Introduction
Osteoarthritis (OA) is also known as degenerative
arthropathy and proliferative OA. The different names are derived
from the pathological manifestations of arthropathy, which include
both cartilage degeneration and the formation of new bone (1,2). The
clinical manifestations of this disease include joint swelling,
pain, joint effusion and hyper-osteogeny (3). The incidence of OA increases with age
and it is a common joint disease in the elderly population
(4). At present, the pathogenesis
of OA has not been determined. Previous studies have demonstrated
that inflammatory responses are involved in the pathogenesis of OA
and that pathological changes in the synovial structure are similar
to that of rheumatoid arthritis at the early stages of onset
(5-7).
At present, OA is diagnosed mainly through the combination of
symptoms and signs with imaging examination. Therefore, the early
diagnosis of OA, differentiation of the severity and the search for
appropriate markers have become important topics in clinical
research.
C-reactive protein (CRP), a member of the pentraxin
family, is composed of five identical subunits linked by
non-covalent bonds (8). CRP is
widely found in vertebrates and invertebrates, and its structure
and sequence demonstrate high evolutionary conservation, suggesting
that this protein has important biological significance (9). CRP is an acute phase protein whose
plasma concentration rises rapidly when the body is affected by
tissue damage or bacterial infection (10-13).
It has been widely used in clinical practice for the diagnosis and
detection of various infectious and autoimmune diseases (11,14).
CRP not only serves as an inflammatory marker but also serves
numerous important physiological functions in innate immune
responses, such as activating complement pathways or regulating
macrophage phagocytosis through Fcγ receptors to help clear cell
debris and apoptotic cells (15-17).
A previous study indicated that monomeric CRP (mCRP) is an
allosteric active form of CRP involved in the inflammatory process,
while pentameric CRP may serve as a protein library capable of
transforming into mCRP (18). In
the present study, the clinical samples of patients with OA were
collected and the differences and associations between CRP, mCRP
and anti-mCRP autoantibodies in the serum of healthy subjects and
patients were analyzed, with the aim of providing evidence for the
pathogenesis of OA and the use of mCRP as a novel clinical
marker.
Materials and methods
Materials and reagents
Human CRP (cat. no. BP300.X; lot nos. 361639 and
404353) purified from ascites was purchased from the Binding Site.
mCRP was prepared by treating CRP with 8 M urea-EDTA (cat. no.
JT8991-1; lot no. 0000221293; Avantor, Inc.) (19) or by recombinant expression and
purification as previously described (20,21).
Sheep anti-human CRP polyclonal antibody (cat. no. PC044; lot nos.
352325 and 076682) was obtained from the Binding Site. Mouse
anti-human CRP monoclonal antibody (mAb) CRP-8 (cat. no. C1688; lot
no. 025M4863V) was obtained from Sigma-Aldrich; Merck KGaA. Mouse
anti-human CRP mAb 1D6 was generated as previously described
(22,23). HRP-labeled goat anti-mouse IgG
(cat. no. 115-035-003; lot no. 125229) was purchased from Jackson
ImmunoResearch Laboratories, Inc. HRP-labeled donkey anti-sheep IgG
(cat. no. ab6900; lot no. GR272029-6) and HRP-labeled mouse
anti-human IgG (cat. no. ab99757) were both obtained from
Abcam.
Patient selection
A total of 206 patients with OA diagnosed between
January 2016 and July 2018 at the Department of Joint Surgery and
Joint Reconstruction of Xi'an Hong Hui Hospital (Xi'an, China) were
enrolled in the present study. Participants were assessed by
rheumatologists and radiologists. Rheumatoid arthritis or any other
form of inflammatory arthritis (such as crystal arthropathy or
septic arthritis) were considered to be exclusion criteria. A total
of 60 age and sex matched healthy individuals were selected from
the same hospital between November 2017 and July 2018. The
clinicopathological characteristics of patients with OA are listed
in Table I. CRP, mCRP and
anti-mCRP autoantibodies were detected by ELISA while other
clinical features were collected hospital case records. The
demographic data of OA patients and healthy individuals are
included in Table II. The plasma
samples of patients were obtained prior to the initiation of
medical treatment. All samples were stored at -80˚C in aliquots.
Informed consent for blood sample collection was signed by all
participants. The present study followed the guidelines of The
Declaration of Helsinki and was approved (approval no. 202003055)
by the Ethics Committee of the Hong Hui Hospital (Xi'an,
China).
 | Table IClinical characteristics of patients
with osteoarthritis. |
Table I
Clinical characteristics of patients
with osteoarthritis.
| | Value (total
number, mean ± SD or median) |
|---|
| Clinical
characteristics | All OA
patients | OA patients that
were KL graded |
|---|
| Sex | | |
|
Female | 159 | 37 |
|
Male | 47 | 12 |
| Age, years | 66.55±8.16 | 68±8.28 |
| Grade | | |
|
KL3 | 14 | 14 |
|
KL4 | 35 | 35 |
| CRP (µg/ml) | 0.77
(0.37-1.66) | 2.17
(0.61-4.97) |
| mCRP (ng/ml) | 12.51
(7.78-24.81) | 15.95
(8.45-24.1) |
| Anti-mCRP
autoantibody (OD) | 0.52±0.37 | 0.46±0.27 |
| Neutrophil ratio
(%) | 73.51±8.49 | 74.16±6.86 |
| Lymphocyte ratio
(%) | 17.1±6.16 | 16.71±6.15 |
| Monocyte ratio
(%) | 7.1 (5.8-8.3) | 7.05
(6.18-8.23) |
| Uric acid
(µmol/l) | 293.06±70.1 | 301.63±75.63 |
| Urea (mmol/l) | 5.5 (4.5-6.6) | 5.7
(4.57-6.65) |
| Creatinine
(µmol/l) | 58 (50-66.25) | 57
(49.75-66.5) |
| Total cholesterol
(mmol/l) | 4.46±0.94 | 4.47±0.97 |
| Triglyceride
(mmol/l) | 1.38
(1.03-1.84) | 1.4 (1.1-1.76) |
| High density
lipoprotein (mmol/l) | 1.23
(1.09-1.39) | 1.26
(1.13-1.4) |
| Low density
lipoprotein (mmol/l) | 2.85
(2.33-3.3) | 2.77
(2.35-3.29) |
| Apolipoprotein A1
(g/l) | 1.35±0.24 | 1.37±0.22 |
| Apolipoprotein B
(g/l) | 0.88
(0.75-1.04) | 0.84 (0.75-1) |
| Glucose
(mmol/l) | 4.88
(4.44-5.42) | 4.87
(4.41-5.81) |
|
Antistreptococcolysin O (IU/ml) | 34.5 (18-59) | 32 (16.5-60.5) |
| Rheumatoid factor
(IU/ml) | 7.2 (3.25-8.9) | 7.3
(3.17-9.05) |
 | Table IIComparisons of patients with OA and
healthy individuals. |
Table II
Comparisons of patients with OA and
healthy individuals.
| | Value (total number
or mean ± SD or median) | |
|---|
| Clinical
characteristic | OA | Healthy | P-value |
|---|
| Sex | | | - |
|
Female | 159 | 46 | |
|
Male | 47 | 14 | |
| Age, years | 66.55±8.16 | 65.35±5.35 | 0.241447 |
| CRP (µg/ml) | 0.77
(0.37-1.66) | 0.3
(0.04-0.72) |
3.83x10-5 |
| mCRP (ng/ml) | 12.51
(7.78-24.81) | 5.04
(3.45-9.77) |
3.72x10-5 |
| Anti-mCRP
Autoantibody (OD) | 0.52±0.37 | 0.46±0.24 | 0.399173 |
Patient classification
The Kellgren-Lawrence (KL) grading system of knee OA
is the grading method of knee OA severity. According to the X-ray
manifestations of the knee joint, OA severity was divided into
grade 0 (normal knee joint), grade 1,2,3 and 4 (the most serious
knee OA) (24,25) and were described as follows: i)
Grade 0: Knee joint X-ray is completely normal. There are no
manifestations of OA. No joint space stenosis and no reactive bone
changes can be observed. ii) Grade 1: Suspected knee joint space
stenosis. Osteophytes may occur, but only slightly. iii) Grade 2:
Small osteophytes and possible joint space narrowing are
identifiable on the X-ray of a standing knee joint. iv) Grade 3: KL
grade 3 of knee OA is characterized by a large number of moderate
osteophytes, clear narrowing of joint space, certain subchondral
bone sclerosis (increased white area with joint edges is
identifiable via X-ray) and possible knee deformity. v) Grade 4: KL
grade 4 of knee OA is characterized by a large number of large
osteophytes, severe narrowing of joint space, obvious subchondral
bone sclerosis and obvious knee deformity. The patients with OA
mentioned in the present study were all patients who received
systematic evaluation and treatment at hospital. In the early
stages of OA, patients have occasional pain in the knee joint,
which affects exercise but rarely daily life. If patients pay
attention to rest and exercise properly, they can alleviate their
symptoms (26,27). For the aforementioned reasons,
there are no hospitalized patients in the early stage of KL grade 1
and KL grade 2. Therefore, patients with KL grade 1 and KL grade 2
were not included in the present study. KL grade 3 and KL grade 4
patients in the middle and late stage exhibit joint degeneration
and pain aggravation, which seriously affects their daily life,
requiring hospitalization or joint replacement (26,27).
These patients were the main research subjects in the present
study.
ELISA assay quantifying CRP
The sheep anti-human CRP polyclonal antibody was
immobilized onto microtiter wells (1:2,000; cat. no. 42592; lot no.
10917007; Corning, Inc.) in coating buffer (10 mM sodium
carbonate/bicarbonate, pH 9.6) overnight at 4˚C. All the following
steps were conducted at 37˚C, and after each incubation step, wells
were washed three times with TBS (10 mM Tris, 140 mM NaCl, pH 7.4)
containing 0.02% NP-40. Wells were blocked with blocking buffer
[TBS containing 1% bovine serum albumin (cat. no. 0322; LABLEAD)]
for 1 h. Samples diluted in blocking buffer were added into wells
for 1 h. Captured CRP was detected with 1D6 mAb (1:100 in blocking
buffer) that specifically recognizes its native conformation and an
HRP-labeled goat anti-mouse IgG (1:20,000 in blocking buffer).
Wells were incubated with TMB buffer [0.1 mg/ml
3,3,5,5-tetramethylbenzidine (cat. no. 0759; LABLEAD) and 0.02%
H2O2 in 0.1 M Na2HPO4,
0.05 M citric acid, pH 4.5-5.5] for 30 min and stopped with 1 M
H2SO4. The optical density (OD) of samples
were measured at 570 and 450 nm using a microplate reader. The OD
value of each sample was calculated as OD450-OD570 nm. A total of
100 µl volume was used at all incubation steps, while 300 µl volume
was used for washing after each incubation step.
ELISA assay quantifying mCRP
Mouse anti-human CRP mAb CRP-8 was immobilized onto
microtiter wells at 0.3 µl/ml in coating buffer overnight at 4˚C.
All the following steps were conducted at 37˚C, and after each
incubation step, wells were washed three times with TBS containing
0.02% NP-40. Wells were blocked with blocking buffer for 1 h.
Samples diluted in blocking buffer were added into wells for 1 h.
Captured mCRP was detected with a sheep anti-human CRP polyclonal
antibody (1:2,000 in blocking buffer) which can both recognize the
CRP, mCRP and an HRP-labeled donkey anti-sheep IgG (1:20,000 in
blocking buffer). Wells were incubated with TMB buffer for 30 min
and stopped with 1 M H2SO4. Absorbance at
OD570 and OD450 nm was measured with a microplate reader. The OD
value of each sample was calculated as OD450-OD570 nm. A total of
100 µl volume was used at all incubation steps, while 300 µl volume
was used for washing after each incubation step.
ELISA assay quantifying anti-mCRP
autoantibody
The mCRP was immobilized onto microtiter wells at 2
µg/ml in coating buffer overnight at 4˚C. All the following steps
were conducted at 37˚C, and after each incubation step wells were
washed 3 times with TBS containing 0.02% NP-40. Wells were blocked
with blocking buffer for 1 h. Samples diluted in blocking buffer
were added into wells for 1 h. Captured anti-mCRP autoantibody was
detected with an HRP-labeled mouse anti-human IgG (1:20,000 in
blocking buffer). Wells were incubated with TMB buffer for 30 min
and stopped with 1 M H2SO4. Absorbance at
OD570 and OD450 nm was measured with a microplate reader. The OD
value of each sample was calculated as OD450-OD570 nm. A total of
100 µl volume was used at all incubation steps, while 300 µl volume
was used for washing after each incubation step.
Statistical analysis
Each sample was analyzed three times. The mean value
of each sample was used for subsequent analysis. Differences
between groups were all analyzed using the Mann-Whitney U-test.
Correlations were all analyzed using the Pearson's correlation
test. The Univariable logistic regression was used to determine
contributors to KL grades in patients with OA. All analyses were
performed with R 4.0.3 (R Core Team) (28). P<0.05 was considered to indicate
a statistically significant difference.
Results
Difference and correlation analysis of
serum CRP, mCRP and anti-mCRP autoantibody
The demographic and clinical data of patients are
summarized in Table I and
continuous characteristics are presented as the mean ± SD or median
(interquartile range). Overall comparisons of patients with OA and
healthy individuals are summarized in Table II. The differences in plasma
levels of CRP, mCRP and anti-mCRP autoantibody between healthy
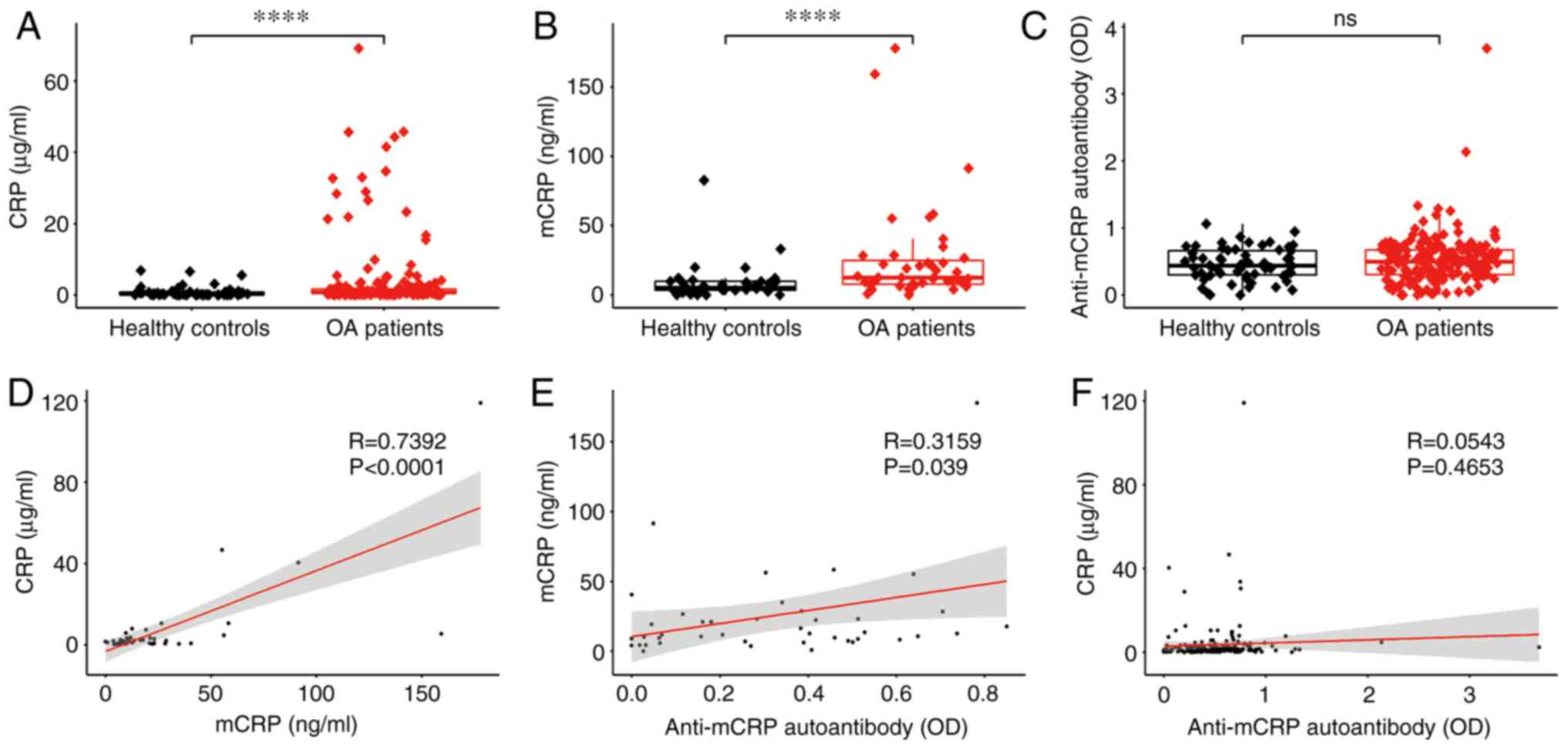
subjects and patients with OA are revealed in Fig. 1. The level of plasma CRP in
patients with OA was significantly higher than that in healthy
individuals (Fig. 1A), as well as
the level of plasma mCRP (Fig.
1B). No significant difference in anti-mCRP autoantibody
between patients with OA and healthy subjects was identified
(Fig. 1C). The relationships among
CRP, mCRP and anti-mCRP autoantibody are also illustrated in
Fig. 1. CRP was strongly
correlated with mCRP (Fig. 1D),
probably due to mCRP being produced by the depolymerization of
pentamer CRP at the inflammatory loci in the lesion area. mCRP was
correlated with anti-mCRP autoantibody (Fig. 1E) most likely due to the fact that
anti-mCRP autoantibody is generated by mCRP stimulation. No
correlation was found between CRP and anti-mCRP autoantibody
(Fig. 1F), which can be explained
by CRP and anti-mCRP autoantibody not being directly related. In
order to further investigate the correlation of CRP and mCRP with
the risk of OA, receiver operating characteristic (ROC) curve
analyses was performed (Fig. 2).
The area under the CRP curve was 0.69, and the area under the mCRP
curve was 0.76, which demonstrated that mCRP has improved
predictive performance.
Differences in CRP, mCRP, anti-mCRP
autoantibody and clinical features between KL grades in patients
with OA
Images of different KL grades are presented in
Fig. 3A and B. Compared with those with KL grade 3,
patients with KL grade 4 had a narrower articular cavity and more
evident joint deformities. CRP and mCRP were revealed to be
significantly higher in KL grade 4 than in KL grade 3 (Fig. 3C and D). These findings suggested that CRP and
mCRP can be used as indicators of disease severity. There was no
significant difference in anti-mCRP autoantibody between KL grades
(Fig. 3E), suggesting that
anti-mCRP autoantibody is not associated with the severity of OA. A
significant difference in creatinine level between KL grades was
also observed (Fig. 3F), while all
other clinical features are not significantly different (data not
shown). Univariate logistic regression was performed (Table III) and the results confirmed
that CRP and mCRP are significantly associated with KL grades. This
finding suggested that CRP and mCRP may be contributors to OA
pathogenesis.
 | Figure 3Differences in CRP, mCRP, anti-mCRP
autoantibody and the clinical features between KL grades in
patients. Typical diagnostic images of (A) KL3 and (B) KL4 grades
in patients with OA. Plasma concentrations of (C) CRP, (D) mCRP,
(E) anti-mCRP autoantibody and (F) creatinine in patients with OA
with KL grades. *P<0.05 as indicated. CRP, C-reactive
protein; mCRP, monomeric CRP; KL, Kellgren-Lawrence; OA,
osteoarthritis; OD, optical density; R, right-hand side; ns, not
significant. |
 | Table IIIUnivariate analysis of
Kellgren-Lawrence grades of patients with osteoarthritis. |
Table III
Univariate analysis of
Kellgren-Lawrence grades of patients with osteoarthritis.
| Clinical
feature | P-value | OR | CI95 lower
limit | CI95 upper
limit |
|---|
| Sex | 0.753 | 1.269 | 0.307 | 6.534 |
| Age | 0.161 | 1.057 | 0.980 | 1.148 |
| CRP | 0.018 | 2.342 | 1.312 | 5.386 |
| mCRP | 0.021 | 1.034 | 1.009 | 1.069 |
| Autoantibody | 0.227 | 0.206 | 0.013 | 2.488 |
| Neutrophils
Ratio | 0.295 | 1.051 | 0.958 | 1.156 |
| Lymphocytes
Ratio | 0.227 | 0.939 | 0.843 | 1.039 |
| Monocytes
Ratio | 0.845 | 1.030 | 0.773 | 1.421 |
| Uric Acid | 0.447 | 1.003 | 0.995 | 1.013 |
| Urea | 0.326 | 1.248 | 0.825 | 2.029 |
| Creatinine | 0.077 | 1.049 | 1.002 | 1.115 |
| Total
Cholesterol | 0.318 | 1.422 | 0.732 | 3.007 |
| Triglyceride | 0.822 | 0.923 | 0.466 | 1.972 |
| High Density
Lipoprotein | 0.569 | 2.321 | 0.871 | 49.317 |
| Low Density
Lipoprotein | 0.431 | 1.369 | 0.656 | 3.250 |
| Apolipoprotein
A1 | 0.602 | 2.215 | 0.119 | 54.015 |
| Apolipoprotein
B | 0.536 | 2.480 | 0.161 | 58.439 |
| Glucose | 0.523 | 0.844 | 0.488 | 1.466 |
|
Antistreptococcolysin O | 0.720 | 0.997 | 0.983 | 1.013 |
| Rheumatoid
Factor | 0.438 | 1.022 | 0.993 | 1.127 |
Associations of CRP, mCRP and
anti-mCRP autoantibody with age and sex in patients
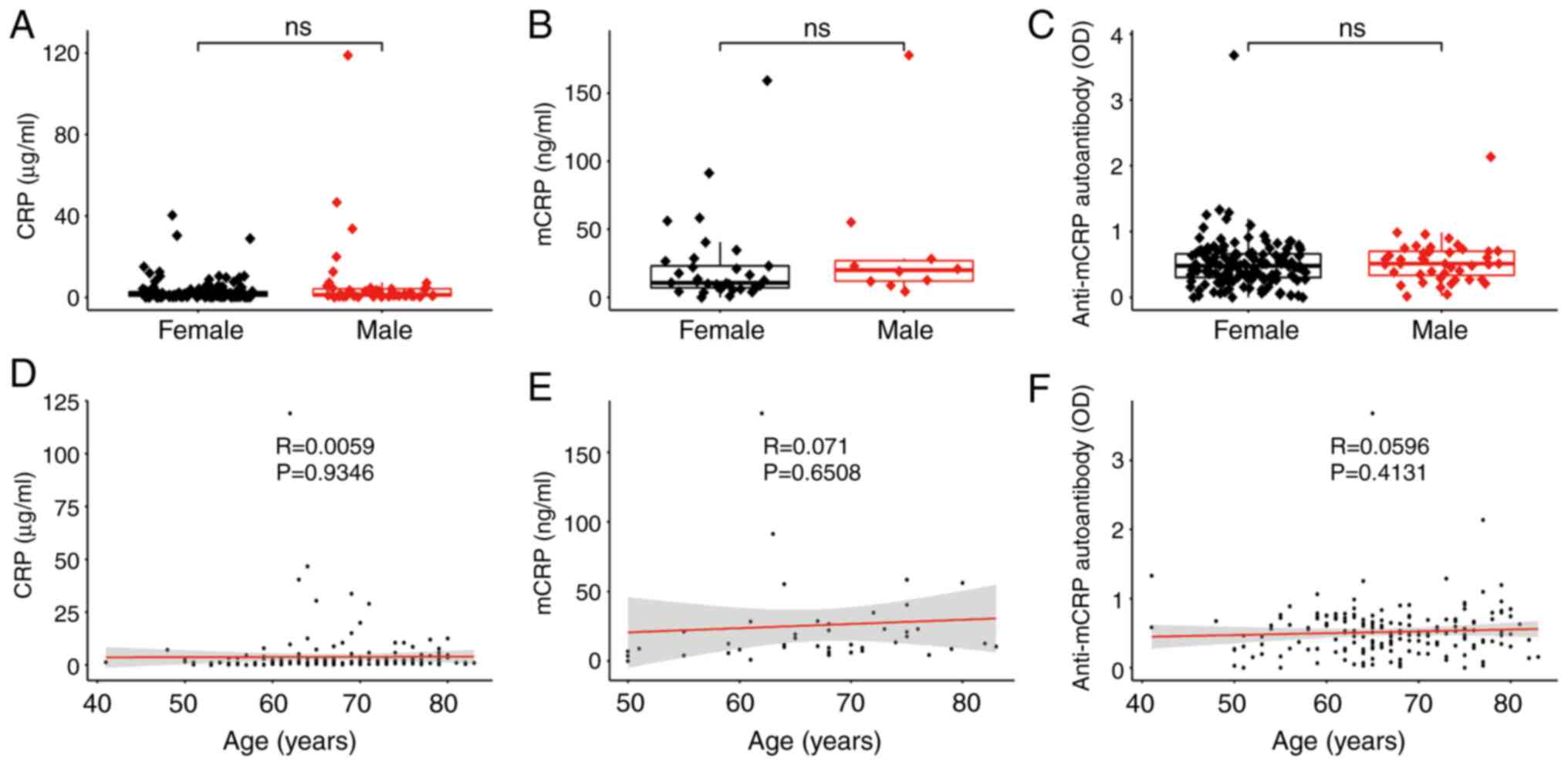
As it can be observed in Fig. 4, the differences in CRP, mCRP and
anti-mCRP autoantibody between sex are all not significant
(Fig. 4A-C). Thus, the patients
were not grouped according to sex for further subgroup analysis.
Since the correlation of CRP, mCRP and anti-mCRP autoantibody with
age was also not significant (Fig.
4D-F), the patients were again not grouped according to their
age.
Discussion
According to a survey performed by the World Health
Organization, the incidence of OA has reached ~10% in people aged
>60 years (29). At present,
the diagnosis of OA mainly depends on imaging methods. Therefore,
the development of convenient early methods of diagnosis for OA is
a direction worthy of study.
CRP is an important marker of the inflammatory
response, which increases rapidly in the acute stage of infection
and tissue injury (10-13,30).
Studies have also revealed that CRP was directly involved in the
development of certain diseases. In myocardial infarction, for
example, CRP mediates tissue damage mainly by activating complement
pathways (31,32). In addition, mCRP is the monomeric
form of CRP and the main active conformation of local tissues
(33-35).
Furthermore, anti-mCRP autoantibody is generated by mCRP
stimulation. Previous studies have determined that anti-mCRP
autoantibody is associated with the degree of injury and prognosis
of patients (36,37). To study the relationship between
CRP, mCRP, anti-mCRP autoantibody and OA, CRP, mCRP and anti-mCRP
autoantibody were selected as observation objects to detect the
difference and correlation between patients and healthy controls.
Significant differences in plasma CRP and mCRP levels between
patients and healthy controls and KL grades were identified,
suggesting that they may be involved in the development of OA and
could be used as severity markers of OA. There was a difference
between CRP and mCRP, due to CRP being a non-specific inflammatory
marker protein secreted by the liver and has a high concentration
in plasma; mCRP is considered to play a role by depolymerizing
pentamer CRP into monomer form in the local tissue of the lesion,
and in addition, most of mCRP still resident in the local tissue
(18,38), so the concentration of mCRP in
blood is low. In clinical application, it is easier to detect CRP
with higher concentration by ELISA; However, compared with CRP,
mCRP may be a more sensitive indicator in a specific disease.
Combined with the ROC curve, mCRP has higher accuracy and
prediction sensitivity.
The mCRP is the monomer form of pentamer CRP and
inflammatory conditions in tissues can promote the dissociation of
CRP to mCRP (39). In the process
of dissociating to mCRP, CRP can expose certain new antigen
epitopes, giving mCRP biological activities different from the
pentameric protein. Previous studies have demonstrated that mCRP
has a stronger biological effect. For example, mCRP, the main
isomer form of CRP, can bind with natural and modified low density
lipoprotein (40) and regulate
complement activation more effectively (41). Furthermore, mCRP has a stronger
activation effect on endothelial cells (7) and neutrophils (42), which means that mCRP formed by the
structural rearrangement of CRP is a favorable complement of CRP
function. These findings suggested that mCRP is the dominant form
of CRP, functioning under pathological and physiological
conditions. The present experimental results delineated that there
was a strong correlation between serum mCRP and CRP in OA and that
there were significant differences in mCRP between patients and
healthy controls, as well as in different stages of OA, suggesting
that CRP in OA may be involved in the development of the disease
through dissociation into mCRP. The mostly commonly investigated
pathways associated with OA are the Wnt, Notch, NF-κB,
PI3K/Akt/mTOR and OPG/RANK/RANKL pathways (43-53).
It has been revealed that mCRP can activate the NF-κB signaling
pathway in mouse and human chondrocytes and induce the expression
of proinflammatory cytokines (54). Boras et al (55) determined that mCRP can co-activate
PI3K signaling pathway with Notch-3. A previous study demonstrated
that mCRP induced osteoclast differentiation by binding to RANKL
(56). The aforementioned studies
demonstrated that the NF-κB, PI3K/Akt/mTOR and OPG/RANK/RANKL
pathways may be associated with mCRP function.
In conclusion, the present results revealed that
there are significant differences in the levels of CRP and mCRP
between patients with OA and healthy controls, and in different
disease stages. Thus, CRP and mCRP may be important serological
markers for the early diagnosis and disease evaluation of OA.
Additionally, it was also identified in the present study that CRP
is significantly correlated with mCRP in OA, which is consistent
with previous studies that CRP plays a vital role in diseases
through dissociation into mCRP (18,39).
However, whether mCRP is directly involved in the pathogenesis of
OA needs further investigation. The present study may have certain
limitations, which are reflected in the relatively small sample
size and lack of KL1 and KL2 patients. Further expanding the number
of patients with KL grades can provide an improved basis and
reliability for follow-up research. Whether the combined analysis
with other serum markers of OA, such as cartilage oligomeric matrix
protein or C-telopeptide fragments of type II collagen, can improve
the accuracy of association and prediction remains to be studied in
the future. At present, there is no validation of the mCRP in
animal models and related mechanism research. In future, using OA
animal models can further investigate whether mCRP regulates the
pathogenesis of OA. The present study suggested that mCRP may be
used as a novel biomarker to help the early diagnosis and severity
evaluation of OA and also lays a foundation for in-depth
exploration of the pathogenesis of OA, particularly with respect to
the role played by mCRP, and future clinical research for
therapeutic targets.
Acknowledgements
The authors would like to thank Professor Yi Wu
(Xi'an Jiaotong University) for his constructive advice regarding
data analysis and manuscript writing.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 31800654) and the
Fundamental Research Funds for the Central Universities (grant no.
sxzy012019076).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and PX designed the research. YL, KX and WL
performed the experiments. XL and PY analyzed the data and wrote
the article. HL, PX and YL confirm the authenticity of all the raw
data. All authors reviewed the results and read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study followed the guidelines of The
Declaration of Helsinki and was approved (approval no. 202003055)
by the ethics committee of the Hong Hui Hospital (Xi'an, China).
Informed consent was obtained from all subjects involved in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ringdahl E and Pandit S: Treatment of knee
osteoarthritis. Am Fam Physician. 83:1287–1292. 2011.PubMed/NCBI
|
|
2
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Towheed TE, Maxwell L, Anastassiades TP,
Shea B, Houpt J, Robinson V, Hochberg MC and Wells G: Glucosamine
therapy for treating osteoarthritis. Cochrane Database Syst Rev.
2005(CD002946)2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Uebelhart D, Malaise M, Marcolongo R, de
Vathaire F, Piperno M, Mailleux E, Fioravanti A, Matoso L and
Vignon E: Intermittent treatment of knee osteoarthritis with oral
chondroitin sulfate: A one-year, randomized, double-blind,
multicenter study versus placebo. Osteoarthritis Cartilage.
12:269–276. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu-Bryan R: Synovium and the innate
inflammatory network in osteoarthritis progression. Curr Rheumatol
Rep. 15(323)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Du Clos TW: Pentraxins: Structure,
function, and role in inflammation. ISRN Inflamm.
2013(379040)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pepys MB and Hirschfield GM: C-reactive
protein: A critical update. J Clin Invest. 111:1805–1812.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Gabay C and Kushner I: Acute-phase
proteins and other systemic responses to inflammation. N Engl J
Med. 340:448–454. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Medzhitov R: Recognition of microorganisms
and activation of the immune response. Nature. 449:819–826.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schwedler SB, Filep JG, Galle J, Wanner C
and Potempa LA: C-reactive protein: A family of proteins to
regulate cardiovascular function. Am J Kidney Dis. 47:212–222.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Casas JP, Shah T, Hingorani AD, Danesh J
and Pepys MB: C-reactive protein and coronary heart disease: A
critical review. J Intern Med. 264:295–314. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Verma S, Devaraj S and Jialal I: Is
C-reactive protein an innocent bystander or proatherogenic culprit?
C-reactive protein promotes atherothrombosis. Circulation.
113:2135–2150; discussion 2150. 2006.PubMed/NCBI
|
|
15
|
Bharadwaj D, Stein MP, Volzer M, Mold C
and Du Clos TW: The major receptor for C-reactive protein on
leukocytes is fcgamma receptor II. J Exp Med. 190:585–590.
1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Marjon KD, Marnell LL, Mold C and Du Clos
TW: Macrophages activated by C-reactive protein through Fc gamma RI
transfer suppression of immune thrombocytopenia. J Immunol.
182:1397–1403. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Y, Lee PY, Sobel ES, Narain S, Satoh M,
Segal MS, Reeves WH and Richards HB: Increased expression of
FcgammaRI/CD64 on circulating monocytes parallels ongoing
inflammation and nephritis in lupus. Arthritis Res Ther.
11(R6)2009.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Wu Y, Potempa LA, El Kebir D and Filep JG:
C-reactive protein and inflammation: Conformational changes affect
function. Biol Chem. 396:1181–1197. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Potempa LA, Maldonado BA, Laurent P, Zemel
ES and Gewurz H: Antigenic, electrophoretic and binding alterations
of human C-reactive protein modified selectively in the absence of
calcium. Mol Immunol. 20:1165–1175. 1983.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Potempa LA, Yao ZY, Ji SR, Filep JG and Wu
Y: Solubilization and purification of recombinant modified
C-reactive protein from inclusion bodies using reversible anhydride
modification. Biophys Rep. 1:18–33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li HY, Wang J, Meng F, Jia ZK, Su Y, Bai
QF, Lv LL, Ma FR, Potempa LA, Yan YB, et al: An intrinsically
disordered motif mediates diverse actions of monomeric C-reactive
protein. J Biol Chem. 291:8795–8804. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ying SC, Gewurz H, Kinoshita CM, Potempa
LA and Siegel JN: Identification and partial characterization of
multiple native and neoantigenic epitopes of human C-reactive
protein by using monoclonal antibodies. J Immunol. 143:221–228.
1989.PubMed/NCBI
|
|
23
|
Ying SC, Shephard E, de Beer FC, Siegel
JN, Harris D, Gewurz BE, Fridkin M and Gewurz H: Localization of
sequence-determined neoepitopes and neutrophil digestion fragments
of C-reactive protein utilizing monoclonal antibodies and synthetic
peptides. Mol Immunol. 29:677–687. 1992.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kohn MD, Sassoon AA and Fernando ND:
Classifications in brief: Kellgren-lawrence classification of
osteoarthritis. Clin Orthop Relat Res. 474:1886–1893.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Petersson IF, Boegard T, Saxne T, Silman
AJ and Svensson B: Radiographic osteoarthritis of the knee
classified by the Ahlback and Kellgren & Lawrence systems for
the tibiofemoral joint in people aged 35-54 years with chronic knee
pain. Ann Rheum Dis. 56:493–496. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Macri EM, Runhaar J, Damen J, Oei EH and
Bierma-Zeinstra SM: Kellgren & Lawrence grading in cohort
studies: Methodological update and implications illustrated using
data from the CHECK cohort. Arthritis Care Res (Hoboken). Jan 15,
2021. (Epub ahead of print). doi: 10.1002/acr.24563.
|
|
27
|
Emrani PS, Katz JN, Kessler CL, Reichmann
WM, Wright EA, McAlindon TE and Losina E: Joint space narrowing and
Kellgren-Lawrence progression in knee osteoarthritis: An analytic
literature synthesis. Osteoarthritis Cartilage. 16:873–882.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
The R Core Team: R: A language and
environment for statistical computing. Reference index. https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf.
Accessed October 10, 2020.
|
|
29
|
Woolf AD and Pfleger B: Burden of major
musculoskeletal conditions. Bull World Health Organ. 81:646–656.
2003.PubMed/NCBI
|
|
30
|
Li HY, Liu XL, Liu YT, Jia ZK, Filep JG,
Potempa LA, Ji SR and Wu Y: Matrix sieving-enforced retrograde
transcytosis regulates tissue accumulation of C-reactive protein.
Cardiovasc Res. 115:440–452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pepys MB, Hirschfield GM, Tennent GA,
Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD,
Polara A, et al: Targeting C-reactive protein for the treatment of
cardiovascular disease. Nature. 440:1217–1221. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Griselli M, Herbert J, Hutchinson WL,
Taylor KM, Sohail M, Krausz T and Pepys MB: C-reactive protein and
complement are important mediators of tissue damage in acute
myocardial infarction. J Exp Med. 190:1733–1740. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Diehl EE, Haines GK III, Radosevich JA and
Potempa LA: Immunohistochemical localization of modified C-reactive
protein antigen in normal vascular tissue. Am J Med Sci. 319:79–83.
2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schwedler SB, Amann K, Wernicke K, Krebs
A, Nauck M, Wanner C, Potempa LA and Galle J: Native C-reactive
protein increases whereas modified C-reactive protein reduces
atherosclerosis in apolipoprotein E-knockout mice. Circulation.
112:1016–1023. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ullah N, Ma FR, Han J, Liu XL, Fu Y, Liu
YT, Liang YL, Ouyang H and Li HY: Monomeric C-reactive protein
regulates fibronectin mediated monocyte adhesion. Mol Immunol.
117:122–130. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li QY, Li HY, Fu G, Yu F, Wu Y and Zhao
MH: Autoantibodies against C-reactive protein influence complement
activation and clinical course in lupus nephritis. J Am Soc
Nephrol. 28:3044–3054. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tan Y, Yu F, Yang H, Chen M, Fang Q and
Zhao MH: Autoantibodies against monomeric C-reactive protein in
sera from patients with lupus nephritis are associated with disease
activity and renal tubulointerstitial lesions. Hum Immunol.
69:840–844. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yao Z, Zhang Y and Wu H: Regulation of
C-reactive protein conformation in inflammation. Inflamm Res.
68:815–823. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL,
Lu W and Zhao J: Cell membranes and liposomes dissociate C-reactive
protein (CRP) to form a new, biologically active structural
intermediate: mCRP(m). FASEB J. 21:284–294. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ji SR, Wu Y, Potempa LA, Qiu Q and Zhao J:
Interactions of C-reactive protein with low-density lipoproteins:
Implications for an active role of modified C-reactive protein in
atherosclerosis. Int J Biochem Cell Biol. 38:648–661.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ji SR, Wu Y, Potempa LA, Liang YH and Zhao
J: Effect of modified C-reactive protein on complement activation:
A possible complement regulatory role of modified or monomeric
C-reactive protein in atherosclerotic lesions. Arterioscler Thromb
Vasc Biol. 26:935–941. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Khreiss T, Jozsef L, Potempa LA and Filep
JG: Loss of pentameric symmetry in C-reactive protein induces
interleukin-8 secretion through peroxynitrite signaling in human
neutrophils. Circ Res. 97:690–697. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fernandez-Torres J, Zamudio-Cuevas Y,
Lopez-Reyes A, Garrido-Rodríguez D, Martínez-Flores K, Lozada CA,
Muñóz-Valle JF, Oregon-Romero E and Martínez-Nava GA: Gene-gene
interactions of the Wnt/β-catenin signaling pathway in knee
osteoarthritis. Mol Biol Rep. 45:1089–1098. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Fan X, Xing L and Tian F: Wnt
signaling: A promising target for osteoarthritis therapy. Cell
Commun Signal. 17(97)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu Z, Ren Y, Mirando AJ, Wang C, Zuscik
MJ, O'Keefe RJ and Hilton MJ: Notch signaling in postnatal joint
chondrocytes, but not subchondral osteoblasts, is required for
articular cartilage and joint maintenance. Osteoarthritis
Cartilage. 24:740–751. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu Z, Chen J, Mirando AJ, Wang C, Zuscik
MJ, O'Keefe RJ and Hilton MJ: A dual role for NOTCH signaling in
joint cartilage maintenance and osteoarthritis. Sci Signal.
8(ra71)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Choi MC, Jo J, Park J, Kang HK and Park Y:
NF-κB signaling pathways in osteoarthritic cartilage destruction.
Cells. 8(734)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang LB, Man ZT, Li W, Zhang W, Wang XQ
and Sun S: Calcitonin protects chondrocytes from
lipopolysaccharide-induced apoptosis and inflammatory response
through MAPK/Wnt/NF-κB pathways. Mol Immunol. 87:249–257.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Musumeci G, Castrogiovanni P, Trovato FM,
Weinberg AM, Al-Wasiyah MK, Alqahtani MH and Mobasheri A:
Biomarkers of chondrocyte apoptosis and autophagy in
osteoarthritis. Int J Mol Sci. 16:20560–20575. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xue JF, Shi ZM, Zou J and Li XL:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of
articular chondrocytes and attenuates inflammatory response in rats
with osteoarthritis. Biomed Pharmacother. 89:1252–1261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Koura HM, Zaki SM, Ismail NA, Salama EE,
El Lebedy DH and Effat LK: Relationship between biochemical bone
markers and bone mineral density in patients with phenylketonuria
under restricted diet. Iran J Pediatr. 24:23–28, Epub 2013 Dec 31.
2014.PubMed/NCBI
|
|
52
|
Liu Y, Ge J, Chen D, Weng Y, Du H, Sun Y
and Zhang Q: Osteoprotegerin deficiency leads to deformation of the
articular cartilage in femoral head. J Mol Histol. 47:475–483.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kovacs B, Vajda E and Nagy EE: Regulatory
effects and interactions of the Wnt and OPG-RANKL-RANK signaling at
the bone-cartilage interface in osteoarthritis. Int J Mol Sci.
20(4653)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sproston NR and Ashworth JJ: Role of
C-reactive protein at sites of inflammation and infection. Front
Immunol. 9(754)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Boras E, Slevin M, Alexander MY, et al:
Monomeric C-reactive protein and Notch-3 co-operatively increase
angiogenesis through PI3K signalling pathway. Cytokine. 69:165–179.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jia ZK, Li HY, Liang YL, Potempa LA, Ji SR
and Wu Y: Monomeric C-reactive protein binds and neutralizes
receptor activator of NF-κB ligand-induced osteoclast
differentiation. Front Immunol. 9(234)2018.PubMed/NCBI View Article : Google Scholar
|