared with that in the OVX group (Fig. 4B and C), consistent with the in vitro and
microCT data. Next, the levels of P1NP and CTX1, which are
important bone formation and bone resorption markers, respectively
(41,42), were measured in the serum. OVX mice
exhibited significantly higher CTX1 levels compared with those in
the Sham mice, but there was no difference in the PTNP levels.
However, 4HR treated mice exhibited a significantly lower level of
CTX1 compared with that in the OVX mice, though no difference was
observed in the P1NP levels (Fig.
4D and E). In conclusion, these
data suggest that 4HR treatment ameliorates OVX-induced
osteoporosis by inhibiting bone resorption without significantly
affecting bone formation.
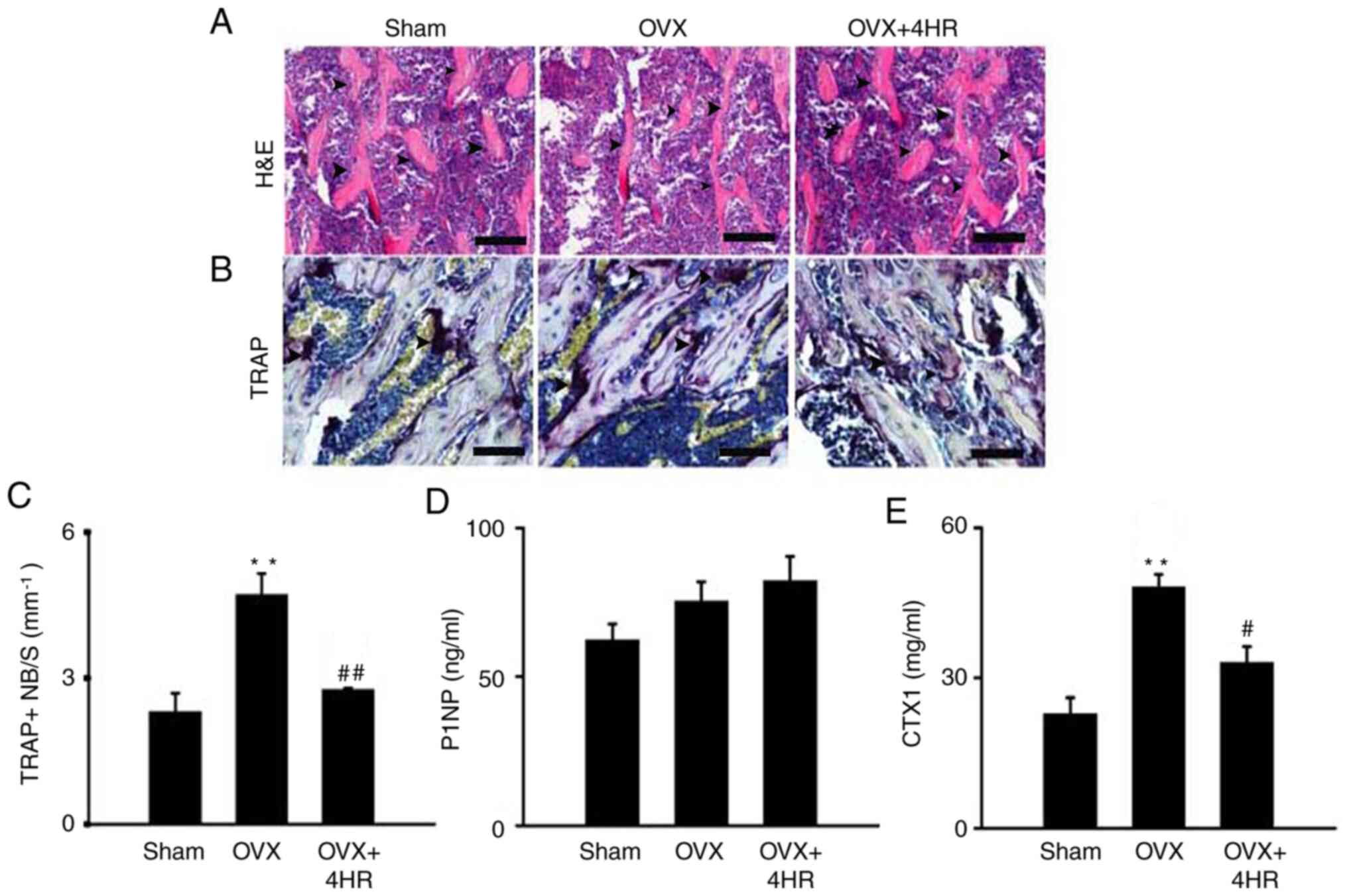 | Figure 44HR administration increases bone
mass and reduces the OC number in vivo. (A) Representative
images of H&E staining of the proximal tibia following OVX with
or without 4HR administration in mice, arrows showed the trabecular
bones. Scale bar, 25 µm. (B) Representative images of TRAP staining
in the proximal tibia in each group 60 days after OVX, arrows
showed the purple multinucleated osteoclasts. Scale bar, 25 µm. (C)
Quantification of TRAP positive cells in the proximal tibia 60 days
after OVX. Data were shown as the OC number per bone surface/mm.
Serum levels of (D) P1NP and (E) CTX1 were measured.
**P<0.01 vs. Sham, #P<0.05 and
##P<0.01 vs. OVX. n=6 per group. 4HR,
4-hexylresorcinol; H&E, hematoxylin and eosin; OVX,
ovariectomy; TRAP, tartrate resistant acid phosphatase; P1NP,
procollagen type 1 amino-terminal propeptide; CTX1, C-terminal
telopeptide of type1; NB/S, number per bone surface. |
Discussion
Osteoclastogenesis is regulated by complex signaling
cascades that are mainly triggered by RANKL (43). In the present study, 4HR application
decreased RANKL-induced osteoclastogenesis in a dose-dependent
manner in BMMs. In addition, 4HR significantly reduced
TRAP-positive cell number and area. TRAP, cathepsin K, NFATc1 and
c-fos are important osteoclastogenesis markers, since they enhance
the differentiation and function of OCs (44). In the present study, BMMs treated
with 4HR exhibited significantly lower TRAP, Cathepsin K, NFATc1
and C-fos expression levels. These findings were confirmed by both
RT-qPCR and western blot analysis.
OC differentiation is regulated by multiple
signaling pathways, one of the most important of which is the NF-κB
pathway in osteoclastogenesis (45). Activated NF-κB signaling pathway was
found to be strongly associated with an increased OC activity, as
evidenced by the increased OC number and activity in an ovariectomy
model in mice (46). 4HR has been
shown to downregulate the NF-κB pathway in human nasopharyngeal
carcinoma cells (21). The present
study showed that 4HR inhibits OC activity in osteoclastogenesis by
suppressing NF-κB, which may in turn exert its anti-osteoporosis
function in vivo. It has also been reported previously that
NF-κB contributes to OVX-induced bone loss (46). In the present study, using the NF-κB
luciferase reporting plasmid, it was demonstrated that 4HR
treatment induced NF-κB signaling suppression in the presence of
RANKL in BMMs. Furthermore, phosphorylated IKKβ was reduced
following 4HR treatment. This suggests that 4HR inhibited
osteoclastogenesis by suppressing the NF-κB pathway.
OVX is a procedure that results in decreased
estrogen that has been valuable for osteoporosis research (39). The main characteristic of this model
is the increased OC number and activity (47). In the present study, administration
of 4HR in mice resulted in a significantly reduced degree of bone
loss induced by OVX as evidenced by microCT and histology data. The
CT results also revealed elevated BV/TV, Tb.N and Tb.Th levels but
lower Tb.Sp levels in the proximal tibia in 4HR treated mice.
H&E staining also showed decreased trabecular bone in the
proximal tibia. To confirm whether the protective effect of 4HR was
a result of osteoclastogenesis inhibition, TRAP staining was
performed to quantify the TRAP-positive cell number in each group.
The results showed that the number of TRAP-positive cells was
increased in OVX mice compared with that in Sham mice. By contrast,
4HR administration markedly decreased the number of TRAP-positive
cells compared with that in OVX mice.
Since estrogen deficiency is one of the main causes
of menopausal osteoporosis (48),
it would be interesting to explore whether the mechanism of 4HR
action is also associated with estrogen levels in vivo,
including increased estrogen receptor activity. However, since
there is currently no evidence of a direct connection, future
studies should investigate this potential relationship (47). The present study on
osteoclastogenesis were consistent with those reported by Choi
et al (23), which showed
that 4HR improved OB function in ovariectomized rats, confirming
the potential use of 4HR for the treatment of osteoporosis. A
limitation of the present study was that it remains unclear if OBs
also participated in the role of 4HR in this OVX model. It is
well-established that the maintenance of normal bone mass is
dependent on the balance between OB and OC activity. No difference
in the level of bone formation marker P1NP was identified among the
three mouse groups, suggesting that 4HR may not have had an effect
on OB activity in the present model. Therefore, future studies
should extend this investigation to OBs.
In conclusion, the present study found that 4HR
exerted a protective effect against osteoporosis by reducing
osteoclastogenesis both in vitro and in vivo. This
may provide a novel insight into the clinical study of osteoporosis
and encourage the further exploration of reagents for the treatment
of osteoporosis.
Acknowledgements
The authors would like to thank Dr Jun Xiao
(Department of Orthopedics, Tongji Hospital, Huazhong University of
Science and Technology; Wuhan, China) for the kind gift of 293T
cells used in the present study.
Funding
Funding: The present study was supported by the Medical Research
Foundation of Wuhan City (grant no. WZ19A05) and the Hygiene and
Health Joint Foundation of Hubei Province (grant no.
WJ2019H412).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ML designed the experiments. WY performed western
blotting, reverse transcription-quantitative PCR, collected
samples, microCT scanning, HE and TRAP staining. TL contributed to
the NF-κB reporter assay. XG and YX performed the OVX surgery. WY
and ML authenticate the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Medical
Ethics Committee of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanis JA: Assessment of fracture risk and
its application to screening for postmenopausal osteoporosis:
Synopsis of a WHO report. WHO study group. Osteoporos Int.
4:368–381. 1994.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the international osteoporosis foundation (IOF)
and the European federation of pharmaceutical industry associations
(EFPIA). Arch Osteoporos. 8(136)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tom SE, Adachi JD, Anderson FA Jr, Boonen
S, Chapurlat RD, Compston JE, Cooper C, Gehlbach SH, Greenspan SL,
Hooven FH, et al: Frailty and fracture, disability, and falls: A
multiple country study from the global longitudinal study of
osteoporosis in women. J Am Geriatr Soc. 61:327–334.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Johnell O and Kanis J: Epidemiology of
osteoporotic fractures. Osteoporos Int. 16 (Suppl 2):S3–S7.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eriksen EF: Cellular mechanisms of bone
remodeling. Rev Endocr Metab Disord. 11:219–227. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kikuta J and Ishii M: Bone imaging:
Osteoclast and osteoblast dynamics. Methods Mol Biol. 1763:1–9.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roodman GD: Cell biology of the
osteoclast. Exp Hematol. 27:1229–1241. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Datta HK, Ng WF, Walker JA, Tuck SP and
Varanasi SS: The cell biology of bone metabolism. J Clin Pathol.
61:577–587. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Trouvin AP and Goëb V: Receptor activator
of nuclear factor-κB ligand and osteoprotegerin: Maintaining the
balance to prevent bone loss. Clin Interv Aging. 5:345–354.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lewiecki EM: Denosumab: A promising drug
for the prevention and treatment of osteoporosis. Womens Health
(Lond). 2:517–525. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eastell R, Christiansen C, Grauer A,
Kutilek S, Libanati C, McClung MR, Reid IR, Resch H, Siris E,
Uebelhart D, et al: Effects of denosumab on bone turnover markers
in postmenopausal osteoporosis. J Bone Miner Res. 26:530–537.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Kendler DL, Roux C, Benhamou CL, Brown JP,
Lillestol M, Siddhanti S, Man HS, San Martin J and Bone HG: Effects
of denosumab on bone mineral density and bone turnover in
postmenopausal women transitioning from alendronate therapy. J Bone
Miner Res. 25:72–81. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gupta G and Aronow WS: Treatment of
postmenopausal osteoporosis. Compr Ther. 33:114–119.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lewiecki EM: Denosumab in postmenopausal
osteoporosis: What the clinician needs to know. Ther Adv
Musculoskelet Dis. 1:13–26. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Silva I and Branco JC: Denosumab: Recent
update in postmenopausal osteoporosis. Acta Reumatol Port.
37:302–313. 2012.PubMed/NCBI
|
|
19
|
Kim MK, Yoon CS, Kim SG, Park YW, Lee SS
and Lee SK: Effects of 4-hexylresorcinol on protein expressions in
RAW 264.7 cells as determined by immunoprecipitation high
performance liquid chromatography. Sci Rep. 9(3379)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rabbani GH, Gilman RH, Kabir I and Mondel
G: The treatment of fasciolopsis buski infection in children: A
comparison of thiabendazole, mebendazole, levamisole, pyrantel
pamoate, hexylresorcinol and tetrachloroethylene. Trans R Soc Trop
Med Hyg. 79:513–515. 1985.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim SG, Lee SW, Park YW, Jeong JH and Choi
JY: 4-hexylresorcinol inhibits NF-κB phosphorylation and has a
synergistic effect with cisplatin in KB cells. Oncol Rep.
26:1527–1532. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
He J, Zhu Q, Dong X, Pan H, Chen J and
Zheng ZP: Oxyresveratrol and ascorbic acid O/W microemulsion:
Preparation, characterization, anti-isomerization and potential
application as antibrowning agent on fresh-cut lotus root slices.
Food Chem. 214:269–276. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi KH, Kim DW, Lee SK, Kim SG and Kim
TW: The administration of 4-hexylresorcinol accelerates orthodontic
tooth movement and increases the expression level of bone turnover
markers in ovariectomized rats. Int J Mol Sci.
21(1526)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao L, Guan H, Song C, Wang Y, Liu C, Cai
C, Zhu H, Liu H, Zhao L and Xiao J: YAP1 is essential for
osteoclastogenesis through a TEADs-dependent mechanism. Bone.
110:177–186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bae S, Lee MJ, Mun SH, Giannopoulou EG,
Yong-Gonzalez V, Cross JR, Murata K, Giguère V, van der Meulen M
and Park-Min KH: MYC-dependent oxidative metabolism regulates
osteoclastogenesis via nuclear receptor ERRα. J Clin Invest.
127:2555–2568. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun X, Gao X, Deng Z, Zhang L, McGilvray
K, Gadomski BC, Amra S, Bao G and Huard J: High bone
microarchitecture, strength, and resistance to bone loss in MRL/MpJ
mice correlates with activation of different signaling pathways and
systemic factors. FASEB J. 34:789–806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Farahzadi R, Fathi E and Vietor I:
Mesenchymal stem cells could be considered as a candidate for
further studies in cell-based therapy of alzheimer's disease via
targeting the signaling pathways. ACS Chem Neurosci. 11:1424–1435.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W,
Ho KK, Qin L, Song H and Mak KK: Reciprocal inhibition of YAP/TAZ
and NF-κB regulates osteoarthritic cartilage degradation. Nat
Commun. 9(4564)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Idris AI: Ovariectomy/orchidectomy in
rodents. Methods Mol Biol. 816:545–551. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huo J and Sun X: Effect of astragalus
polysaccharides on ovariectomy-induced osteoporosis in mice. Genet
Mol Res. 15:2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gao X, Usas A, Tang Y, Lu A, Tan J,
Schneppendahl J, Kozemchak AM, Wang B, Cummins JH, Tuan RS and
Huard J: A comparison of bone regeneration with human mesenchymal
stem cells and muscle-derived stem cells and the critical role of
BMP. Biomaterials. 35:6859–6870. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ,
Kim JY and Kim HH: MicroRNA-124 regulates osteoclast
differentiation. Bone. 56:383–389. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Takada J, Dinavahi R, Miyauchi A, Hamaya
E, Hirama T, Libanati C, Nakamura Y, Milmont CE and Grauer A:
Relationship between P1NP, a biochemical marker of bone turnover,
and bone mineral density in patients transitioned from alendronate
to romosozumab or teriparatide: A post hoc analysis of the
STRUCTURE trial. J Bone Miner Metab. 38:310–315. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu S, Zhang Y, Wang J, Li K, Tan K, Liang
K, Shen J, Cai D, Jin D, Li M, et al: TSC1 regulates osteoclast
podosome organization and bone resorption through mTORC1 and
Rac1/Cdc42. Cell Death Differ. 25:1549–1566. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang Y, Xu S, Li K, Tan K, Liang K, Wang
J, Shen J, Zou W, Hu L, Cai D, et al: mTORC1 inhibits NF-κB/NFATc1
signaling and prevents osteoclast precursor differentiation, in
vitro and in mice. J Bone Miner Res. 32:1829–1840. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lorenzo J, Horowitz M and Choi Y:
Osteoimmunology: Interactions of the bone and immune system. Endocr
Rev. 29:403–440. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lin TH, Pajarinen J, Lu L, Nabeshima A,
Cordova LA, Yao Z and Goodman SB: NF-κB as a therapeutic target in
inflammatory-associated bone diseases. Adv Protein Chem Struct
Biol. 107:117–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Abu-Amer Y: NF-κB signaling and bone
resorption. Osteoporos Int. 24:2377–2386. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu TT, Liu DM, Xuan Y, Zhao L, Sun LH,
Zhao DD, Wang XF, He Y, Guo XZ, Du R, et al: The association
between the baseline bone resorption marker CTX and incident
dysglycemia after 4 years. Bone Res. 5(17020)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Thurairaja R, Iles RK, Jefferson K,
McFarlane JP and Persad RA: Serum amino-terminal propeptide of type
1 procollagen (P1NP) in prostate cancer: A potential predictor of
bone metastases and prognosticator for disease progression and
survival. Urol Int. 76:67–71. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Boyce BF, Xiu Y, Li J, Xing L and Yao Z:
NF-κB-mediated regulation of osteoclastogenesis. Endocrinol Metab
(Seoul). 30:35–44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Silva I and Branco JC: Rank/Rankl/opg:
Literature review. Acta Reumatol Port. 36:209–218. 2011.PubMed/NCBI
|
|
46
|
Onal M, Xiong J, Chen X, Thostenson JD,
Almeida M, Manolagas SC and O'Brien CA: Receptor activator of
nuclear factor κB ligand (RANKL) protein expression by B
lymphocytes contributes to ovariectomy-induced bone loss. J Biol
Chem. 287:29851–29860. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Salamanna F, Borsari V, Contartese D,
Nicoli Aldini N and Fini M: Link between estrogen deficiency
osteoporosis and susceptibility to bone metastases: A way towards
precision medicine in cancer patients. Breast. 41:42–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kanis JA, Cooper C, Rizzoli R and
Reginster JY: Scientific Advisory Board of the European Society for
Clinical and Economic Aspects of Osteoporosis (ESCEO) and the
Committees of Scientific Advisors and National Societies of the
International Osteoporosis Foundation (IOF). European guidance for
the diagnosis and management of osteoporosis in postmenopausal
women. Osteoporos Int. 30:3–44. 2019.PubMed/NCBI View Article : Google Scholar
|