Introduction
Good's syndrome (GS), also known as
thymoma-associated immune deficiency syndrome, is a rare primary
immune deficiency disease that occurs in individuals aged 40-60
years. Common clinical features are thymoma, hypogammaglobulinemia,
low peripheral B-lymphocyte count and inverted
CD4+/CD8+ T-cell ratio. Thymoma is the most
common tumor type in the anterior mediastinum and most thymomas may
cause immune dysfunction. Furthermore, 5-10% of patients with
thymoma have increased susceptibility to bacterial and
opportunistic viral and fungal infections (1-3).
In 1954, this phenomenon was first described by Dr Robert Good
(4). Later, immunological studies
demonstrated that most patients also had hypogammaglobulinemia,
B-cell reduction or deficiency and inversion of the CD4/CD8 ratio,
which now part of the definition of Good's syndrome. A prominent
feature of patients with GS is loss of B cells. Furthermore, the
prognosis is poor and mortality is high. Infections are the most
common cause of death (5,6). However, Thongngarm et al
(7) examined the clinical course
and outcomes for nine patients with GS after treatment with
intravenous immunoglobulin at Thailand's largest national tertiary
referral hospital. They reported that seven patients had favorable
clinical outcomes.
Case report
Written informed consent was obtained from the
patient for the publication of this case report and accompanying
images. The patient was an unemployed 42-year-old Han Chinese male.
In summary, the patient underwent thymectomy and after 6 months, a
relapsed respiratory tract infection and was diagnosed with
Alcaligenes sp. infection as detected by three blood
cultures. After analysis of the clinical and immunological
characteristics, the patient was finally diagnosed with GS. The
patient's condition was stable after receiving anti-infection
treatment and regular supplementation with gamma globulin. This was
the second reported case of GS combined with Alcaligenes sp.
to date. A detailed case description is provided below.
The patient had a fever for 3 days. The body
temperature fluctuated between 36.5 and 38.5˚C, accompanied by
throat discomfort, cough, phlegm and muscle soreness. The patient
was admitted to the Infectious Disease Unit of the First Hospital
of Quanzhou Affiliated to Fujian Medical University (Quanzhou,
China) in November 2017.
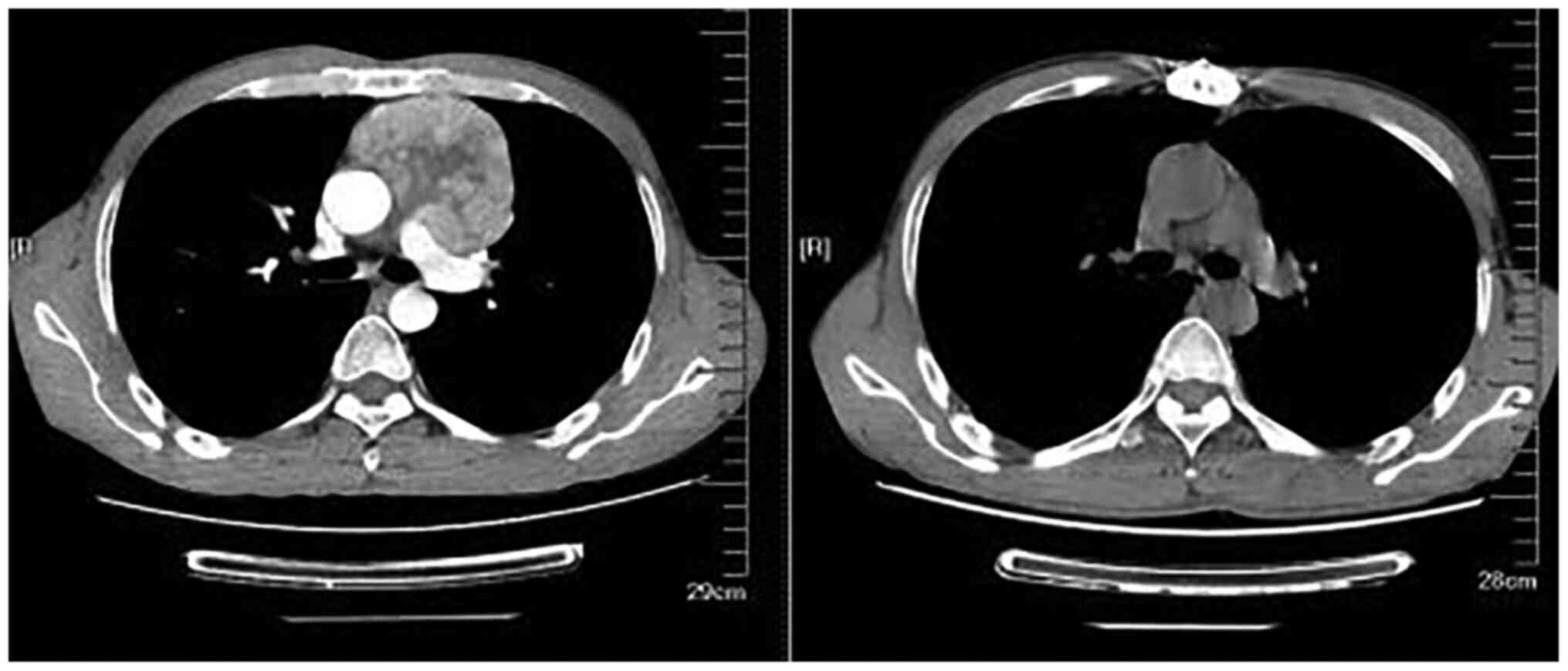
In May 2017, the patient underwent enhanced CT of
the lungs due to cough for >1 month (Fig. 1), which revealed occupying lesions
in the left anterior mediastinum, slightly enlarged lymph nodes and
two hilus pulmonis. Therefore, thymectomy was performed the First
Hospital of Quanzhou Affiliated to Fujian Medical University
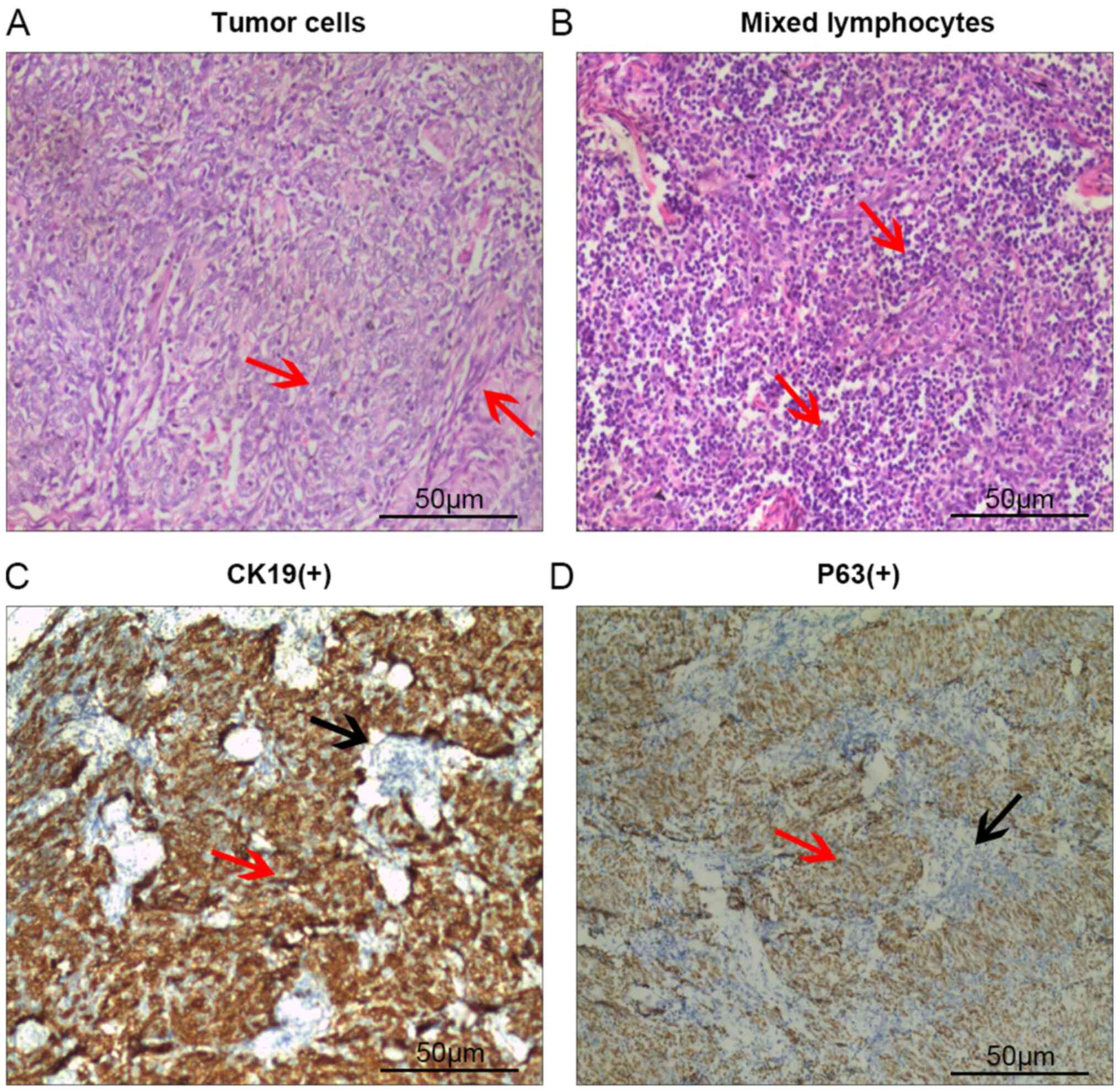
(Quanzhou, China). The post-operative pathological diagnosis was
type AB thymoma (Fig. 2). The
patient had a severe infection after the surgery and recovered
after anti-infection treatment and supplementation with gamma
globulin. In June 2017, humoral immune function suggested that
immunoglobulin (Ig)G, IgM and IgA levels were markedly reduced
(Table I), which was left
untreated. The patient was previously healthy and none of his
family members had any similar symptoms. The patient had no history
of smoking, drinking or infectious diseases.
 | Table IResults of multiple immune tests and
intervention measures at different time-points. |
Table I
Results of multiple immune tests and
intervention measures at different time-points.
| Time-point | IgG, g/l (RR,
7.51-15.6) | IgM, g/l (RR,
0.46-3.04) | IgA, g/l (RR,
0.82-4.53) | Intervention
measures |
|---|
| June 2017 | 1.66 | <0.0417 | 0.0938 | 20 d after thymoma
resection, intravenous supplementation with gamma globulin at 5.0 g
was performed once |
| November 2017 | 2.08 | <0.0417 | 0.103 | Gamma globulin was
not infused |
| January 2018 | 10.2 | 0.0470 | 0.140 | Intravenous
supplementation of gamma globulin (5 g/d for 7 d) and subcutaneous
injection of thymalfasin (1.6 µg q3d) |
| February 2018 | 6.55 | 0.14 | 0.141 | Intravenous
supplementation of gamma globulin (5 g/d for 2 d) and subcutaneous
injection of thymalfasin (1.6 µg q3d) |
Physical examination on admission revealed the
following: The body temperature was 37.8˚C. The patient was in a
good mental state and had a low BMI of 17.5. He had pharyngeal
congestion but no rales in the lungs. The auxiliary examination
after admission revealed the following: Among the routine blood
parameters (8), the leukocyte count
increased (13.10x109/l, normal range:
3.5-9.5x109/l), the neutrophil percentage increased
(76.40%, normal range 40-75%), the lymphocyte count was normal
(2.06x109/l, normal range: 1.1-3.2x109/l),
the erythrocyte count was normal (4.99 g/l x1012/l,
normal range: 4.3-5.8x1012/l), hemoglobin was normal
(142 g/l, normal range: 130-175 g/l) and the platelet count was
normal (305x109/l, normal range:
125-350x109/l). The C-reactive protein level increased
(148.45 mg/l, normal range <8 mg/l). In regards to the humoral
immune function, IgG, IgM and IgA levels were markedly decreased
(Table I). The pulmonary CT scan
revealed changes to the postoperative thymoma and inflammation in
the left lower lung after thymectomy. The bilateral double-tube
blood culture indicated the presence of Alcaligenes sp.
[blood culture and identification method: Specimens were cultured
in an FX400 blood culture instrument (BD Biosciences), positive
specimens were transferred to the blood plate and strains were
identified using a Phoenix 100 automatic bacterial identification
system and its supporting bacteria identification card (BD
Biosciences)]. The strains were susceptible to piperacillin and
resistant to cefotaxime and levofloxacin. Anti-infection treatment
using ticarcillin-clavulanate potassium (intravenous infusion at
3.2 g q6h) was performed for 1 week. After 1 day of infusion the
body temperature decreased to normal. After 1 week, the cough and
phlegm improved and blood culture was negative upon reanalysis.
Hene, the patient was discharged. The patient received sequential
oral amoxicillin (1.0 bid for 7 days) and he discontinued the
medication voluntarily after 3 days.
At two weeks after discharge, the patient was
readmitted to the hospital due to fever (maximum temperature,
38.2˚C), cough and phlegm. After admission, the bilateral
double-tube blood culture revealed Alcaligenes sp. (the
methods of cultivation and identification were the same as those
described above). A pulmonary CT scan indicated an improvement in
the inflammation of the left lower lobe. Furthermore,
anti-infection treatment with the same medicines
(ticarcillin-clavulanate potassium intravenous infusion at 3.2 g
q6h) was performed and the course of the treatment was prolonged to
2 weeks. The body temperature decreased to normal following 1 day
of infusion. The blood culture was negative and hence, the patient
was discharged. Sulfamethoxazole tablets (2 tablets bid for 7 days)
were orally administered sequentially.
At two weeks after discharge, the patient had a
fever with a temperature of 37.8˚C, a sore throat and a mild cough.
Hence, he was admitted for the third time. A similar blood culture
confirmed the presence of Alcaligenes sp. (the methods of
cultivation and identification were the same as those described
earlier). An expanded examination was performed to further detect
any potential lesions. No significant abnormality was observed by
transthoracic echocardiography and enhanced CT. Electronic
gastroscopy and colonoscopy indicated fungal esophagitis. The
humoral immunoassay indicated a decrease in IgG, IgM and IgA levels
(Table I). Analysis of T-lymphocyte
subsets suggested a decrease in the numbers of B and
CD4+ T cells and the CD4/CD8 ratio, while the number of
CD8+ T cells was increased (Table II). Thus, besides the previous
thymoma, recurrent bloodstream infection and B-cell deficiency,
CD4+ T-cell reduction, CD8+ T-cell elevation,
inversion of CD4/CD8 ratio and hypogammaglobulinemia had been
confirmed by a peripheral blood test. The patient was finally
diagnosed with GS. He was diagnosed with a secondary (secondary to
a respiratory tract infection) recurrent bloodstream infection
(Alcaligenes sp.), respiratory infection and fungal
esophagitis. The same medications as those received previously
(ticarcillin-clavulanate potassium intravenous infusion at 3.2 g
q6h) were administered for 2 weeks, followed by intravenous
supplementation with gamma globulin (5 g/d for 7 days) and
subcutaneous injection of thymalfasin (1.6 µg q3d) simultaneously
to improve immunity. Furthermore, antifungal treatment was
performed by oral administration of fluconazole (300 mg qd for 14
days). The patient understood and accepted the symptoms of GS and
compliance was good. After discharge, the patient voluntarily
infused gamma globulin at 5 g/d for 2 days and thymalfasin (1.6 µg
q3d) was subcutaneously injected in an outpatient setting. The
patient was followed up once every 1-2 months and no infection
developed within 1 year. However, hypogammaglobulinemia and
decreased CD4+ T cells persisted (Table I).
 | Table IIResults for lymphocyte subsets (7
months after thymoma operation). |
Table II
Results for lymphocyte subsets (7
months after thymoma operation).
| Item | Percentage (RR) | Concentration,
cells/µl or ratio (RR) | Trend |
|---|
| Total T cells
(CD3+) | 90.28
(60.8-80.1) | 2,421.08
(937.6-1,717.3) | ↑ |
| CD4+ T
cells | 13.90 (44-66.9) | 336.62
(518.8-963.9) | ↓ |
| CD8+ T
cells | 83.36
(25.9-47.2) | 2,018.11
(326.7-640.2) | ↑ |
| CD4/CD8
(CD3+CD4+/CD3+CD8+) | | 0.17 (1.01-2.64) | ↓ |
| Total B cells
(CD19+) | 0.06 (7.3-19) | 1.62
(140.6-402.3) | ↓ |
| NK cells
(CD3-CD16+CD56+) | 9.66 (7.1-24.5) | 258.94
(112.1-481.6) | |
Discussion
GS is an adult-onset T-cell and B-cell combined
immunodeficiency, which is associated with thymoma, manifesting as
recurrent infection, paraneoplastic syndrome and autoimmune disease
(9,10). Its clinical manifestations are
diverse. It has no specificity, irrespective of symptoms associated
with thymoma or infections caused by immune deficiency. Early
diagnosis is difficult, besides the insufficient awareness of
clinicians. A meta-analysis performed by Dong et al
(3) in China indicated that the
time interval between symptom onset and the diagnosis of GS ranged
from 5 days to 11 years. The humoral immunity of the patient of the
present study was significantly lower at the time-point of surgery.
A severe infection occurred after the surgery, but it was not
recognized as GS. The disease remained undetected until the
occurrence of the recurrent bloodstream infection caused by
Alcaligenes sp. the third time 6 months later, as no
difficult-to-clear primary or migratory lesions were observed
during a comprehensive examination. The observations suggested that
hypogammaglobulinemia may provide a good clue for clinical
diagnosis when treating patients with a previous history of thymoma
combined with recurrent infections.
Infections are the most common clinical
manifestation and cause of death in patients with GS, particularly
respiratory, skin and intestinal infections (1,3,6). A
previous study demonstrated that the degree of B-cell and T-cell
dysfunction and hypogammaglobulinemia were the most important
factors affecting the intensity of infection (9). In the present case, the humoral
immunity of peripheral blood was tested four times and the T-cell
subset was tested once. The results suggested that the total B-cell
(CD19+) count was significantly decreased (1.6
cells/µl). In addition, IgG fluctuated between 1.66 and 2.08 g/l
without an infusion of Ig, IgA fluctuated between 0.09 and 0.14 g/l
and IgM was <0.046 g/l. Furthermore, the
CD3+CD4+ T-lymphocyte count was decreased
(336 cells/µl) and the CD4/CD8 ratio was inverted (0.17). However,
the CD3+CD8+ T-cell count was significantly
increased (2,018 cells/µl). These results were consistent with
those of a meta-analysis by Chen et al (11), which also indicated that the numbers
of different types of regulatory T cells in the peripheral blood
were higher compared with those in healthy controls. The
distribution of immune cells in the peripheral blood was different
from that in healthy individuals, suggesting that patients with GS
are in an immunosuppressed state. However, the intrinsic mechanism
underlying the progression of GS should be further
investigated.
GS appears to be more complex compared with other
immunodeficiency diseases due to the combined immunodeficiency of T
cells and B cells. Patients frequently have an infection caused by
capsular bacteria. The patient in the present study had an
Alcaligenes sp. infection. Alcaligenes widely exists
in nature and is a normal constituent of the intestinal flora of
humans and animals. Alcaligenes also exists in the
respiratory tract, skin and water, and may lead to opportunistic
infections. The patient in the present study had pharyngeal
discomfort, cough and pharyngeal congestion prior to the onset of
the disease; it was considered to be a pulmonary invasion of blood
flow. Stark (12) reported on the
first case of one patient with GS who had recurrent septicemia
caused by alkaline oxidizing xylose due to an indwelling venous
catheter. The patient also had osteomyelitis of the fibula,
suggesting that the presence of opportunistic pathogens, besides
common capsular bacteria, in patients with GS should not be
ignored.
At present, no specific treatment for GS is
available. Thymectomy may prevent local invasive growth and
metastasis of thymoma but it does not improve hypogammaglobulinemia
and B-cell deficiency (13,14), which even leads to the deterioration
of immune function in certain cases (15). However, supplementation with Igs to
maintain serum IgG at an appropriate concentration and hence reduce
infection is currently the most effective treatment for GS. In the
present study, the disease recurred after the first antibiotic
treatment in hospital, it may be related to an inadequate course of
treatment, but the recurrence still occurred after the second
hospitalization. Gamma globulin was supplemented after the patient
was diagnosed with GS. The patient's condition was then stable with
no further recurrence. In a short duration (2 months), the same
pathogenic bacteria (Acaligens sp.) invaded three times,
indicating that the potential lesions in the patient were not
eradicated. It was further confirmed that anti-infection treatment
was not sufficient for treating GS; regular supplementation with
gamma globulin was required to maintain the IgG/IgM level and hence
reduce the occurrence of opportunistic infections. Thongngarm et
al (7) suggested that early
diagnosis and prompt gamma globulin replacement may change the
natural course of this condition and may be successful in keeping
patients infection-free. However, it is still necessary to pay
attention to intracellular infection and tumor occurrence due to
cellular immunodeficiency.
The prognosis of GS is poor. The 5- and 10-year
survival rates are 70 and 33%, respectively. The prognosis depends
mainly on the severity of relevant infectious diseases, blood
system diseases and autoimmune diseases and not on the behavior of
thymoma (1,3,16).
Therefore, follow-up should be strengthened to actively prevent
infection.
In conclusion, GS should be alerted for adult
patients with a previous history of thymoma and recurrent
infections, particularly patients with an opportunistic pathogen
infection. Furthermore, humoral immunity and cellular immune
function indicators should be carefully screened to facilitate
early diagnosis and treatment. For patients diagnosed with GS
combined with infection, the treatments are mainly surgical
resection of thymoma, Ig replacement therapy and anti-infection
treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC performed the examination, treatment and data
collection of the patient, as well as the conception, design,
drafting and revising of the manuscript. JL participated in data
collection and analysis, as well as the design, drafting and
revision of the manuscript. YL and YG helped to collect patients'
data and revise the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kelesidis T and Yang O: Good's syndrome
remains a mystery after 55 years: A systematic review of the
scientific evidence. Clin Immunol. 135:347–363. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tarr PE, Sneller MC, Mechanic LJ,
Economides A, Eger CM, Strober W, Cunningham-Rundles C and Lucey
DR: Infections in patients with immunodeficiency with thymoma (Good
syndrome). Report of 5 cases and review of the literature. Medicine
(Baltimore). 80:123–133. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dong JP, Gao W, Teng GG, Tian Y and Wang
HH: Characteristics of Good's Syndrome in China: A systematic
review. Chin Med J (Engl). 130:1604–1609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Good RA: Absence of plasma cells from bone
marrow and lymph nodes following antigenic stimulation in patients
with a gamma globulinemia. Rev Hematol. 9:502–503. 1954.PubMed/NCBI
|
|
5
|
Zaman M, Huissoon A, Buckland M, Patel S,
Alachkar H, Edgar JD, Thomas M, Arumugakani G, Baxendale H, Burns
S, et al: Clinical and laboratory features of seventy-eight UK
patients with Good's syndrome (thymoma and hypogammaglobulinaemia).
Clin Exp Immunol. 195:132–138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Joven MH, Palalay MP and Sonido CY: Case
report and literature review on Good's syndrome, a form of acquired
immunodeficiency associated with thymomas. Hawaii J Med Public
Health. 72:56–62. 2013.PubMed/NCBI
|
|
7
|
Thongngarm T, Boonyasiri A, Pradubpongsa
P, Tesavibul N, Anekpuritanang T, Kreetapirom P and
Sompornrattanaphan M: Features and outcomes of immunoglobulin
therapy in patients with Good syndrome at Thailand's largest
tertiary referral hospital. Asian Pac J Allergy Immunol.
37:109–115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
National Health Commission of the People's
Republic of China. Available from: http://www.nhc.gov.cn/wjw/s9492/201110/53169.shtml.
|
|
9
|
Tavakol M, Mahdaviani SA, Ghaemi MR, Vaezi
M, Dorudinia A, Jamaati H and Velayati AA: Good's
Syndrome-association of the late onset combined immunodeficiency
with thymoma: Review of literature and case report. Iran J Allergy
Asthma Immunol. 17:85–93. 2018.PubMed/NCBI
|
|
10
|
Bernard C, Frih H, Pasquet F, Kerever S,
Jamilloux Y, Tronc F, Guibert B, Isaac S, Devouassoux M,
Chalabreysse L, et al: Thymoma associated with autoimmune diseases:
85 cases and literature review. Autoimmun Rev. 15:82–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen X, Zhang JX, Shang WW, Xie WP, Jin SX
and Wang F: Aberrant peripheral immune function in a good syndrome
patient. J Immunol Res. 2018(6212410)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stark JJ: Alcaligenes xylosoxidans
osteomyelitis without trauma in a patient with Good's syndrome. Eur
J Intern Med. 18(447)2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaufman AJ, Palatt J, Sivak M, Raimondi P,
Lee DS, Wolf A, Lajam F, Bhora F and Flores RM: Thymectomy for
myasthenia gravis: Complete stable remission and associated
prognostic factors in over 1000 cases. Semin Thorac Cardiovasc
Surg. 28:561–568. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Idress A and Wahla AS: Good syndrome, a
rare disease that physicians cannot afford to overlook; case report
and review of literature. J Pak Med Assoc. 63:1541–1543.
2013.PubMed/NCBI
|
|
15
|
Ohuchi M, Inoue S, Hanaoka J, Igarashi T,
Tezuka N, Ozaki Y and Teramoto K: Good syndrome coexisting with
leukopenia. Ann Thorac Surg. 84:2095–2097. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kelleher P and Misbah SA: What is Good's
syndrome? Immunological abnormalities in patients with thymoma. J
Clin Pathol. 56:12–16. 2003.PubMed/NCBI View Article : Google Scholar
|