1. Introduction
The biochemical agent ribose is present as L and D
enantiomers (1). The L-ribose
enantiomer is unstable and thus, D-ribose is the primary functional
isoform of ribose (2). D-ribose is
a highly water-soluble 5-carbon sugar, also known as D-furanose,
which is present in different types of RNA molecule, including
mRNA, transfer RNA and ribosomal RNA (3,4).
D-ribose was first identified as a physiologically
important molecule in humans in 1958; however, its physiological
and pathological roles in humans, and in particular in diseases,
are still being studied (5). Until
1970, D-ribose had only been known to serve as a means of
increasing blood sugar levels in states of low energy (6). D-ribose was subsequently indicated to
be clinically beneficial for treating certain diseases, such as
congestive heart failure (CHF) (7-10).
Although D-ribose is not stored in cells, it is essential in cell
resynthesizing (7,11,12),
remedial synthesis and ischemia and hypoxia (13-15).
D-ribose may also be supplemented intravenously, via oral therapy
or via other exogenous means, and is utilized in several scenarios,
including the clinic (9,16), in athletes (17,18)
and in healthcare (19), and energy
is rapidly recharged via the synthesis of adenosine triphosphate
(ATP).
In the present review, the potential physiological
functions of D-ribose, its toxic effects, clinical value and its
utility for the treatment of several diseases are discussed.
2. Physiological roles of D-ribose
Conversion of D-ribose to ATP
ATP, an adenine nucleotide, is the primary molecule
that is utilized as a means of readily available energy and is an
essential molecule for life. The principal nucleotide that
generates ribose is D-ribose, such that ribose-5-phosphate joins
the pentose phosphate pathway to produce ribokinesis ATP (Fig. 1). In addition, ribose 5-phosphate
may be used in various forms for glycolysis and pyrimidine and/or
purine nucleotide synthesis. D-ribose may serve as the substrate
for the formation of phosphoribosyl pyrophosphate (PRPP), the
precursor for de novo ATP synthesis; therefore, D-ribose may
be used to produce ATP in order to meet the demands of the body
under certain circumstances (13).
 | Figure 1Overview of the biochemical processes
to synthesize ATP from D-ribose. The generation of ATP from
D-ribose involves PRPP, IMP, AMP and ADP. ATP, adenosine
triphosphate; ADP, adenosine diphosphate; AMP, adenosine
monophosphate; IMP, inosine monophosphate; PRPP, phosphoribosyl
pyrophosphate; HPRT, hypoxanthine phosphoribosyl transferase; PPi,
pyrophosphoric acid; R5-P, ribulose 5-phosphate. |
Effects of D-ribose on the heart
CHF is a severe clinical syndrome of the heart,
which may be the result of various heart diseases developing to a
severe stage. Due to the reduced function of ventricular pumping or
filling, the cardiac output cannot meet the metabolic needs; thus,
blood perfusion of tissues and organs is insufficient and pulmonary
circulation or systemic circulation congestion develop as a result.
CHF is the end-stage event of heart disease progression and one of
the primary causes of death in patients with various cardiac
diseases (20). Shecterle et
al (21) reported that lower
concentrations of ATP in cardiac myocytes induced ventricular
diastolic dysfunction. In order to maintain adequate ATP supplies
in the human heart, the adenosine diphosphate (ADP)/ATP ratio must
be kept as low as possible. If the absolute concentration of ADP or
Pi increases, or the relative ratio of ADP/ATP increases, cardiac
dysfunction may occur. This abnormal energy state is caused by a
limited ability to convert ADP to ATP in the circulation.
Therefore, a sufficiently high energy supply of phosphate is
essential to maintain cell integrity and function. Indeed, the
relatively slow synthesis of adenine nucleotides underlies a
decrease in ATP concentration in myocardial cells following
myocardial ischemia and the impact of ATP deficiency is long-term
(22). Thus, it has been proposed
that supplementing ischemic myocardium with D-ribose may accelerate
PRPP synthesis directly to increase ATP levels in myocardial cells
and thus to reduce diastolic ventricular dysfunction. A
time-dependent association between ATP levels and diastolic
function following myocardial ischemic injury has been reported
(23). Supplementation with
D-ribose during and after ischemia may quickly restore the levels
of ATP and improve the phenomenon of diastolic dysfunction caused
by ischemia, such as systemic hypertension and angina pectoris. The
possible mechanisms by which D-ribose improves function and limits
damage may be due to its ability to bypass the rate-limiting step
in the pentose phosphate pathway, resulting in an increase in the
content of PRPP nucleophosphate. PRPP is indispensable in the ab
initio and remedial pathway of ATP synthesis. Therefore,
D-ribose may improve the recovery of myocardial ATP content
following ischemia by increasing the de novo synthesis rate
of adenine nucleotides. In addition, following myocardial ischemia,
D-ribose improves distal myocardial function. Furthermore, D-ribose
supplementation may also efficiently target ‘hibernating’
myocardium and reduce cardiomyocyte loss of ATP attributed to
ischemia in patients with coronary artery disease, as well as
enhance early diastolic filling and the respiratory performance of
patients with CHF. However, the effects of D-ribose as a myocardial
protective agent prior to ischemia have remained to be assessed, to
the best of our knowledge.
Use of D-ribose to counter
fatigue
The human body requires a large amount of energy to
perform its activities. Ribose is an excellent source that may be
converted to ATP and therefore alleviate excessive fatigue. The
fatigue that is being referred to in the present review is caused
by exercise. Yuan et al (24) used mouse models to assess the
effects of D-ribose and other anti-fatigue products, such as
ginseng and maca extract, on physical fatigue caused by exercise.
They determined that D-ribose was only effective against short-term
fatigue in mice compared with ginseng and maca. This may be due to
the fact that D-ribose increases aerobic respiration by increasing
the concentration of ATP in cardiomyocytes, thus reducing lactic
acid produced by glycolysis during anaerobic respiration. When the
human body cannot produce sufficient energy via glucose metabolism
and lipid metabolism, protein degradation occurs to provide
additional energy and the increase in ammonia entering the
ornithine cycle leads to an increase in plasma urea nitrogen
levels. D-ribose may also reduce the plasma urea nitrogen content
and the decomposition rate of proteins by increasing liver glycogen
reserves. However, the detailed mechanisms have remained to be
fully determined and require to be further explored. D-ribose
cannot be used to counter liver glycogen shortages, whereas ginseng
and maca class compounds may be capable of this.
Just as combination therapy may be used to treat
cancer (25), it has been indicated
that D-ribose may also be used in combination with other drugs to
counter physical fatigue. D-ribose combined with ginseng extract
and morphine produced better results compared with either product
alone (26,27) and exerted long-term anti-fatigue
effects (28). Likewise, studies
have indicated that D-ribose serves an important role in both
patients with fatigue caused by CHF (9) and mental fatigue (29) by regulating the metabolism of
high-energy phosphate. It was also determined that the swimming
time of mice treated with D-ribose alone was significantly longer
than of those treated with a combination of caffeine and D-ribose
(30). According to the measurement
of different substances in the gastrocnemius of fatigued mice
immediately after swimming, the combined action of D-ribose and
caffeine significantly increased the concentration of ATP, ADP and
AMP in the gastrocnemius of mice in comparison with those in the
D-ribose alone group. The concentration of inosine monophosphate
(IMP) decreased in the former and increased in the latter. The
concentration of AMP in the caffeine alone group increased, but
there was no significant difference in IMP. After three days of
recovery, the ADP concentration of the D-ribose alone group
decreased and the IMP concentration increased significantly, and
the ADP concentration in the combined caffeine and D-ribose group
decreased significantly. The IMP concentration was not significant
compared with the same group before the 3 days recovery. These
results support the view that D-ribose is essential for fatigue
relief.
D-ribose-mediated regulation of blood
sugar levels
Insulin is intricately involved in the regulation of
blood sugar levels and exogenous insulin is irreplaceable for
diabetics (31,32). Insulin resistance (a lack of
response to insulin) is the major barrier in the treatment of
patients with diabetes (33-35).
Increasing the response of cells to insulin is the goal of the
majority of studies on diabetes. Hong et al (36) indicated that D-ribose reduced
fasting blood glucose levels considerably after 10 and 20 days of
intraperitoneal injection, suggesting that D-ribose was able to
control blood sugar levels by modulating the sensitivity to insulin
in mice. Therefore, on the one hand, D-ribose increased the
sensitivity of cells to insulin, but on the other hand, D-ribose is
a monosaccharide. After ingestion, it causes an increase in insulin
secretion, resulting in an increase in oxidative decomposition of
sugars and a decrease in blood glucose levels. In addition, using
an oral glucose tolerance test, the above study also determined
that D-ribose rapidly improved glucose tolerance. By measuring
serum insulin levels, it was indicated that the serum insulin
concentration was significantly increased after 10 or 20 days of
treatment with D-ribose and then returned to normal levels.
Although these results indicate that D-ribose may, in part,
increase insulin and glucose tolerance, this method may not be
applicable to humans due to physiological differences between mice
and humans.
3. Clinical applications of D-ribose
Amelioration of CHF using
D-ribose
Both in developed and developing countries, CHF
remains a serious health problem and is the leading cause of death
worldwide, particularly amongst elderly populations of developed
countries (37). A large amount of
work has been performed to understand the pathogenesis of CHF and
to improve targeted therapeutic medicines (38,39).
Pauly and Pepine (8,12) indicated that D-ribose
supplementation is associated with rapid ATP production in cardiac
cells under ischemic and hypoxic conditions, and that it may
preserve cardiovascular energy levels. Exposure to transient
ischemia-reperfusion in isolated rat hearts suggested that the
recovery rate of ATP levels doubled following D-ribose treatment;
the ATP levels in the isolated perfused rat heart with simulation
of ischemia for 15 min and reflux for 10-15 min was only 66-69% of
the baseline of the control heart, whereas in the D-ribose-treated
hearts, it was 89-96% of the baseline levels. This suggests that
D-ribose may effectively increase ATP synthesis and thus its
levels, reducing the damage caused by cardiac ischemia and tissue
hypoxia. However, D-ribose is not the preferred substrate for
cardiac energy production and cannot provide comparable oxidative
energy compared with glucose or pyruvate. D-ribose serves a role in
providing PRPP and adenine nucleotide in models of reversible
myocardial injury and related hypertrophy and regional infarction
(12). The beneficial effects of
D-ribose are attributed to ATP supplementation by increasing the
availability of PRPP and increasing ab initio ATP synthesis.
As other pathways, such as ion homeostasis, substrate utilization,
proteolytic protein degradation, oxidative stress and mitochondrial
function are all affected by transient ischemic injury, metabolic
supplementation of D-ribose is most favorable when PRPP is the
primary limiting factor.
Likewise, Ma et al (40) reported that D-ribose increased ATP
concentrations during myocardial ischemia-reperfusion injury in
myocardial cells. Of note, inflammation of myocardial cells caused
by free radicals and enzymes without oxygen also significantly
increased following D-ribose therapy. The mechanism underlying this
is that D-ribose, via increasing the recovery of myocardial energy,
reduced the activation of NF-κB by oxygen free radicals, such as
hydrogen peroxide, thereby decreasing the expression of chemokines
in activated neutrophils and significantly attenuating the
activation, infiltration and degranulation of neutrophils, as well
as reducing the release of myeloperoxidase in myocardial tissues.
The activity of other myocardial enzymes is significantly reduced,
thus significantly reducing the inflammatory response. In addition,
D-ribose supplementation may also reduce dobutamine-induced left
ventricular ischemic dysfunction, providing a more predictive
assessment of function after surgical revascularization.
Association between D-ribose and
chronic diabetic complications
The prevalence and incidence of diabetes is
increasing and has become a major public health concern worldwide
(41). However, it is not the
disease itself that eventually leads to a decrease in the quality
of life, physical impairments or even death of patients with
diabetes, but several chronic complications (41). Several clinically used methods may
exacerbate the complications of chronic diabetes. Increased
screening of patients is thus required to manage the conditions and
a telemedicine project has been suggested to reduce the impairments
caused by chronic complications of patients with diabetes and to
improve their quality of life (42). However, studies such as that by
Salci et al (43) tested
preventative measures (For example, blood pressure control,
psychosocial concerns and detection and treatment of chronic
complications) for chronic diabetes mellitus complications and the
results indicated that they were not sufficiently useful. This
indicates there are various deviations in implementing certain
precautionary steps and also demonstrates the nature and difficulty
of controlling and treating patients with diabetes (44).
There is a requirement for improved methods to
diagnose chronic complications in patients with diabetes early and
more effectively. Certain clinicians use glycosylated hemoglobin as
a biomarker (45,46). A study reported that patients with
diabetes had anti-D-autoantibodies against riboglycosylated
hemoglobin, which may be used as a marker for early diabetes
detection, and inhibition of its output may be used to minimize
diabetes progression and the incidence and development of
subsequent complications (47).
Furthermore, Chen et al (23) also studied the physiological
functions of D-ribose in the pathogenesis of type 2 diabetes,
suggesting that D-ribose may react with hemoglobin to generate
glycosylated hemoglobin. Of note, the glycated serum protein (GSP)
caused by D-ribose is more effective than other sugars, such as
D-glucose. In addition, Chen et al (48) reported that D-ribose may also
enhance serum protein glycosylation to produce GSP, leading to a
series of complications of chronic diabetes. The authors also
indicated that serum protein glycosylation caused tissue damage in
the previous 1-3 weeks, whereas hemoglobin glycosylation caused
tissue damage in the previous 8-12 weeks. These two types of
non-enzymatic glycosylation related tissue damage are the leading
causes of the complications of chronic diabetes. D-ribose may lead
to the formation of advanced glycation end products (AGE) and
complications of chronic diabetes, such as diabetic nephropathy
(36).
Regarding the possible mechanism, as compared with
glucose, D-ribose is able to rapidly interact with the amino
residues of non-enzymatic protein to form early glycosylation
products (for instance, reversible Schiff base) in patients with
diabetes; these early glycosylation products may be further
rearranged, dehydrated and condensed to form AGE, which bind to
receptors for AGE (RAGE), which results in increased expression of
vascular endothelial growth factor and vascular cell adhesion
factor to enhance vascular permeability, angiogenesis and local
inflammation. Furthermore, the activation of the AGE-RAGE pathway
in monocytes may also increase the secretion of various cytokines
and cause a series of oxidative stress reactions, so as to serve a
role in the chronic complications of diabetes (49).
Although D-ribose may lower blood sugar levels over
a short period of time, it may also trigger complications of
chronic diabetes following long-term accumulation. The mechanism by
which D-ribose is implicated in complications of chronic diabetes
is via a D-ribose-induced increase in glycosylated hemoglobin,
further resulting in the generation of AGEs. Therefore, high
D-ribose concentrations are associated with chronic diabetic
complications. These results indicate that D-ribose is a
double-edged sword in the management of diabetes. It may be
possible to measure the content of glycosylated proteins or
D-ribose in patients with diabetes to predict potential clinical
complications and thus introduce effective preventative steps;
however, further studies are required to confirm this
hypothesis.
Drug therapy
As indicated above, the potential importance of
D-ribose in two world-leading diseases, CHF and diabetes, is well
established. The efficacy of D-ribose in other diseases has also
been assessed. The term restless leg syndrome was coined in 1672
and was previously known as Willis Ekbom disease since 1944
(50,51). Restless leg syndrome is
characterized by uncomfortable feelings and discomfort, frequently
when resting in the lower limbs, followed by an uncontrollable
movement for relief. It is a movement-dominated sensory nerve
condition which can cause sleep disorders. This disease severely
affects the quality of life of patients and has thus attracted
significant attention. It has been indicated that intake of
D-ribose does not completely eradicate the symptoms of the disease
(52); however, it may improve a
patient's quality of life, reduce the severity of the effects and
prevent disease progression. The effect of D-ribose, which may
rapidly replace ATP in muscle cells, has also been used for the
treatment of fibromyalgia and chronic syndrome. Teitelbaum et
al (53) and another study
(15) reported that D-ribose may
significantly alleviate fibromyalgia and chronic symptoms and
improve quality of life. Likewise, Gebhart and Jorgenson (54) indicated that if traditional therapy
fails in patients with muscle fiber pain, D-ribose may be
considered, which has certain beneficial effects on the disease.
Furthermore, according to one study, D-ribose may have cosmetic
properties (55). Indeed, D-ribose
enhances the metabolism of skin cells and regulates ATP production.
The results were also confirmed by Shecterle and St Cyr (56).
D-ribose has numerous applications; however, there
are side-effects to its use. Several studies have indicated that
administration of certain concentrations of D-ribose in various
animal models is healthy and does not cause any adverse effects
regarding behavior, hematology, biochemistry, histology or general
pathology (57-59).
Thus, it may be hypothesized that D-ribose has beneficial effects
on the function of local muscle cells or tissue cells, is able to
relieve symptoms and has no apparent toxicity in any area and is
thus appropriate for widespread use.
4. Potential hazards of high concentrations
of D-ribose
The correlation between high
concentration of D-ribose and various system diseases
The uses of D-ribose for treating or detecting
certain diseases described in detail above have several advantages;
however, if the concentration of D-ribose in the body is too high,
adverse effects may occur. The potential implications of high
concentrations of D-ribose are discussed in detail below.
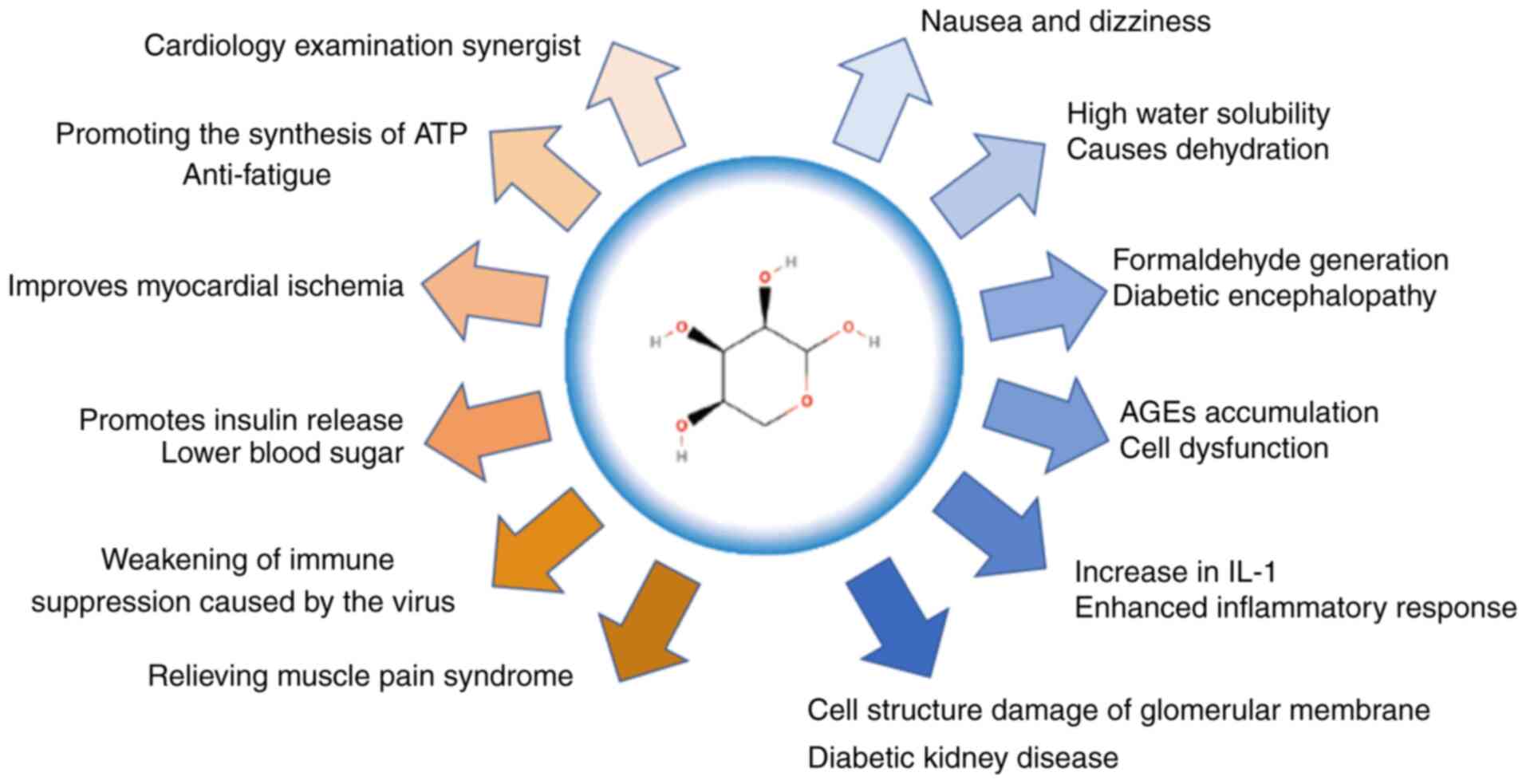
AGEs may exhibit notable cytotoxic effects, a major
cause of chronic diabetes complications (Fig. 2) (60). Several studies have also suggested
that the clinical complications of type 2 diabetes are related to
the accumulation of AGEs and the inflammatory response mediated by
RAGE (49,61). However, high D-ribose levels are
associated with AGE accumulation. By assessing the impact of
D-ribose on human nonenzymatic glycosylation of myoglobin, Yamamoto
and Yamamoto (49) determined that
D-ribose may accelerate glycosylation of myoglobin and other
proteins more efficiently than D-glucose. Yu et al (62) also suggested that the urine
concentration of D-ribose in patients with type 2 diabetes was
substantially higher than that of normal individuals, which also
indicated high levels of D-ribose in the body of patients with
diabetes. In addition, high D-ribose concentrations may also cause
chronic diabetes complications by other means. D-ribose may
glycosylate insulin to form ribosyl insulin, activate Caspase-9 and
-3/-7, trigger transcription factor NF-κB and produce intracellular
reactive oxygen species (ROS) that may cause cytotoxic damage to
the surrounding tissues and cells, eventually leading to the
manifestation of chronic complications (63). Thus, high concentrations of D-ribose
may be a cause of the complications of chronic diabetes (Fig. 3). D-ribose may cause a variety of
chronic complications, such as nervous system-related diseases and
diabetic nephropathy. How D-ribose causes these chronic
complications is discussed further below.
Nervous system-related diseases
Alzheimer's disease is a disorder of the central
nervous system characterized by progressive cognitive dysfunction
and behavioral impairment. It is a common disease amongst the
elderly, results in severe memory losses and renders an individual
unable to socially function properly. It has become a significant
issue impacting global public health systems and sustainable social
growth. In the course of researching the pathogenesis and treatment
of Alzheimer's disease, several scientists have identified a
certain association between D-ribose and Alzheimer's disease. Javed
et al (64) studied the
pathogenesis of Alzheimer's disease caused by D-ribose and
indicated that detecting D-ribose concentrations in the plasma may
help doctors to better characterize the disorder. Han et al
(65) inferred that excessive
D-ribose concentrations increased the levels of glycosylated
proteins and accumulation of AGEs in mice, resulting in
corresponding cytotoxicity. Wei et al (66) reported that D-ribose induced protein
misfolding and aggregation faster than other monosaccharides and
induced apoptosis in SHSY5Y neural cells. The results indicated
that D-ribose may affect certain age-associated diseases. Studies
have also suggested that uncontrolled diabetes may increase the
risk of Alzheimer's disease and vascular dementia (67). In addition, D-ribose serves an
important role in type 2 diabetic encephalopathy. D-ribose has been
demonstrated to cause the rapid saccharification of α-synuclein to
form a molten spherical polymer, leading to oxidative stress and
high cytotoxicity (68). It was
hypothesized that diabetic encephalopathy is linked to
D-ribose-induced Tau protein glycosylation (69). To test this hypothesis, Wu et
al (70) examined age-related
cognitive decline and diabetic encephalopathy using intragastric
long-term D-ribose administration in mice to induce type 2 diabetic
encephalopathy. The results indicated that D-ribose glycosylation
was enhanced. In addition, it has been shown in a previous study
that AGEs were able to inhibit brain-derived neurotrophic factor
expression and tropomyosin-associated kinase B, resulting in
hyperphosphorylation of the Tau protein. It is therefore proposed
that selective inhibition of ribose glycosylation may be used as a
therapeutic strategy for the prevention of Alzheimer's disease and
type 2 diabetic encephalopathy (71). Wang et al (72) also indicated that in alkaline
environments, D-ribose may decompose to formaldehyde by the reverse
aldol reaction, and that by determining the content of formaldehyde
in various parts of mice, it was suggested that formaldehyde
produced by D-ribose accumulates in the brain of the mice and that
the accumulation of endogenous formaldehyde to pathological
concentrations may lead to disturbance of consciousness and
cognition. However, it was also hypothesized that accumulation of
D-ribose-induced AGEs is an important means underlying the
occurrence and development of diabetic encephalopathy (62). Han et al (65) reported that D-ribose glycosylation
led to the accumulation of AGEs in the nervous system, leading to
spatial cognitive impairment and astrocyte-mediated RAGE-dependent
inflammation. This indicates that D-ribose-induces cognitive
dysfunction linked to type 2 diabetic encephalopathy (73). The study by Gheith et al
(74) agreed with this hypothesis
and proved that the accumulation of AGEs may lead to neuronal
dysfunction and death. According to a recent study, high levels of
D-ribose in type 1 diabetes may suggest that D-ribose is involved
in complications associated with type 1 diabetes, including
encephalopathy (62). Several of
the above studies have indicated possible damage to the nervous
system caused by high concentrations of D-ribose in the body and
provided an alternate means of understanding the potential
pathogenesis of Alzheimer's disease and diabetic encephalopathy.
Whether Alzheimer's disease and diabetic encephalopathy may be
prevented or delayed by reducing the concentration of D-ribose or
by cutting off the D-ribose pathway involving glycosylation of
related proteins and generation of AGEs remains to be determined.
To assess this, additional in-depth studies, novel treatment agents
and clinical trials are required.
Role of a high concentration of
D-ribose in the pathogenesis of diabetic nephropathy
Diabetic nephropathy is one of the most severe
diabetic complications and a leading cause of end-stage renal
disease worldwide. Within 20-25 years of diabetes onset, 20-40% of
patients with diabetes develop diabetic nephropathy (74). Therefore, research on the
pathogenesis and care for diabetic nephropathy has gained
increasing attention from numerous academics, including analysis of
poly (ADP-ribose) polymerase-1 (PARP-1). Various experiments have
suggested that inhibiting PARP-1 is able to prevent or alleviate
diabetic nephropathy (75,76). Furthermore, it has been indicated
that inhibiting aldose reductase activity was also able to minimize
nitrosation stress and PARP activation of glomerular mesangial
cells in a high-glucose environment (77). Studies on sodium-glucose
transporter-2 (SGLT-2) and its involvement in renal tubules have
also been performed. It has been indicated that SGLT-2 inhibition
combined with renin-angiotensin system therapy inhibition was able
to better protect the long-term health of the kidney and help delay
the development of renal diseases in patients with type 2 diabetes
(78,79).
Furthermore, an increasing number of studies are
assessing the pathways that underlie AGE-mediated diabetic
nephropathy (80,81) and the AGE-RAGE-oxidative stress
system theory (82). Several
studies have indicated increased expression of RAGE in advanced
chronic kidney disease, which indirectly demonstrates the close
relationship between AGEs and diabetes (83). The increase in AGE-modified plasma
proteins is associated with the production of ROS caused by RAGE,
the activation of transcription factor NF-κB and the pathological
changes in gene expression levels in several cell types (84). It is well established that D-ribose
influences the incidence and development of diabetic nephropathy
via AGE accumulation. To confirm this point, Zhang et al
(85) used murine mesangial cells
as an experimental model. They indicated that D-ribose induced
nonenzymatic glycosylation of related proteins and led to the
accumulation of AGE-induced glomerular cell dysfunction. At the
same time, it was also determined that D-ribose upregulated Bax
protein expression, downregulated Bcl-2 protein expression and
disrupted the Caspase-9/-3 pathway to facilitate glomerular
mesangial cell apoptosis. Increasing D-ribose concentrations may
also lead to increased ROS and cytokine levels, leading to podocyte
inflammation and renal fibrosis. In addition, Hong et al
(36) formulated the
AGE-RAGE-oxidative stress system theory and other mechanisms by
which D-ribose causes diabetic nephropathy. One approach was the
induction of both exogenous and endogenous D-ribose to form and
distinguish NOD-, LRR- and pyrin domain-containing protein 3
inflammatory bodies in podocytes and to secrete podocytes via
extracellular vesicles (EVs). Therefore, releasing cytokine IL-1β
may trigger glomerular injury, and D-ribose may also reduce
interactions between lysosomes and multivesicular bodies (MVBs),
increase the fusion of MVBs and plasma membrane and increase
cytokine IL-1β release via EV secretion. Another potential
mechanism is the increase of the D-ribose content of ceramide in
the lysosome to control the lysosome-ceramide sphingolipid
mechanism of interaction with MVBs, enhancing the ability of
podocytes to release EVs and increasing the release of IL-1β to
enhance the inflammatory response (86). Together, in addition to daily blood
sugar regulation, patients with diabetes should consider monitoring
the concentration of D-ribose daily to avoid diabetic nephropathy.
This may improve the quality of life of patients with diabetes.
5. Conclusions and future perspectives
Currently, research on the clinical use D-ribose is
still in the relatively early stages and a considerable amount of
further investigation is required. However, based on the body of
literature available, it is well established that D-ribose has
several and broad prospects in various areas, including medicine
and healthcare, as well as sports and athletics. For instance, in
emergencies, such as during myocardial ischemia-reperfusion injury
caused by cardiac operations or cardiac arrest, D-ribose may
directly accelerate PRPP synthesis, thereby rapidly increasing the
ATP concentration and other mechanisms in myocardial cells by
injecting a certain amount of D-ribose to reduce the occurrence of
myocardial ischemic injury. At the same time, further research and
confirmation of the appropriate concentration ranges of D-ribose to
use during these procedures and whether it may be used in
conjunction with other first-aid rescue medications, such as those
for cardiac arrest, is required.
Furthermore, a series of existing studies on the
relationship between D-ribose and diabetes indicated that the
concentration of D-ribose in patients with diabetes is proportional
to the incidence and severity of diabetic complications. For the
majority of people with diabetes, the primary factors impacting the
quality of life or reducing their lifespan is not diabetes itself,
but the resulting complications. Whether D-ribose may be used as a
medication to treat diabetic complications and the effects of the
D-ribose concentration on the body should be further studied.
Simultaneously, improving D-ribose concentration determination
technologies is particularly important. Thus, assessing D-ribose
concentrations in patients with early diabetes may be used to
predict the probability and extent of diabetic complications. In
addition, reducing D-ribose intake in everyday life in patients
with diabetes may be of direct significance. Perhaps, D-ribose
analogues or drugs that target D-ribose may be used in advance to
minimize patient harm from diabetic complications and reduce the
occurrence of diabetic complications. Ultimately, D-ribose may be
used to improve the quality of life of patients with diabetes and
extend their lifespan.
Finally, D-ribose is also used as an anti-fatigue
medication to enhance muscle exercise intensity in athletes and is
commonly used as a food additive in food and health products.
However, there is still no definitive conclusion on its safe range.
In addition, whether excessive use of D-ribose poses other
potential health hazards, as well as whether D-ribose is suitable
for everyone requires further study.
Acknowledgements
Not applicable.
Funding
This study was supported by the Hunan Provincial Natural Science
Foundation of China (grant no. 2019JJ50509), the Hunan Province
Education Department (grant no. 18C0479) and the University of
South China (grant nos. 190XQD025 and X2019167).
Availability of data and materials
Not applicable.
Authors' contributions
SL, JW, YX and LZ contributed to the conception of
the study and wrote the manuscript. HQ and MN contributed towards
the conception of the study and revised it critically for important
intellectual content. JF, NY and ZZ contributed to the preparation
of the manuscript and illustration of the figures. All authors read
and approved the final manuscript and agreed to be accountable for
all aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bailey JM: The consequences for amino acid
homochirality if L-ribose RNA and not D-ribose RNA had evolved
first. Biochem Soc Trans. 25(S651)1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ban J, Shabbir S, Lim M, Lee B and Rhee H:
Synthesis of l-ribose from d-ribose by a stereoconversion through
sequential lactonization as the key transformation. Synthesis.
49:4299–4302. 2017.
|
|
3
|
Chen B, Jamieson ER and Tullius TD: A
general synthesis of specifically deuterated nucleotides for
studies of DNA and RNA. Bioorg Med Chem Lett. 4:3093–3096.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Illangasekare M, Turk R, Peterson GC,
Lladser M and Yarus M: Chiral histidine selection by D-ribose RNA.
RNA. 16:2370–2383. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huisman TH, Martis EA and Dozy A:
Chromatography of hemoglobin types on carboxymethylcellulose. J Lab
Clin Med. 52:312–327. 1958.PubMed/NCBI
|
|
6
|
Steinberg T, Poucher RL, Sarin RK, Rees RB
and Gwinup G: Oral administration of D-ribose in diabetes mellitus.
Diabetes. 19:11–16. 1970.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pierce JD, Mahoney DE, Hiebert JB,
Thimmesch AR, Diaz FJ, Smith C, Shen Q, Mudaranthakam DP and Clancy
RL: Study protocol, randomized controlled trial: Reducing symptom
burden in patients with heart failure with preserved ejection
fraction using ubiquinol and/or D-ribose. BMC Cardiovasc Disord.
18(57)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
MacCarter D, Vijay N, Washam M, Shecterle
L, Sierminski H and St Cyr JA: D-ribose aids advanced ischemic
heart failure patients. Int J Cardiol. 137:79–80. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bayram M, St Cyr JA and Abraham WT:
D-ribose aids heart failure patients with preserved ejection
fraction and diastolic dysfunction: A pilot study. Ther Adv
Cardiovasc Dis. 9:56–65. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bao-Hui Y, Qi D, Gui-Qin L and Zheng-Ping
W: The physiological function of D-ribose and its application. Inst
Biopharm Res. 210–212. 2016.
|
|
11
|
zur Nedden S, Doney AS and Frenguelli BG:
Modulation of intracellular ATP determines adenosine release and
functional outcome in response to metabolic stress in rat
hippocampal slices and cerebellar granule cells. J Neurochem.
128:111–124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pauly DF and Pepine CJ: D-Ribose as a
supplement for cardiac energy metabolism. J Cardiovasc Pharmacol
Ther. 5:249–258. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Addis P, Shecterle LM and St Cyr JA:
Cellular protection during oxidative stress: A potential role for
D-ribose and antioxidants. J Diet Suppl. 9:178–182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Busca A and Parra-Herran C: The role of
pathologic evaluation of endometrial ablation resections in
predicting ablation failure and adenomyosis in hysterectomy. Pathol
Res Pract. 212:778–782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nakamura K, Nakayama K, Ishikawa M,
Katagiri H, Katagiri A, Ishibashi T, Sato E, Asakawa Y and Kyo S:
Efficacy of multiple microwave endometrial ablation technique for
menorrhagia resulting from adenomyosis. J Obstet Gynaecol Res.
41:1769–1772. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Conway VD, Race BA and Chigrinskiy EA:
Role of ribose deficit in rat testicular metabolism under
conditions of overtraining. Bull Exp Biol Med. 150:649–651.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kreider RB, Melton C, Greenwood M,
Rasmussen C, Lundberg J, Earnest C and Almada A: Effects of oral
D-ribose supplementation on anaerobic capacity and selected
metabolic markers in healthy males. Int J Sport Nutr Exerc Metab.
13:76–86. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Seifert JG, Brumet A and St Cyr JA: The
influence of D-ribose ingestion and fitness level on performance
and recovery. J Int Soc Sports Nutr. 14(47)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wei Y, Han CS, Zhou J, Liu Y, Chen L and
He RQ: D-ribose in glycation and protein aggregation. Biochim
Biophys Acta. 1820:488–494. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Omran H, Illien S, MacCarter D, St Cyr J
and Lüderitz B: D-Ribose improves diastolic function and quality of
life in congestive heart failure patients: A prospective
feasibility study. Eur J Heart Fail. 5:615–619. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shecterle LM, Terry KR and St Cyr JA:
Potential clinical benefits of D-ribose in ischemic cardiovascular
disease. Cureus. 10(e2291)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wallen WJ, Belanger MP and Wittnich C:
Preischemic administration of ribose to delay the onset of
irreversible ischemic injury and improve function: Studies in
normal and hypertrophied hearts. Can J Physiol Pharmacol. 81:40–47.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Chen X, Su T, Chen Y, He Y, Liu Y, Xu Y,
Wei Y, Li J and He R: d-Ribose as a contributor to glycated
haemoglobin. EBioMedicine. 25:143–153. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuan BH, Liu GQ, Liu M, Li DC, Han J and
Wang ZP: Study on the anti-fatigue effect of compound D-ribose on
mice. Sci Technol Food Ind. 349–353. 2016.
|
|
25
|
Tsakiris N, Papavasileiou M, Bozzato E,
Lopes A, Vigneron AM and Préat V: Combinational drug-loaded lipid
nanocapsules for the treatment of cancer. Int J Pharm.
569(118588)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Derosa G, Pasqualotto S, Catena G,
D'Angelo A, Maggi A and Maffioli P: A randomized, double-blind,
placebo-controlled study to evaluate the effectiveness of a food
supplement containing creatine and D-ribose combined with a
physical exercise program in increasing stress tolerance in
patients with ischemic heart disease. Nutrients.
11(3075)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jhawar SR, Goyal S, Thandoni A, Wu H,
Hassan S, Schiff DS, Allen J, Stogniew M, Tarapore R, Stein M, et
al: Combination radiation therapy and imipridone ONC201 for the
treatment of solid tumors. Int J Radiat Oncol Biol Phys. 99 (Suppl
2):E598–E599. 2017.
|
|
28
|
Kajiwara H: Motif 2 in adenosine kinase
homologous ginseng polypeptide showed affinity to D-ribose by
capillary zone electrophoresis and surface plasmon resonance. J
Chromatogr A. 817:173–179. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ataka S, Tanaka M, Nozaki S, Mizuma H,
Mizuno K, Tahara T, Sugino T, Shirai T, Kajimoto Y, Kuratsune H, et
al: Effects of oral administration of caffeine and D-ribose on
mental fatigue. Nutrition. 24:233–238. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuanbao Hui LQ, Min L, Cheng L, Jun H and
Ping W: Study on anti- fatigue effect of compound D-ribose on mice.
Sci Technol Food Ind. 22:349–353. 2016.
|
|
31
|
Kitamoto T, Sakurai K, Lee EY, Yokote K,
Accili D and Miki T: Distinct roles of systemic and local actions
of insulin on pancreatic β-cells. Metabolism. 82:100–110.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Czech MP: Insulin action and resistance in
obesity and type 2 diabetes. Nat Med. 23:804–814. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Sun S, Jia H, Qi Y, Zhang J, Lin
L, Chen Y, Wang W and Ning G: The optimized calculation method for
insulin dosage in an insulin tolerance test (ITT): A randomized
parallel control study. Front Endocrinol (Lausanne).
11(202)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Petersen MC and Shulman GI: Mechanisms of
insulin action and insulin resistance. Physiol Rev. 98:2133–2223.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Adeva-Andany M, Souto-Adeva G,
Ameneiros-Rodríguez E, Fernández-Fernández C, Donapetry-García C
and Domínguez-Montero A: Insulin resistance and glycine metabolism
in humans. Amino Acids. 50:11–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hong J, Wang X, Zhang N, Fu H and Li W:
D-ribose induces nephropathy through RAGE-dependent NF-κB
inflammation. Arch Pharm Res. 41:838–847. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rassaf T and Totzeck M: Modern concepts in
cardio-oncology. J Thorac Dis. 10 (Suppl 35):S4386–S4390.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Williams JW, Huang LH and Randolph GJ:
Cytokine circuits in cardiovascular disease. Immunity. 50:941–954.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shaito A, Thuan DTB, Phu HT, Nguyen THD,
Hasan H, Halabi S, Abdelhady S, Nasrallah GK, Eid AH and Pintus G:
Herbal medicine for cardiovascular diseases: Efficacy, mechanisms,
and safety. Front Pharmacol. 11(422)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
MA LF, Yang SQ and Yang QJ: Protective
effect of exogenous D-ribose on myocardial ischemia/reperfusion
injury of rat hearts. J Chongqing Med Univ. 6:689–692. 2011.(In
Chinese).
|
|
41
|
Sen S and Chakraborty R: Treatment and
diagnosis of diabetes mellitus and its complication: Advanced
approaches. Mini Rev Med Chem. 15:1132–1133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nagai R, Murray DB, Metz TO and Baynes JW:
Chelation: A fundamental mechanism of action of AGE inhibitors, AGE
breakers, and other inhibitors of diabetes complications. Diabetes.
61:549–559. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Salci MA, Meirelles BHS and Silva DMVGD:
Prevention of chronic complications of diabetes mellitus according
to complexity. Rev Bras Enferm. 70:996–1003. 2017.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
44
|
You Y, Liu F, Gao S, Lin Y and Ge B:
D-ribose Induced Rapid Non-enzymatic Glycation of Human Myoglobin.
J Nanhua Univ. 44499–503. (535)2016.
|
|
45
|
Chehregosha H, Khamseh ME, Malek M,
Hosseinpanah F and Ismail-Beigi F: A view beyond HbA1c: Role of
continuous glucose monitoring. Diabetes Ther. 10:853–863.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yap CW, Ang YG, Quek TPL, Heng BH and Chew
DEK: Re-examining the sensitivity of HbA1c to screen for diabetes
mellitus. J Diabetes. 10:380–385. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Siddiqui Z, Faisal M, Alatar AR and Ahmad
S: Prevalence of auto-antibodies against
D-ribose-glycated-hemoglobin in diabetes mellitus. Glycobiology.
29:409–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen Y, Yu L, Wang Y, Wei Y, Xu Y, He T
and He R: d-Ribose contributes to the glycation of serum protein.
Biochim Biophys Acta Mol Basis Dis. 1865:2285–2292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yamamoto Y and Yamamoto H: RAGE-mediated
inflammation, type 2 diabetes, and diabetic vascular complication.
Front Endocrinol (Lausanne). 4(105)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
brutorum WTDa: An account of some books.
Philos Trans R Soc Lond. 7:4071–4078. 1672.
|
|
51
|
Ekbom KA: Asthenia crurum paraesthetica
(Irritable legs). Acta Med Scand. 118:197–209. 1944.
|
|
52
|
Shecterle L, Kasubick R and Cyr JS:
D-ribose benefits restless legs syndrome. J Altern Complement Med.
14:1165–1166. 2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Teitelbaum J, Jandrain J and McGrew R:
Treatment of chronic fatigue syndrome and fibromyalgia with
D-ribose-an open-label, multicenter study. Open Pain J. 5:32–37.
2012.
|
|
54
|
Gebhart B and Jorgenson JA: Benefit of
ribose in a patient with fibromyalgia. Pharmacotherapy.
24:1646–1648. 2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Arnaud JP: Cosmetic use of D-ribose and
method thereof. United States Patent Application Publication,
2007.
|
|
56
|
Shecterle LM and St Cyr JA: Dermal
benefits of topical D-ribose. Clin Cosmet Investig Dermatol.
2:151–152. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Griffiths JC, Borzelleca JF and St Cyr J:
Sub-chronic (13-week) oral toxicity study with D-ribose in Wistar
rats. Food Chem Toxicol. 45:144–152. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ismail ZB, Abu-Baker N, Alzoubi K,
Al-Zhgoul M, Al-Essa MK, Khlouf S, Al-Saleh A, Al-Omari B,
Abu-Tayeh R, Shomaf M, et al: Evaluation of α-D-ribofuranose
(D-ribose) toxicity after intravenous administration to rabbits.
Hum Exp Toxicol. 31:820–829. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sinatra ST and Caiazzo C: (D)-Ribose
supplementation in the equine: Lack of effect on glycated plasma
proteins suggesting safety in humans. J Am Coll Nutr. 34:108–112.
2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Peppa M, Uribarri J and Vlassara H:
Glucose, advanced glycation end products, and diabetes
complications: What is new and what works. Clin Diabetes.
21:186–187. 2003.
|
|
61
|
Yamamoto Y and Yamamoto H: Receptor for
advanced glycation end-products-mediated inflammation and diabetic
vascular complications. J Diabetes Investig. 2:155–157.
2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yu L, Chen Y, Xu Y, He T, Wei Y and He R:
D-ribose is elevated in T1DM patients and can be involved in the
onset of encephalopathy. Aging (Albany NY). 11:4943–4969.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Iannuzzi C, Borriello M, Carafa V, Altucci
L, Vitiello M, Balestrieri ML, Ricci G, Irace G and Sirangelo I:
D-ribose-glycation of insulin prevents amyloid aggregation and
produces cytotoxic adducts. Biochim Biophys Acta. 1862:93–104.
2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Javed M, Ahmad MI, Javed H and Naseem S:
D-ribose and pathogenesis of Alzheimer's disease. Mol Biol Rep.
47:2289–2299. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Han C, Lu Y, Wei Y, Liu Y and He R:
D-ribose induces cellular protein glycation and impairs mouse
spatial cognition. PLoS One. 6(e24623)2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wei Y, Chen L, Chen J, Ge L and He RQ:
Rapid glycation with D-ribose induces globular amyloid-like
aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC
Cell Biol. 10(10)2009.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Xu WL, von Strauss E, Qiu CX, Winblad B
and Fratiglioni L: Uncontrolled diabetes increases the risk of
Alzheimer's disease: A population-based cohort study. Diabetologia.
52:1031–1039. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chen L, Wei Y, Wang X and He R:
Ribosylation rapidly induces alpha-synuclein to form highly
cytotoxic molten globules of advanced glycation end products. PLoS
One. 5(e9052)2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wei Y, Chen L and He RQ: D-ribosylated tau
forms globular aggregates with high cytotoxicity. Alzheimer's
Dementia. 5(395)2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wu B, Wei Y, Wang Y, Su T, Zhou L, Liu Y
and He R: Gavage of D-Ribose induces Aβ-like deposits, Tau
hyperphosphorylation as well as memory loss and anxiety-like
behavior in mice. Oncotarget. 6:34128–34142. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wu B, Wang Y, Shi C, Chen Y, Yu L, Li J,
Li W, Wei Y and He R: Ribosylation-derived advanced glycation end
products induce tau hyperphosphorylation through brain-derived
neurotrophic factor reduction. J Alzheimers Dis. 71:291–305.
2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang Y, Shi C, Chen Y, Yu L, Li Y, Wei Y,
Li W and He R: Formaldehyde produced from d-ribose under neutral
and alkaline conditions. Toxicol Rep. 6:298–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Han C, Lu Y, Wei Y, Wu B, Liu Y and He R:
D-ribosylation induces cognitive impairment through RAGE-dependent
astrocytic inflammation. Cell Death Dis. 5(e1117)2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gheith O, Farouk N, Nampoory N, Halim MA
and Al-Otaibi T: Diabetic kidney disease: World wide difference of
prevalence and risk factors. J Nephropharmacol. 5:49–56.
2015.PubMed/NCBI
|
|
75
|
Shevalye H, Maksimchyk Y, Watcho P and
Obrosova IG: Poly(ADP-ribose) polymerase-1 (PARP-1) gene deficiency
alleviates diabetic kidney disease. Biochim Biophys Acta.
1802:1020–1027. 2010.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Shevalye H, Stavniichuk R, Xu W, Zhang J,
Lupachyk S, Maksimchyk Y, Drel VR, Floyd EZ, Slusher B and Obrosova
IG: Poly(ADP-ribose) polymerase (PARP) inhibition counteracts
multiple manifestations of kidney disease in long-term
streptozotocin-diabetic rat model. Biochem Pharmacol. 79:1007–1014.
2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Drel VR, Pacher P, Stevens MJ and Obrosova
IG: Aldose reductase inhibition counteracts nitrosative stress and
poly(ADP-ribose) polymerase activation in diabetic rat kidney and
high-glucose-exposed human mesangial cells. Free Radic Biol Med.
40:1454–1465. 2006.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Trevisan R and Dodesini AR: The
hyperfiltering kidney in diabetes. Nephron. 136:277–280.
2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zeni L, Norden AGW, Cancarini G and Unwin
RJ: A more tubulocentric view of diabetic kidney disease. J
Nephrol. 30:701–717. 2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kumar Pasupulati A, Chitra PS and Reddy
GB: Advanced glycation end products mediated cellular and molecular
events in the pathology of diabetic nephropathy. Biomol Concepts.
7:293–309. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Yuan Y, Sun H and Sun Z: Advanced
glycation end products (AGEs) increase renal lipid accumulation: A
pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis.
16(126)2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yamagishi S and Matsui T: Advanced
glycation end products, oxidative stress and diabetic nephropathy.
Oxid Med Cell Longev. 3:101–108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Hou FF, Ren H, Owen WF Jr, Guo ZJ, Chen
PY, Schmidt AM, Miyata T and Zhang X: Enhanced expression of
receptor for advanced glycation end products in chronic kidney
disease. J Am Soc Nephrol. 5:1889–1896. 2004.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lapolla A, Fedele D, Seraglia R and Traldi
P: The role of mass spectrometry in the study of non-enzymatic
protein glycation in diabetes: An update. Mass Spectrom Rev.
25:775–797. 2006.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zhang N, Zhao S, Hong J, Li W and Wang X:
Protective effects of kaempferol on D-ribose-induced mesangial cell
injury. Oxid Med Cell Longev. 2019(7564207)2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Hong J, Bhat OM, Li G, Dempsey SK, Zhang
Q, Ritter JK, Li W and Li PL: Lysosomal regulation of extracellular
vesicle excretion during d-ribose-induced NLRP3 inflammasome
activation in podocytes. Biochim Biophys Acta Mol Cell Res.
1866:849–860. 2019.PubMed/NCBI View Article : Google Scholar
|