Introduction
Stroke is among the most important diseases that
endanger human life and health in the world today, which affects 15
million people per year in the world, causing 5 million deaths and
5 million cases of disability (1).
Stroke is the number one cause of death in China, with an
increasing incidence of 8.7%/year and direct economic losses of
more than CN¥100 billion annually (2-4).
Central poststroke pain (CPSP) occurs after ischemic or hemorrhagic
stroke and is lesion-related, with continuous or intermittent pain
accompanied by paresthesia. CPSP is one of the central neuropathic
pain syndromes and one of the severe sequelae of stroke (5). While epidemiologic reports have
revealed that hemorrhagic strokes represent only 8-18% of all
strokes, they contribute to higher mortality rates than ischemic
stroke (6). Approximately 8-14% of
patients who have experiences a stroke suffer from CPSP,
particularly after hemorrhagic stroke (7). At present, there is a significant
lack of understanding of the pathogenesis of CPSP (8). Understanding the underlying mechanism
of hemorrhagic-induced thalamic pain could offer new approaches for
managing this disorder.
The inflammasome is a complex composed of multiple
proteins, mainly consisting of caspase-1, apoptosis-associated
speck-like protein containing a CARD (ASC) and NOD-like receptor
(NLR). The inflammasome regulates the body's innate immune system
and senses microorganisms, metabolites and stress responses
(9). At present, the NLR family
pyrin domain containing 3 (NLRP3) inflammasome, which is present in
the cytoplasm and can detect and recognize intracellular microbial
infection or sterile inflammation caused by other molecules, is the
most thoroughly studied inflammasome (10). A large number of endogenous and
exogenous substances can cause NLRP3 inflammasome oligomerization
and caspase-1 activation, which therefore stimulates the secretion
and maturation of IL-18 and IL-1β that participate in the
regulation of the body's inflammatory response (11). Over the past few years, the
association between the inflammasome and pain related to the
production of proinflammatory cytokines has received increasing
attention from the medical community. Previous studies have
suggested that inflammasome dysfunction is closely related to
complex regional pain syndromes (12), inflammatory headaches (13), gouty arthritis (14), lumbar disc herniation (15) and spinal cord pain (16).
MicroRNAs (miRNAs/miRs) are endogenous small
noncoding RNAs with a size of ~22 nucleotides. miRNAs serve
important roles in essential biological activities, including cell
proliferation, differentiation and apoptosis, and can be paired
with the 3'untranslated region (UTR) of mRNA target genes to
negatively regulate the transcription process (17). Cumulative evidence suggests that
the expression of a considerable number of miRNAs in the nervous
system is differentially regulated during the development of
neuropathic pain (18,19).
miR-223 is most highly expressed in bone marrow
cells and negatively expressed in several illnesses, including
inflammation, lymphoma, leukemia, influenza and hepatitis B
(20). Previously, studies have
reported that miR-223 can impede inflammation to prevent collateral
impairment (21,22). Moreover, NLRP3 mRNA is a confirmed
target of miR-223(23). One study
demonstrated that miR-223 can negatively target NLRP3, which
stimulates the production of specific macrophages, and miR-223 is
currently considered a regulatory molecule that participates in the
development of inflammatory responses (24). However, none of the aforementioned
previous studies have discussed whether miR-223 targeting of NLRP3
is involved in CPSP.
In the present study, the function of miR-223 in
thalamic hemorrhage-induced CPSP processing in the central nervous
system (CNS) was investigated. Furthermore, to provide innovative
insight into the molecular mechanisms of CPSP, whether miR-223
directly interacts with and regulates NLRP3 inflammasome expression
was further explored.
Materials and methods
Animals
The research protocol and animal experiments were
approved by the Animal Care and Use Committee of the Medical
College of Yangzhou University (Yangzhou, China; approval no.
SYXK2017-0044) and conformed to the standards for animal use and
care formulated by the Government of China (25). All experiments were carried out
according to the protocol of the International Association for Pain
Research (26). A total of 148 CD1
male mice (age, ~7-8 weeks,weight, ~25-30 g) were purchased from
the Comparative Medical Center of Yangzhou University. All the mice
were held in captivity in animal facilities and were maintained
under a basic cycle of 12-h light/dark cycles, temperature (23±1˚C)
and humidity (50±5%) with free access to food and water.
Appropriate efforts were made to minimize suffering and only a
small number of animals were used. To reduce variability within and
between individuals in the measurement of behavior outcomes, the
mice were trained to perform the behavioral test for 1-2 days
before the experiment. For the behavioral tests, the experimenter
did not know the treatment conditions. After the experiment, the
animals were euthanized. Euthanasia was performed by cervical
dislocation.
Hemorrhage-induced thalamic pain
model
Isoflurane (5% induction; 2% maintenance) was
administered to anesthetize the mice, which were then laid in a
stereotactic frame. Collagenase Ⅳ (Coll IV; 0.01 U/10 nl, dissolved
in saline solution; Sigma-Aldrich; Merck KGaA) was injected into
the right ventral posterior medial (VPM) and ventral posterior
lateral (VPL) nuclei of the thalamus (3.01-4.25 mm on the ventral
side of the skull surface, posterior 1.30-1.95 mm on the lateral
side of the midline and anterior-posterior to bregma 0.82-2.30 mm)
under the guidance of stereotactic orientation (27) using a glass micropipette. The sham
operation cohort was injected with 10 nl sterile physiological
saline. Following administration, the glass micropipette was held
in position for 10 min to enable the Coll IV to fully disperse and
then the glass micropipette was gradually removed. Following
microinjection, iodophor and sterile saline were used to perfuse
the surgical area, which was later stitched with a wound clip.
NLRP3-small interfering (si)RNA
microinjection
A total of 2 µl NLRP3-siRNA (160 µM;
5'-GTACTTAAATCGTGAAACA -3'; Guangzhou RiboBio Co., Ltd.) solution
was diluted using 1 µl sterile 20% glucose solution (4X), mixed
gently and spun down briefly. Subsequently, 1 µl of TurboFect In
vivo Transfection Reagent (Thermo Fisher Scientific, Inc.) was
added to the diluted NLRP3-siRNA solution and mixed immediately by
pipetting. The sample was incubated for 15-20 min at room
temperature and then incubated on ice. The mixed NLRP3-siRNA sample
(500 nl; 20 µM) was microinjected into the VPM/VPL nuclei of the
thalamus with a glass micropipette connected to a microsyringe
pump, as aforementioned. Sequences for NLRP3-siRNA and the
corresponding negative control are presented in Table I.
 | Table ISequences for NLRP3-siRNA and the
corresponding negative control. |
Table I
Sequences for NLRP3-siRNA and the
corresponding negative control.
| Gene | Sequence
(5'-3') |
|---|
| NLRP3- | F:
CCUGGAAGACAUAGACUUUTT |
| siRNA | R:
AAAGUCUAUGUCUUCCAGGTT |
| Negative | F:
UUCUCCGAACGUGUCACGUTT |
| control-siRNA | R:
ACGUGACACGUUCGGAGAATT |
miR-223 agomir and antagomir
microinjection
CY 09 (Bio-Techne), was used to determine whether
thalamic pain mimicked by the miR-223 antagomir could be rescued
and to offer a reference for the treatment of thalamic pain. CY 09
(10 mg/kg) or vehicle (equal volume of saline) was pre-administered
via the tail vein 30 min before microinjection of miR-223 antagomir
into the unilateral thalamus. Subsequently, CY 09 (10 mg/kg) or
vehicle was administered via the tail vein every day until day 7.
miR-223 agomir (5'-UGUCAGUUUGUCAAAUACCCCA-3') and its scrambled
negative control (5'-UCGUUUUUACACGAUCACGGUUU-3'), and miR-223
antagomir (5'-UGGGGUAUUUGACAAACUGACA-3') and its scrambled negative
control (5'-GAUCCUCGGUCCUAGUAGUUA-3') were synthesized by Shanghai
GenePharma Co., Ltd. Prior to thalamus microinjection, the samples
were mixed with Invivofectamine® 3.0 Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The mixed miR-223
agomir, antagomir or control samples (500 nl; 20 µM) were
introduced into the thalamus using a glass micropipette linked to a
microsyringe pump. The micropipette was detached 10 min after
administration. The surgical area was washed using sterile saline
and the cut was stitched.
Behavioral tests
Pain behavior tests, including cold, thermal and
mechanical tests, were performed. First, the claw withdrawal
frequency in response to mechanical stimulation was assessed as
previously described (28). In
brief, the mice were kept alone in a plexiglass compartment on a
raised screen and allowed to adapt for 30 min. Two-adjusted von
Frey filaments (calibrated at 0.07 and 0.4 g; Stoelting Co.) were
utilized to rouse the hind paw for ~1-2 sec and this was
repetitively performed 10 times at an interval of 5 min between the
two hind paws. Quick withdrawal of the claws was regarded as a
positive response. The withdrawal response of the claw for each of
the 10 stimuli was quantified as a percentage of response
frequency: (Sum of claw withdrawal times/10 trials) x100% =
response frequency.
Subsequently, an Analgesia Meter (model, 336; IITC
Life Science Inc.) was used to assess claw retraction latency to
heat as previously described (28-31).
In brief, mice were kept in a Plexiglas compartment on a glass
plate. The beam radiated from the lightbox and spread to the
midpoint of the sole surface of each of the hind paws. Rapid
lifting of the rear claw was considered a gesture for turning off
the light. The duration of illumination beam time was considered
the claw latency time. In each case, five replicates were conducted
with an interval of 5 min. A 20 sec stoppage time was utilized to
prevent tissue damage.
Finally, the claw withdrawal latency for harmful
cold (0˚C) was measured utilizing a cold aluminum plate as
previously described (28-31).
In brief, each mouse was kept in a Plexiglas compartment on a flat
plate and the temperature was continuously monitored using a
thermometer. The time interval between placement and the mouse jump
sign was considered to be the claw jump delay. Because over time,
mice gradually tolerate cold stimulation, each of the experiments
was replicated in triplicate at 10-min intervals, as previously
described (32). A 20 sec stoppage
time was utilized to prevent tissue damage.
At 30 min post-completion of the pain behavior
tests, the motor function test was performed, investigating
placement, grip and righting reflex, as described previously
(23). For placement reflexes, the
hind legs were placed somewhat lower than the forelimbs, while the
back of the hind paws touched the periphery of the bench. Then,
whether the rear paw was reflexively positioned on the desktop was
recorded. For grip reflex, the animal was laid on a wire grill and
whether the hind claw grasped the line was noted. The animal was
laid on his back on a horizontal plane for the righting reflex to
record whether the mouse could instantly turn to the correct
upright position. All the tests were replicated five times at 5-min
intervals and the results were logged by computing the number of
regular reflex actions/test.
Reverse transcription-quantitative PCR
(RT-qPCR)
For RT-qPCR, total RNA was extracted from the
thalamus using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and RT was performed using ThermoScript
Reverse Transcriptase (Thermo Fisher Scientific, Inc.). RT was
performed in accordance with previously published reports (33,34).
Amplification of the template (4 µl) was performed via qPCR. Each
sample was run in triplicate in a 20 µ reaction with 250 nM forward
and reverse primers, 10 µl SsoAdvanced Universal SYBR Green
Supermix (Bio-Rad Laboratories), and 20 ng cDNA. PCR reactions were
performed with an initial 3-min incubation at 95˚C, followed by 40
cycles at 95˚C for 10 sec, 60˚C for 30 sec, and 72˚C for 30 sec in
a Bio-Rad CFX96 real-time PCR system. The primers are presented in
Table II. U6 was employed as an
internal control for normalization. Three samples of 20 µl each
were analyzed. A 7500 Fast Real-Time PCR Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to perform
qPCR. The 2-ΔΔCq method was used to quantify expression
levels (35).
 | Table IISequence of primers (mouse) used for
reverse transcription-quantitative PCR. |
Table II
Sequence of primers (mouse) used for
reverse transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| U6 | F:
GCTTCGGCAGCACATATACTAAAAT |
| | R:
CGCTTCACGAATTTGCGTGTCAT |
| miR-223 | F:
CGCTCCGTGTATTTGACAAGC |
| | R:
AGCCACACTTGGGGTATTTGA |
Western blotting
Western blotting was performed as previously
reported (33,34,36).
Briefly, following protein concentration quantification by BCA
assay, tissue from the thalamus were homogenized with ice-cold
lysis buffer (10 mM Tris, 5 mM EGTA, 0.5% Triton X-100, 2 mM
benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, 40 µM leupeptin,
150 mM NaCl). The crude homogenate was centrifuged at 4˚C for 15
min at 1,000 g. The supernatants were collected for cytoplasmic
protein detection. The pellets were further sonicated and dissolved
in nucleus-soluble ice-cold buffer (1 M Tris-HCl, 1% SDS, and 0.1%
Triton X-100). After the protein concentration was measured, total
protein was heated for 5 min at 99˚C. Subsequently, SDS-PAGE using
a 10% gel was performed to separate proteins, with 30 mg
protein/well. Separated proteins were transferred to a PVDF
membrane via wet transfer. The membrane was blocked with 5% skimmed
milk in TBS with 0.1% Tween-20 for 1 h. Subsequently, membranes
were incubated overnight at 4˚C with the following primary
antibodies: rabbit anti-caspase-1 (1:1,000; Cell Signaling
Technology, Inc. cat. no. 24232), rabbit anti-ASC (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 67824), rabbit anti-NLRP3
(1:1,000; Cell Signaling Technology, Inc. cat. no. 15101), rabbit
anti-IL-18 (1:1,000; Abcam; cat. no. ab243091), mouse anti-IL-1β
(1:1,000; Cell Signaling Technology, Inc. cat. no. 63124), rabbit
anti-phosphorylated (p)-ERK1/2 (1:2,000; Cell Signaling Technology,
Inc. cat. no. 4370), rabbit anti-ERK1/2 (1:2,000; Cell Signaling
Technology, Inc. cat. no. 4695), rabbit anti-GAPDH (1:5,000; Cell
Signaling Technology, Inc. cat. no. 2118) and mouse anti-glial
fibrillary acidic protein (GFAP; 1:2,000; Cell Signaling
Technology, Inc. cat. no. 3670). Following the primary incubation
membranes were incubated with goat HRP-conjugated anti-rabbit or
anti-mouse antibody (1:3,000; Jackson ImmunoResearch Laboratories,
Inc. cat. nos. 115-005-003, 111-005-003). TBS with 0.1% Tween-20
was used as washing reagent. The secondary antibody was incubated
for 1-2 h at room temperature. Proteins were visualized using
western peroxide reagent, Clarity Western ECL Substrate (Bio-Rad
Laboratories, Inc.) and a ChemiDoc XRS system (Bio-Rad
Laboratories, Inc.) with Image Lab 4.0 software (Bio-rad
Laboratories, Inc.). Semi-quantification of band density was
performed using ImageJ 1.8 software (National Institutes of
Health).
Dual-luciferase reporter assay
The possible binding site between NLRP3 and miR-223
was identified using the online forecasting software TargetScan 7.2
(http://www.targetscan.org/mmu_72/).
PC-12 cells were cultured with high-glucose DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 5% FBS (Gibco; Thermo Fisher
Scientific, inc.) and 1% gentamicin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were incubated at 37˚C with 5%
CO2. PC-12 cells with a fusion rate of 60-70% were
transfected with a luciferase reporter plasmid and miR-223 agomir
or negative control (agomir scramble) (both Shanghai GenePharma
Co., Ltd.). A plasmid vector encoding a mutant locus of NLRP3
(NLRP3 mut) for miR-223 binding and a plasmid vector encoding the
wild-type locus of NLRP3 (NLRP3 wt) for miR-223 binding were
designed and constructed. The NLRP3-3'-UTR primer sequences were
synthesized by Shanghai GenePharma Co., Ltd. and are presented in
Table III. Following sequence
verification by Sanger sequencing, PC-12 cells were co-transfected
with the NLRP3 mut plasmid (80 ng) or NLRP3 wt plasmid and the
miR-223 agomir or negative control using Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Luciferase activity was
quantified as the ratio of firefly to Renilla luciferase activity,
which was evaluated using a Luciferase Assay System (Promega
Corporation) following 48 h of transfection. The relative
luciferase activity is presented as the ratio of the assessed
luciferase activity to that of the Renilla control.
 | Table IIISequences of primers for NLRP3-3'UTR
for the dual-luciferase reporter assay. |
Table III
Sequences of primers for NLRP3-3'UTR
for the dual-luciferase reporter assay.
| Primer | Sequence
(5'-3') |
|---|
| Forward |
ACCTCAACAGTCGCTACACG |
| Reverse |
TAGACTCCTTGGCGTCCTGA |
Statistical analysis
The mice were randomly allocated to different
experimental cohorts. Data are presented as the mean ± SD. One-way
ANOVA and two-way ANOVA were used to statistically compare the
data. If the ANOVA results revealed significant differences, paired
comparisons between the mean values were assessed using Tukey's
post hoc test. All data were analyzed using SigmaPlot 12.5 (Systat
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-223 expression levels decrease and
NLRP3/ASC/caspase-1 protein expression levels increase following
Coll Ⅳ-induced thalamic pain
Following the establishment of the
hemorrhage-induced thalamic pain model, the results demonstrated
that the hemorrhage was localized predominantly around the VPL and
VPM of the thalamus, without extending into the internal capsule.
No bleeding was observed in other brain regions following
microinjection of Coll IV at the dose and volume used. As expected,
microinjection of saline into the thalamus did not result in
significant bleeding and a normal thalamic structure was
exhibited.
Thalamus hemorrhage mice displayed intense and
persistent abnormal mechanical pain, hyperalgesia and abnormal cold
contralateral pain. The claw retraction frequency using 0.07 g and
0.4 g von Frey filaments (Fig. 1A
and B) increased significantly
compared with the saline control. Moreover, the claw retraction
latency for heat (Fig. 1C) and
cold (Fig. 1D) stimulation
decreased significantly on the contralateral side following Coll IV
microinjection into the thalamus compared with the saline control.
These pain reactions occurred 1 day following microinjection and
lasted for a minimum of 14 days following Coll IV microinjection.
Saline microinjection had no effect on the frequency or latency of
contralateral base claw contraction. Furthermore, neither Coll IV
nor saline microinjection changed the frequency or latency of
ipsilateral claw retraction (Fig.
1E-G).
 | Figure 1Hemorrhage-induced thalamic pain
decreases miR-223 expression levels and activates the NLRP3
inflammasome. Microinjection of Coll IV, rather than saline, into
the ventral posterior lateral and ventral posterior medial nuclei
contributed to a significant increase in paw withdrawal frequency
in response to (A) 0.07 g and (B) 0.4 g von Frey filaments and a
significant decrease in paw withdrawal latency to (C) thermal and
(D) cold stimuli on the contralateral side of the mice. (E-G) Both
saline and Coll IV were injected into the ventral posterior lateral
nuclei and ventral posterior medial nuclei, which did not change
the ipsilateral side behavior. n=8 mice/group. (H) miR-223
expression levels were significantly reduced at day 1, 3, 7 and 14
days in the Coll IV-treated cohort. (I-L) The NLRP3 inflammasome
was activated and caspase-1, ASC and NLRP3 were significantly
elevated at day 1, 3, 7 and 14 in the Coll IV-treated group. n=3
mice/group. *P<0.05 vs. saline group at the
corresponding time points. miR, microRNA; NLRP3, NLR family pyrin
domain containing 3; Coll IV, collagenase IV; ASC,
apoptosis-associated speck-like protein containing a CARD. |
Subsequently, whether miR-223 and NLRP3 inflammasome
proteins were altered in the thalamus following hemorrhage-induced
thalamic pain was investigated. In mice, hemorrhage-induced
thalamic pain time-dependently induced a significant reduction in
miR-223 expression levels (Fig.
1H) and a significant increase in NLRP3/ASC/caspase-1 protein
expression levels (Fig. 1I-L) in
the thalamus on the ipsilateral side of mice that received Coll IV
compared with the saline group. Therefore, miR-223 expression
levels decrease and NLRP3/ASC/caspase-1 protein expression levels
increase following Coll Ⅳ-induced thalamic pain.
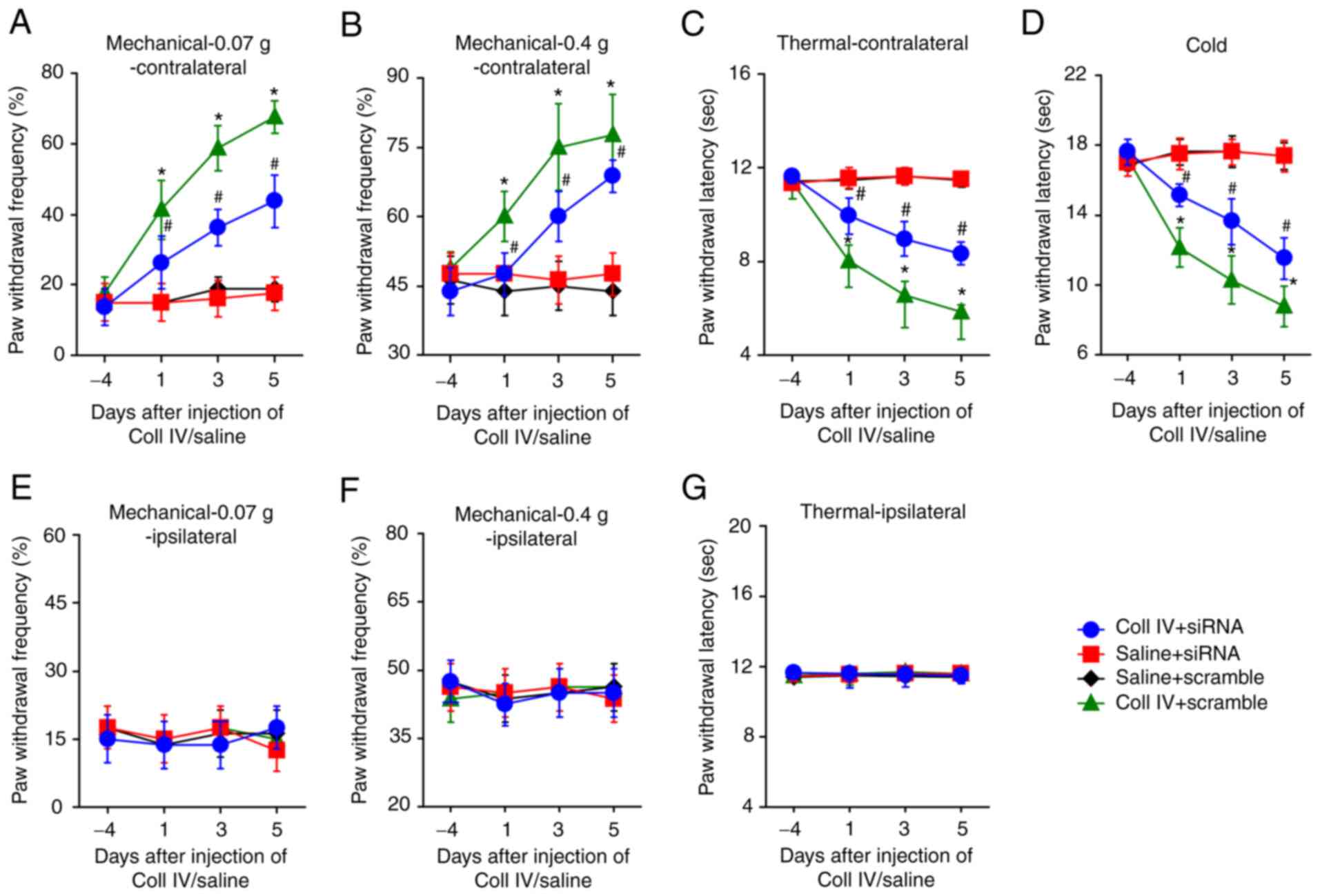
NLRP3-siRNA microinjection attenuates
Coll Ⅳ-induced thalamic pain
Subsequently, whether NLRP3-siRNA microinjection
into the ipsilateral thalamus could attenuate thalamus
hemorrhage-induced pain was investigated. siRNA microinjection was
performed 3 days prior to saline or Coll IV microinjection at the
same coordinate points of the ipsilateral thalamus. The results
demonstrated that thermal hyperalgesia, mechanical allodynia and
cold allodynia significantly developed at day 1, 3 and 5 following
Coll IV microinjection on the contralateral side of the thalamus in
Coll IV + scramble group compared with the saline + siRNA group
(Fig. 2A-D). Pretreatment with
NLRP3-siRNA via microinjection significantly attenuated the
increase in paw withdrawal frequency in response to 0.07 g and 0.4
g von Frey filaments and reduced the paw withdrawal latency to heat
and cold at 1, 3 and 5 days following Coll IV microinjection on the
contralateral side in the Coll IV + siRNA compared with the Coll IV
+ scramble group. Moreover, microinjection of NLRP3-siRNA had no
effect on the basal PWF or PWL on the ipsilateral side in the Coll
IV + siRNA group (Fig. 2E-G) or on
both the contralateral and ipsilateral sides of the saline + siRNA
group throughout the experiment. Therefore, NLRP3-siRNA
microinjection attenuates Coll IV-induced thalamic pain.
NLRP3-siRNA microinjection decreases
NLRP3 inflammasome activation
Following the behavioral tests, brain tissues were
obtained for western blotting. The results demonstrated that
NLRP3/ASC/caspase-1 protein expression levels in the ipsilateral
thalamus were significantly elevated in the Coll IV + scramble
group compared with the saline + scramble group (Fig. 3A). However, NLRP3/ASC/caspase-1
protein expression levels were significantly reduced in the Coll IV
+ siRNA group compared with the Coll IV + scramble group.
Furthermore, the protein expression levels of the
inflammatory factors, IL-18 and IL-1β, in the ipsilateral thalamus
were investigated. The results demonstrated that IL-18 and IL-1β
protein expression levels were significantly elevated in the Coll
IV + scramble group compared with the saline + scramble group
(Fig. 3B). However, IL-1β and
IL-18 protein expression levels were significantly reduced in the
Coll IV + siRNA group compared with the Coll IV + scramble
group.
The western blotting results also demonstrated that
GFAP, a marker for astrocyte hyperactivation, and p-ERK1/2, a
marker for central sensitization, were significantly elevated in
the ipsilateral thalamus in the Coll IV + scramble group compared
with the saline + scramble group (Fig.
3C). However, this was significantly reversed in the Coll IV +
siRNA group compared with the Coll IV + scramble group. Therefore,
NLRP3-siRNA microinjection decreases NLRP3 inflammasome
activation.
miR-223 agomir microinjection
attenuates Coll Ⅳ-induced thalamic pain
Subsequently, whether microinjection of miR-223
agomir into the ipsilateral thalamus could attenuate thalamus
hemorrhage-induced pain was examined. miR-223 agomir microinjection
was performed 3 days prior to saline or Coll IV microinjection at
similar coordinate points of the ipsilateral thalamus. The results
demonstrated that thermal hyperalgesia, mechanical allodynia and
cold allodynia significantly developed at day 1, 3 and 5 following
Coll IV microinjection on the contralateral side in the Coll IV +
agomir scramble group compared with the saline + agomir-scramble
group (Fig. 4A-D).
Pre-administration of miR-223 agomir via microinjection
significantly attenuated the increase in PWF in response to 0.07 g
and 0.4 g von Frey filaments and the reduction in PWL to heat and
cold stimuli at day 1, 3 and 5, in the Coll IV + miR-233 agomir
group compared with the Coll IV + agomir-scramble group.
Furthermore, microinjection of the miR-223 agomir had no effect on
the basal PWF and PWL on the ipsilateral side in the Coll IV +
miR-223 agomir group (Fig. 4E-G),
or on both the contralateral and ipsilateral sides in the saline +
miR-223 agomir group throughout the experiments. Therefore, miR-223
agomir microinjection attenuates Coll Ⅳ-induced thalamic pain.
miR-223 reduces NLRP3 inflammasome
activation by binding to the NLRP3-3'-UTR
To examine the possible molecular mechanism involved
in miR-223 regulation of thalamic pain, bioinformatic analysis was
performed to identify direct target genes of miR-223. The results
indicated that the NLRP3-3'-UTR contained a conserved miR-223
binding site and an RNA mutant sequence of the 3'-UTR of NLRP3 was
indicated in the alignment (Fig.
5A). The interplay between the 3'-UTR of NLRP3 and miR-223 was
confirmed by co-transfecting PC-12 cells with the NLRP3 wt plasmid
or NLRP3 mut plasmid and miR-223 agomir or agomir scramble using
Lipofectamine 2000 transfection reagent. The results from the
dual-luciferase reporter assay indicated that miR-223 significantly
reduced the luciferase activity of the wt 3'-UTR compared with the
miR-233 group but had no effect on the mut 3'-UTR of NLRP3. These
results therefore validated that NLRP3 may be a target gene of
miR-223 (Fig. 5B).
 | Figure 5miR-223 targets the NLRP3 3'UTR and
affects the protein expression levels of caspase-1, ASC, NLRP3,
IL-18, IL-1β, p-ERK1/2 and GFAP. (A) Binding site of miR-223 within
the NLRP3 3'-UTR. (B) miR-223 agomir significantly reduced the
relative luciferase activity in PC12 cells transfected with the
NLRP3 3'-UTR. (C) miR-223 agomir significantly increased miR-223
expression levels. (D) Administration of miR-223 agomir
significantly reduced the protein expression levels of caspase-1,
ASC and NLRP3. (E) miR-223 agomir also significantly reduced the
expression of IL-18 and IL-1β. (F) After microinjection of miR-223
agomir, p-ERK1/2 and GFAP protein expression levels significantly
decreased. n=3 mice/cohort. *P<0.05 vs. saline +
agomir-scramble; #P<0.05 vs. Coll IV +
agomir-scramble. miR, microRNA; NLRP3, NLR family pyrin domain
containing 3; UTR, untranslated region; GFAP, glial fibrillary
acidic protein; p, phosphorylated; ASC, apoptosis-associated
speck-like protein containing a CARD; wt, wild-type; mut,
mutant. |
Moreover, the results demonstrated that miR-223
expression levels were significantly lower in the agomir-scramble +
Coll IV group compared with the agomir-scramble + saline group
(Fig. 5C). Furthermore, miR-223
expression levels were significantly elevated in the ipsilateral
thalamus in both the miR-223 agomir + Coll IV- or saline-treated
cohort compared with the agomir-scramble + Coll IV and
agomir-scramble + saline groups, respectively. Subsequently, the
protein expression levels of activated caspase-1, ASC and NLRP3 in
the thalamus of thalamic pain model mice, pretreated with miR-223
agomir or agomir scramble on the day 5 following surgery, were
evaluated via western blotting. The results demonstrated that
caspase-1, ASC and NLRP3 protein expression levels were
significantly increased in the thalamus in the Coll IV +
agomir-scramble group compared to the saline + agomir scramble
group (Fig. 5D). Moreover,
overexpression of miR-223 (Coll IV + agomir), compared with the
Coll IV + agomir-scramble group, significantly reduced the
caspase-1, ASC and NLRP3 protein expression levels in the thalamus.
The IL-18 and IL-1β protein expression levels in the thalamus were
also assessed using western blot analysis. The results demonstrated
that the protein expression levels of both cytokines were
significantly elevated in the Coll IV + agomir-scramble group
compared with the saline + agomir-scramble group (Fig. 5E). However, IL-18 and IL-1β protein
expression levels were significantly reduced in the Coll IV +
agomir group compared to the Coll IV + agomir-scramble group. These
results were further verified by the interplay between NLRP33 and
miR-223, which illustrated that miR-223 may negatively regulate
NLRP3 expression and thereby inhibit inflammatory activity
(caspase-1, ASC and NLRP3) and cytokine maturation.
It was also demonstrated that GFAP and p-ERK1/2
protein expression levels were significantly higher in the Coll IV
+ agomir-scramble group compared with saline + agomir-scramble
group (Fig. 5F). However, the high
expression levels of GFAP and p-ERK1/2 were significantly reversed
by miR-223 overexpression in the Coll IV + agomir group compared
with the Coll IV + agomir-scramble group. These findings indicated
that miR-223 may negatively regulate central sensitization and
astrocyte hyperactivation. Therefore, miR-223 reduces NLRP3
inflammasome activation by binding to the NLRP3-3'-UTR.
miR-223 antagomir mimics thalamic pain
and activates the NLRP3 inflammasome
It was then determined whether mimicking thalamic
pain through a decrease in miR-223 via microinjection of the
miR-223 antagomir into the unilateral thalamus altered the
nociceptive thresholds in naïve mice. The results demonstrated that
miR-223 expression levels in the microinjected thalamus from
miR-223 antagomir-injected mice were significantly decreased
compared with naïve + antagomir scramble group (Fig. 6H). Microinjection of miR-223
antagomir, but not scramble antagomir, produced significantly
augmented paw withdrawal responses to cold, heat and mechanical
stimuli on the contralateral side compared with the naïve group
(Fig. 6A-D). On the ipsilateral
side, the basal paw withdrawal responses did not changed in either
the miR-223 antagomir-microinjected or scramble
antagomir-microinjected mice (Fig.
6E-G).
The results also demonstrated that the NLRP3
inflammasome was activated, with significantly elevated
NLRP3/ASC/caspase-1 protein expression levels in the miR-223
antagomir-microinjected compared with the naïve group, but not in
the antagomir scramble-microinjected mice (Fig. 6I). Furthermore, the IL-18 and IL-1β
protein expression levels were significantly increased in the
miR-223 antagomir-microinjected mice compared with the
scramble-microinjected mice (Fig.
6J). p-ERK1/2 and GFAP protein expression levels were also
demonstrated to be significantly upregulated following miR-223
antagomir microinjection into the thalamus compared with the naïve
group (Fig. 6K). Therefore,
miR-223 antagomir mimics thalamic pain and activates the NLRP3
inflammasome.
Mimicked thalamic pain induced by the
miR-223 antagomir is rescued by an NLRP3 inflammasome inhibitor
In the present study it had been demonstrated that
miR-223 targeted the NLRP3 3'-UTR and negatively regulated NLRP3.
To determine whether a decrease in miR-223 expression levels
produced thalamic pain by activating the NLRP3 inflammasome, a
specific NLRP3 inflammasome inhibitor (37). Vehicle pretreatment 30 min before
microinjection of miR-223 antagomir still yielded significantly
increased paw withdrawal responses to cold, heat and mechanical
stimuli on the contralateral side compared with the naïve group
(Fig. 7A-D). However, this
behavioral change was significantly reversed following CY 09
administration when compared with the naïve + miR-223 antagomir +
vehicle group. These results suggested that CY 09 pretreatment 30
min before miR-223 antagomir microinjection significantly
attenuated paw withdrawal responses to cold, heat and mechanical
stimuli on the contralateral side. On the ipsilateral side, the
basal paw withdrawal responses did not change in any cohort
(Fig. 7E-G).
Subsequently, whether CY 09 could influence the
IL-18, IL-1β, GFAP and p-ERK1/2 protein expression levels was
investigated. The results first determined that miR-223 expression
levels were significantly reduced in the miR-223 antagomir
microinjection groups, compared with the naïve group, but not in
the antagomir scramble microinjection cohorts. Moreover, CY 09 did
not affect the expression of miR-223 (Fig. 8A). It was demonstrated that in the
miR-223 antagomir mimic thalamic pain groups, IL-18 and IL-1β
protein expression levels were significantly reduced with CY 09
pretreatment compared to vehicle + miR-233 antagomir group
(Fig. 8B). These results suggested
that CY 09 may specifically inhibit inflammasome activity and
subsequently reduce IL-18 and IL-1β protein expression levels.
Furthermore, it was determined that GFAP and p-ERK1/2 protein
expression levels were also considerably reduced in the miR-223
antagomir + CY 09 pretreatment group compared with the miR-223
antagomir + vehicle pretreatment group (Fig. 8C). These findings indicated that
activation of the NLRP3 inflammasome may contribute to central
sensitization and astrocyte hyperactivation. Therefore, mimicked
thalamic pain induced by the miR-223 antagomir is rescued by an
NLRP3 inflammasome inhibitor.
Discussion
Thalamic hemorrhagic stroke is a common
cerebrovascular event with severe consequences for CPSP patients
(38). Evidence has confirmed that
inflammation and the immune response participate in the
pathophysiology of hemorrhagic stroke and activated glial cells
accumulate at the site of bleeding injury (39). As a severe sequela of stroke, CPSP
currently lacks effective treatments due to its unknown mechanism.
Therefore, it is important to explore the underlying mechanism of
CPSP. In the present study, a CPSP model was induced by thalamic
hemorrhage using Coll IV injection with using the research method
from a previous study as reference (27). The results demonstrated that
thalamic hemorrhagic stroke, resulting from microinjection of Coll
IV into the VPL nucleus, led to pain hypersensitivities, such as
thermal hyperalgesia, mechanical allodynia and cold allodynia in a
mouse model, which may imitate thalamic pain arising from
hemorrhagic stroke in the clinic. Comprehension of the underlying
mechanism of hemorrhage-induced thalamic pain allows for the
development of innovative therapeutic strategies for preventing and
treating the disease. The present study also indicated that
astrocyte activation (detected via GFAP) may occur in the Coll
IV-induced CPSP model. The results demonstrated that miR-223 and
the NLRP3 inflammasome participated in the development of thalamic
hemorrhagic stroke-induced pain. miR-223 expression levels were
significantly decreased in the CPSP model, and injection of a
miR-223 agomir significantly attenuated thalamic pain (including
mechanical, thermal and cold sensitivity) and significantly lowered
proinflammatory cytokine (IL-18 and IL-1β) protein expression
levels. However, microinjection with the miR-223 antagomir into the
VPL nucleus of naïve mice mimicked thalamic pain and significantly
increased proinflammatory cytokine (IL-18 and IL-1β) protein
expression levels. The present study also indicated that miR-223
may ease CPSP by targeting the NLRP3 inflammasome signaling
cascade.
Previous studies have reported that miRNAs have a
vital function in neuropathic pain (40-42).
For example, miR-195 upregulation occurs in spinal microglia of
rats with spinal nerve ligation and exacerbates neuropathic pain by
impeding autophagy following peripheral nerve injury (43). However, the role of miRNAs in CPSP
are still unknown. miR-223 has been extensively examined and been
found to contribute to vital functions in numerous diseases,
including cancer (44,45) and inflammatory diseases (46). Moreover, miR-223 has a critical
function in the nervous system. Cressatti et al (47) demonstrated that the salivary
miR-223 level is considerably reduced in patients with Parkinson's
disease. However, the association between miR-223 and CPSP remains
to be elucidated. In the present study, it was demonstrated that
miR-223 has a suppressive function by targeting NLRP3 in mice with
CPSP. The results determined that miR-223 expression levels were
significantly reduced following thalamic hemorrhage, overexpression
of miR-223 significantly ameliorated CPSP and inhibition of miR-223
in naïve mice mimicked CPSP. These results indicated that the
downregulation of miR-223 after thalamic hemorrhage may contribute
to CPSP development. These findings indicated that miR-223 may
inhibit CPSP by inhibiting NLRP3-triggered neuroinflammation,
therefore offering fresh insight into the molecular pathogenesis of
CPSP.
Among all NLR inflammasomes, NLRP3 is the most
comprehensively understood because it has been widely studied in
respect to certain diseases, such as inherited autoinflammatory
syndromes (48) and certain
metabolic diseases (49).
Recently, NLRP3 inflammasome function has been studied in the CNS
and the NLRP3 inflammasome has been found to contribute to crucial
functions in the development of bacterial meningitis and
Alzheimer's disease (50). In the
present study, the NLRP3 inflammasome proteins ASC and NLRP3, as
well as the downstream factors caspase-1, IL-18 and IL-1β, were
significantly elevated in the thalamus of CPSP mice. Furthermore,
the results demonstrated that by blocking NLRP3 inflammasome
activity or expression pain hypersensitivities, such as cold
allodynia, mechanical allodynia and thermal hyperalgesia, were
significantly attenuated. These findings indicated that CPSP may
induce NLRP3 inflammasome activation and subsequent cleavage of
IL-18 and IL-1β. Thalamus injection of miR-223 agomir 3 days before
Coll IV injection significantly ameliorated thalamus
hemorrhage-induced CPSP, such as cold allodynia, mechanical
allodynia and thermal hyperalgesia. The analgesic effects of
overexpressed miR-223 were potentially associated with a
significant reduction in caspase-1, ASC, NLRP3, IL-18 and IL-1β
protein expression levels. Furthermore, the results of the
dual-luciferase reporter assay indicated that miR-223 may target
the 3'UTR of NLRP3 mRNA to inhibit NLRP3 expression. The rescue
experiment performed in the present study demonstrated that
mimicking CPSP via miR-223 inhibition in naïve mice was
significantly reversed by administration of an NLRP3 inhibitor.
These results indicated that miR-223 downregulation may result in
CPSP via the upregulation of NLRP3. Further, it was also
demonstrated that CPSP induced the activation of astrocytes and
neuronal damage. Microinjection of the miR-223 agomir into the
thalamus significantly reversed this result, likely due to
mediation of the role of the miR-223 agomir by neurons and the
activation of astrocytes by injured neurons. Together, these
results suggested that miR-223 and NLRP3 inflammasomes may
participate in the pathogenesis of CPSP.
IL-1β is produced as an antecedent, pro-IL-1β, which
then must be cleaved by caspase-1 to become biologically active
(51). The present study
demonstrated that the NLRP3 inflammasome, the best-characterized
inflammasome activated by cellular stress or infection, led to CPSP
development via induction of IL-1β cleavage. miR-223 overexpression
significantly inhibited this signaling pathway and may therefore
serve as an efficacious analgesic in pain management.
In summary, the experimental data in the present
study demonstrated that miR-223 inhibited the activity of the NLRP3
inflammasome (caspase-1, ASC and NLRP3), which ameliorated thalamus
hemorrhage-induced CPSP in mice via the downregulation of NLRP3.
However, the mechanisms of NLRP3 and miR-223 in CPSP occurrence,
development and prognosis require further validation because
miR-223 expression at the cellular level was not ascertained in the
present study.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The National Natural
Science Foundation of China (grant nos. 81571936 and 81601679).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG conceived the project and supervised all
experiments. TH, CW, YZ and JG designed the project. XC and YL
performed the molecular and biochemical experiments. YX constructed
the animal models, performed behavioral tests and revised the
manuscript. YG analyzed the data. TH wrote the manuscript draft. JG
edited the manuscript. TH and YX confirm the authenticity of all
the raw data. All authors have read, discussed and approved the
final manuscript.
Ethics approval and consent to
participate
The research protocol and animal experiments were
approved by the Animal Care and Use Committee of the Medical
College of Yangzhou University (Yangzhou, China; approval no.
SYXK2017-0044).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maida CD, Norrito RL, Daidone M,
Tuttolomondo A and Pinto A: Neuroinflammatory mechanisms in
ischemic stroke: Focus on cardioembolic stroke, background, and
therapeutic approaches. Int J Mol Sci. 21(6454)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Paolucci S, Iosa M, Toni D, Barbanti P,
Bovi P, Cavallini A, Candeloro E, Mancini A, Mancuso M, Monaco S,
et al: Prevalence and time course of post-stroke pain: A
multicenter prospective hospital-based study. Pain Med. 17:924–930.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harrison RA and Field TS: Post stroke
pain: Identification, assessment, and therapy. Cerebrovasc Dis.
39:190–201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Klit H, Finnerup NB and Jensen TS: Central
post-stroke pain: Clinical characteristics, pathophysiology, and
management. Lancet Neurol. 8:857–868. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vukojevic Z, Dominovic Kovacevic A, Peric
S, Grgic S, Bjelica B, Basta I and Lavrnic D: Frequency and
features of the central poststroke pain. J Neurol Sci. 391:100–103.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Feigin VL, Lawes CM, Bennett DA and
Anderson CS: Stroke epidemiology: A review of population-based
studies of incidence, prevalence, and case-fatality in the late
20th century. Lancet Neurol. 2:43–53. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kumar G and Soni CR: Central post-stroke
pain: Current evidence. J Neurol Sci. 284:10–17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kumar B, Kalita J, Kumar G and Misra UK:
Central poststroke pain: A review of pathophysiology and treatment.
Anesth Analg. 108:1645–1657. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leemans JC, Cassel SL and Sutterwala FS:
Sensing damage by the NLRP3 inflammasome. Immunol Rev. 243:152–162.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li WW, Guo TZ, Liang D, Shi X, Wei T,
Kingery WS and Clark DJ: The NALP1 inflammasome controls cytokine
production and nociception in a rat fracture model of complex
regional pain syndrome. Pain. 147:277–286. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen L, Li X, Huang L, Wu Q, Chen L and
Wan Q: Chemical stimulation of the intracranial dura activates
NALP3 inflammasome in trigeminal ganglia neurons. Brain Res.
1566:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Smith HS, Bracken D and Smith JM: Gout:
Current insights and future perspectives. J Pain. 12:1113–1129.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang A, Wang K, Ding L, Bao X, Wang X,
Qiu X and Liu J: Bay11-7082 attenuates neuropathic pain via
inhibition of nuclear factor-kappa B and nucleotide-binding
domain-like receptor protein 3 inflammasome activation in dorsal
root ganglions in a rat model of lumbar disc herniation. J Pain
Res. 10:375–382. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qian J, Zhu W, Lu M, Ni B and Yang J:
D-β-hydroxybutyrate promotes functional recovery and relieves pain
hypersensitivity in mice with spinal cord injury. Br J Pharmacol.
174:1961–1971. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu J: Control of protein synthesis and
mRNA degradation by microRNAs. Curr Opin Cell Biol. 20:214–221.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sakai A, Saitow F, Miyake N, Miyake K,
Shimada T and Suzuki H: miR-7a alleviates the maintenance of
neuropathic pain through regulation of neuronal excitability.
Brain. 136:2738–2750. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leinders M, Üçeyler N, Pritchard RA,
Sommer C and Sorkin LS: Increased miR-132-3p expression is
associated with chronic neuropathic pain. Exp Neurol. 283:276–286.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gilicze AB, Wiener Z, Tóth S, Buzás E,
Pállinger É, Falcone FH and Falus A: Myeloid-derived microRNAs,
miR-223, miR27a, and miR-652, are dominant players in myeloid
regulation. BioMed Res Int. 2014(870267)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang J, Bai X, Song Q, Fan F, Hu Z, Cheng
G and Zhang Y: miR-223 inhibits lipid deposition and inflammation
by suppressing toll-like receptor 4 signaling in macrophages. Int J
Mol Sci. 16:24965–24982. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cardoso AL, Guedes JR and de Lima MC: Role
of microRNAs in the regulation of innate immune cells under
neuroinflammatory conditions. Curr Opin Pharmacol. 26:1–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bauernfeind F, Rieger A, Schildberg FA,
Knolle PA, Schmid-Burgk JL and Hornung V: NLRP3 inflammasome
activity is negatively controlled by miR-223. J Immunol.
189:4175–4181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haneklaus M, Gerlic M, Kurowska-Stolarska
M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA and
Masters SL: Cutting edge: miR-223 and EBV miR-BART15 regulate the
NLRP3 inflammasome and il-1β production. J Immunol.
189(3795-3795-3799)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
General Administration of Quality
Supervision, Inspection and Quarantine of the People's Republic of
China, Standardization Administration of China. Laboratory animal -
Requirements of environment and housing facilities GB
14925-2010[S]. China Quality Inspection Press, Beijing, pp1-18,
2010.
|
|
26
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cai W, Wu S, Pan Z, Xiao J, Li F, Cao J,
Zang W and Tao YX: Disrupting interaction of PSD-95 with nNOS
attenuates hemorrhage-induced thalamic pain. Neuropharmacology.
141:238–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Z, Mao Y, Liang L, Wu S, Yuan J, Mo K,
Cai W, Mao Q, Cao J, Bekker A, et al: The transcription factor
C/EBPβ in the dorsal root ganglion contributes to peripheral nerve
trauma-induced nociceptive hypersensitivity. Sci Signal.
10(eaam5345)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li Z, Gu X, Sun L, Wu S, Liang L, Cao J,
Lutz BM, Bekker A, Zhang W and Tao YX: Dorsal root ganglion myeloid
zinc finger protein 1 contributes to neuropathic pain after
peripheral nerve trauma. Pain. 156:711–721. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari
V, Atianjoh FE, Lee CY, Liang L, Zang W, Njoku D, et al: Opioid
receptor-triggered spinal mTORC1 activation contributes to morphine
tolerance and hyperalgesia. J Clin Investig. 124:592–603.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao
JY, Liang L, Wang W, Guan X, Kao SC, Tiwari V, et al: A long
noncoding RNA contributes to neuropathic pain by silencing Kcna2 in
primary afferent neurons. Nat Neurosci. 16:1024–1031.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Huang T, Fu G, Gao J, Zhang Y, Cai W, Wu
S, Jia S, Xia S, Bachmann T, Bekker A and Tao YX: Fgr contributes
to hemorrhage-induced thalamic pain by activating NF-κB/ERK1/2
pathways. JCI Insight. 5(e139987)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fang XZ, Huang TF, Wang CJ, Ge YL, Lin SY,
Zhang Y and Gao J: Preconditioning of physiological cyclic stretch
attenuated HMGB1 expression in pathologically mechanical
stretch-activated A549 cells and ventilator-induced lung injury
rats through inhibition of IL-6/STAT3/SOCS3. Int Immunopharmacol.
31:66–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Y, Huang T, Jiang L, Gao J, Yu D, Ge
Y and Lin S: MCP-induced protein 1 attenuates sepsis-induced acute
lung injury by modulating macrophage polarization via the JNK/c-Myc
pathway. Int Immunopharmacol. 75(105741)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu D, Fang X, Xu Y, Xiao H, Huang T, Zhang
Y, Ge Y, Li Y, Zong L and Gao J: Rev-erbα can regulate the
NF-κB/NALP3 pathway to modulate lipopolysaccharide-induced acute
lung injury and inflammation. Int Immunopharmacol. 73:312–320.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jiang H, He H, Chen Y, Huang W, Cheng J,
Ye J, Wang A, Tao J, Wang C, Liu Q, et al: Identification of a
selective and direct NLRP3 inhibitor to treat inflammatory
disorders. J Exp Med. 214:3219–3238. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang Y, Yang F, Yang F, Li CL, Wang Y, Li
Z, Lu YF, Yu YQ, Fu H, He T, et al: Gabapentinoid Insensitivity
after Repeated Administration is Associated with Down-Regulation of
the α(2)δ-1 Subunit in rats with central post-stroke pain
hypersensitivity. Neurosci Bull. 32:41–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang J: Preclinical and clinical research
on inflammation after intracerebral hemorrhage. Prog Neurobiol.
92:463–477. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pan Z, Shan Q, Gu P, Wang XM, Tai LW, Sun
M, Luo X, Sun L and Cheung CW: miRNA-23a/CXCR4 regulates
neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome
axis. J Neuroinflammation. 15(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang Z, Liu F, Wei M, Qiu Y, Ma C, Shen L
and Huang Y: Chronic constriction injury-induced microRNA-146a-5p
alleviates neuropathic pain through suppression of IRAK1/TRAF6
signaling pathway. J Neuroinflammation. 15(179)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shi J, Jiang K and Li Z: miR-145
ameliorates neuropathic pain via inhibiting inflammatory responses
and mTOR signaling pathway by targeting Akt3 in a rat model.
Neurosci Res. 134:10–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z,
Ding J, Jia L and Yuan W: Increased miR-195 aggravates neuropathic
pain by inhibiting autophagy following peripheral nerve injury.
Glia. 61:504–512. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Correction to title, results and
conclusion in. Down-regulated IncRNA F630028O10Rik contributes to
suppress lung cancer in mice through inhibiting miR-223-3p and VEGF
signaling pathway. Chest. 150(261)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang F, Xu Y, Liu C, Ma C, Zou S, Xu X,
Jia J and Liu Z: NF-κB/miR-223-3p/ARID1A axis is involved in
Helicobacter pylori CagA-induced gastric carcinogenesis and
progression. Cell Death Dis. 9(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Calvente CJ, Tameda M, Johnson CD, del
Pilar H, Lin YC, Adronikou N, De Mollerat Du Jeu X, Llorente C,
Boyer J and Feldstein AE: Neutrophils contribute to spontaneous
resolution of liver inflammation and fibrosis via microRNA-223. J
Clin Investig. 129:4091–4109. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cressatti M, Juwara L, Galindez JM, Velly
AM, Nkurunziza ES, Marier S, Canie O, Gornistky M and Schipper HM:
Salivary microR-153 and microR-223 levels as potential diagnostic
biomarkers of idiopathic Parkinson's disease. Mov Disord.
35:468–477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hoffman HM, Mueller JL, Broide DH,
Wanderer AA and Kolodner RD: Mutation of a new gene encoding a
putative pyrin-like protein causes familial cold autoinflammatory
syndrome and Muckle-Wells syndrome. Nat Genet. 29:301–305.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
49
|
Wen H, Ting JP and O'Neill LA: A role for
the NLRP3 inflammasome in metabolic diseases-did Warburg miss
inflammation? Nat Immunol. 13:352–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu SB, Mi WL and Wang YQ: Research
progress on the NLRP3 inflammasome and its role in the central
nervous system. Neurosci Bull. 29:779–787. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Guo S, Yang C, Diao B, Huang X, Jin M,
Chen L, Yan W, Ning Q, Zheng L, Wu Y and Chen Y: The NLRP3
inflammasome and IL-1β accelerate immunologically mediated
pathology in experimental viral fulminant hepatitis. PLoS Pathog.
11(e1005155)2015.PubMed/NCBI View Article : Google Scholar
|