Introduction
Infantile hemangioma (IH) is the most common benign
tumor among children, and occurs in 1-12% of infants worldwide
(1). There are three
characteristic stages in this disease, including the proliferating,
involuting and involuted periods (2). The possible complications of IH
include immediate side effects such as pain, purpura, edema,
ulceration and crusting, as well as long-lasting complications such
as hyperpigmentation, hypopigmentation, and atrophic or
hypertrophic scarring (3).
However, no specific treatment has been proposed for IH thus far,
since the mechanisms behind the initiation and spontaneous
regression of the tumor are unknown.
The isolation of hemangioma-derived mesenchymal stem
cells (Hem-MSCs) has indicated that IH may originate from
multipotential stem cells and may differentiate into endothelial
cells, pericytes, adipocytes and fibroblasts (4). Adipocytes and fibroblasts are closely
associated with the resident fibro-fatty tissues after the
involution of the tumor. Multiple pathways are involved in this
pathogenesis, including the vascular endothelial growth factor
(VEGF), basic fibroblast growth factor (b-FGF) and peroxisome
proliferator-activated receptor-γ (PPAR-γ) signaling pathways
(5).
Celecoxib, a non-steroidal anti-inflammatory drug,
has attracted considerable attention due to its preventive role in
numerous cancer types (6). As a
selective inhibitor of cyclooxygenase-2 (COX-2), it can inhibit
angiogenesis by reducing the production of prostaglandin E2 (PGE2),
which promotes tumor progression through different mechanisms
(7). The potential mechanisms
behind its action may be the inactivation of the VEGF signaling
pathway, which is highly expressed in multiple types of cancer and
is closely associated with angiogenesis (8). In addition, celecoxib can reduce
fibroblast proliferation and decrease collagen expression (9). COX-2 inhibition can reduce the
overall deposition of collagen in tumors and increase tendon
healing (10,11).
Therefore, it was hypothesized that celecoxib can
inhibit cell proliferation, induce adipogenesis and reduce
fibroblast formation in IH. The present study was undertaken to
investigate the effect of celecoxib on the pathogenesis of IH in an
IH mouse model.
Materials and methods
Ethics approval
The present study was approved by the Ethics Board
of The Children's Hospital of Nanjing Medical University (China)
(approval no. 201705097-1). Written informed consent was obtained
regarding the handling of samples from the parents according to the
Declaration of Helsinki. The experimental animal protocol was
reviewed and approved by the Animal Ethics and Welfare Committee of
Nanjing Medical University (China) (approval no.
IACUC-1902023).
Preparation of hemangioma
specimens
Five fresh IH samples from different patients in the
proliferating phase were obtained between March 2019 to May 2019
from The Children's Hospital in Nanjing, China, under a protocol
for human subjects that was approved by the Committee on Clinical
Investigation of Nanjing Medical University (approval no.
201705097-1). Tissues were immediately used for cell isolation of
Hem-MESs. The postoperative pathological evaluation of IH samples
showed proliferative IH
(CD34+/CD31+/SMA+/Glut-1+/D2-40-).
Hem-MSC isolation and culture
Hem-MSCs were isolated from clinically resected
specimens as previously described, with certain modifications
(12). Briefly, the hemangioma
samples were rinsed in PBS, cut into small pieces (1x1x1
mm3) and dispersed on the surface of a plastic culture
dish. The cells were cultured in Dulbecco's modified Eagle's
medium-low glucose (DMEM-L; HyClone; Cytiva), supplemented with 10%
fetal bovine serum (FBS) (HyClone; Cytiva), 100 U/ml penicillin and
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) for
4 days (with 5% CO2 at 37˚C). Next, the medium was
changed every other day, and the cells were passaged when reaching
90% confluency. Generation 3-5 were used for the experiments. MSCs
from all 5 specimens were used for the experiments as the amount of
MSCs between generation 3-5 was limited. Cells were labeled with
FITC- or phycoerythrin (PE)-conjugated isotype-matched control
antibodies, FITC-conjugated antibodies against human CD45
(eBioscienceTM, cat. # 11-0459-42) and CD90
(eBioscienceTM), and/or PE-conjugated antibodies against
human CD34 (eBioscienceTM, cat. # 12-0349-42) and CD105
(eBioscienceTM, cat. # 12-1057-42) (Thermo Fisher
Scientific, Inc.). Samples were analyzed on a FACSVantage SE flow
cytometer (BD Pharmingen; BD Biosciences) and analyzed with WinMDI
software (v2.9).
In vitro cell proliferation assay with
celecoxib
Celecoxib was purchased from Pfizer, Inc. and was
dissolved in 100% dimethyl sulfoxide (DMSO), and then diluted with
DMEM-L for subsequent experiments. The final concentration of DMSO
for all treatments was maintained below 0.1%.
Hem-MSCs were counted by Neubauer Chamber under a
microscope (Olympus, magnification x100) and then seeded into a
96-well plate at a concentration of 104 cells per well.
Next, the cells were cultured with different concentrations of
b-FGF (0.1-0.0001 ng/ml; Peprotech®) and celecoxib
(0.1-1,000 µg/ml) for 24 h. Subsequently, the culture medium was
replaced with fresh medium containing 10 µl CCK-8 solution
(Dojindo). After a 2-h incubation, the absorbance at 450 nm was
detected by ELISA using a microplate reader to assess the cell
counts. The data were analyzed by SPSS 21 (IBM Corp.).
In vitro adipogenic
differentiation
Hem-MSCs were plated at the same density
(106/ml) in separate dishes and divided into four
groups: (A) control group; (B) adipogenic induction group; (C)
celecoxib group; (D) adipogenic induction + celecoxib group. After
24 h, the cells were induced to differentiate into adipocytes in an
adipogenic induction medium for human bone marrow MSCs (BM-MSCs)
(HUXMD-90031; Cyagen Biosciences). The medium was changed every 3
days. After induction for 7 or 14 days, the cells were stained with
Oil Red O (O8010; Solarbio) to detect the adipocytes.
Gene expression was analyzed by reverse
transcription-quantitative PCR (RT-qPCR). Total RNA from cells in
the different groups was isolated and reverse transcribed into cDNA
as previously described (13).
According to the manufacturer's instruction, total RNA was
extracted by Trizol total RNA isolation kit (TaKaRa Biotechnology).
The reverse transcription was performed using PrimeScript™ Reverse
Transcriptase (TaKaRa Biotechnology). qPCR was carried out with
SYBR Green Master Mix (Vazyme Biotech Co., Ltd.) on a QuantStudio 3
Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The cycling program consisted of a preliminary denaturation
(95˚C for 10 min), followed by 40 cycles (95˚C for 15 sec and 60˚C
for 1 min). The relative mRNA levels were normalized to the levels
of β-actin and calculated using the comparative cycle threshold
(ΔΔCq) method (14). The primers
used in qPCR were as follows: human-COX-2, forward
5'-ATGCTGACTATGGCTACAAAAGC-3' and reverse
5'-TCGGGCAATCATCAGGCAC-3'; human-PPAR-γ, forward
5'-GGGATCAGCTCCGTGGATCT-3' and reverse
5'-TGCACTTTGGTACTCTTGAAGTT-3'; human-CCAAT-enhancer-binding protein
(CEBP)α forward 5'-AGGAGGATGAAGCCAAGCAGCT-3' and reverse
5'-AGTGCGCGATCTGGAACTGCAG-3' and β-actin forward
5'-AGCGAGCATCCCCCAAAGTT-3' and reverse
5'-CGGCACGAAGGCTCATCATT-3'.
In vivo treatment with b-FGF and
celecoxib
A total of 27 specific-pathogen-free, 6-week-old,
male, BALB/C-nu/nu mice (16±2 g) were purchased from Nanjing
Medical University (China). The mice were raised under controlled
12-h light-dark cycles, with a constant temperature (22-24˚C) and
humidity (55-60%) in a pathogen-free animal research center at
Nanjing Medical University (Nanjing, China). The animals had
continuous free access to sterilized food (γ-ray-irradiated food)
and autoclaved water.
After a 1-week acclimation, the experiments were
initiated. The mice were randomly separated into three groups:
control, b-FGF and celecoxib groups. To generate xenograft tumors,
Hem-MSCs were trypsinized, washed and re-suspended in Corning
Matrigel (354277; Corning Life Sciences). Next, 1x107
cells in 0.3 µl Matrigel were inoculated subcutaneously into the
back of each mouse as previously described (15). In the b-FGF group, the cells were
treated with 0.01 ng/ml b-FGF for 24 h before injection, while in
the celecoxib treatment group, celecoxib (0.1 mg/g/day) was
administered to the mice via oral intake for 4 weeks (16). The animal health and behavior were
monitored every day. At the end of weeks 1, 2 and 4, three mice in
each group were sacrificed and their tumors were harvested
(3x3x3=27). The maximum allowed tumor dimension was ~1 cm. The mice
were sacrificed by injection of pentobarbital sodium (100 mg/kg).
The death of the mice was indicated with no heart beat and breath
detected after 10 min.
Histopathological analysis
Tumors were isolated from the sacrificed mice and
immediately fixed in 10% buffered formalin for histopathology
processing. Tissue samples were embedded in paraffin and 5-µm
sections were cut using a manual microtome. Sections were stained
with hematoxylin and eosin (H&E). Silver staining (SBJ-0285;
Gorden-Sweet protocol) was used to observe the fibers in the tumor
as previously described (17).
Immunohistochemical analysis
Immunohistochemical staining with anti-fibroblast
antibodies was performed to evaluate expression patterns of
fibroblasts in the tumor. After retrieval by citric acid (10 mM
citrate buffer, pH 6.0) (eBioscience; Thermo Fisher Scientific,
Inc.) in a microwave, tissue sections from the IH model mice were
incubated with a primary antibody against S100 calcium binding
protein (dilution 1:300 in 10% normal goat serum) (Abcam, cat. no.
ab242659), a marker of fibroblasts (18), overnight at 4˚C. Next, the sections
were incubated with an anti-rabbit IgG (biotin, cat. no. a0516,
dilution 1:500) antibody, and detected using DAB with hematoxylin
counterstaining. Images were obtained by microscopy (Olympus, x100
magnification).
Statistical analysis
Statistical analysis was performed using GraphPad
7.0 statistical software (GraphPad Software, Inc.). Differences
between two groups were evaluated using the Student's t-test. A
value of P<0.05 was considered significant.
Results
Histopathology of IH
Histopathological examination in the present study
revealed that the proliferative phase IH is composed of
well-defined, unencapsulated masses of capillaries lined by plump
endothelial cells. These lesional capillaries are separated by
fibers and collagen. H&E and ammoniacal silver staining showed
that proliferative-phase IHs are complex cellular mixtures with a
large number of collagen (Fig.
1).
Hem-MSCs isolated from clinically resected specimens
were spindle-shaped, tightly packed and evenly distributed under an
inverted microscope at x100 magnification. Flow cytometric analyses
showed that these cells expressed the mesenchymal cell markers CD90
and CD103, but did not express markers for endothelial cells, such
as CD34 or CD45 (Fig. 2). The
cell-surface markers on Hem-MSCs indicate a mesenchymal cell
phenotype.
Celecoxib inhibits cell
proliferation
As shown in Fig. 3,
the results of Cell Counting Kit-8 assay revealed that b-FGF
significantly promoted Hem-MSC cell proliferation with a relatively
low cell toxicity at a concentration of 0.01 ng/ml. Celecoxib had a
cytotoxic effect on Hem-MSCs and inhibited cell proliferation at
various concentrations. When the concentration of celecoxib was
lower than 0.1 mg/ml, the difference in viability at the different
concentrations was not very large. Thus, we selected 0.1 mg/ml for
use in the following in vitro experiments. In vivo,
the celecoxib was received via oral intake as previously described
(16).
Celecoxib induces adipogenesis
In vitro, lipid droplets stained with Oil Red
O were apparent on day 7 (week 1), and continued to accumulate
through day 14 (week 2) in the celecoxib group (Fig. 4C) and the two induction groups
[adipogenic induction group (Fig.
4B) and the adipogenic induction + celecoxib group (Fig. 4D)] when compared to the control
group (Fig. 4A). Celecoxib was
able to induce the formation of lipid drops without induction
medium.
The expression levels of genes involved in
adipogenic differentiation [cyclooxygenase-2 (COX-2),
human-CCAAT-enhancer-binding protein (CEBP)α, and peroxisome
proliferator-activated receptor-γ (PPAR-γ)] were increased in the
celecoxib group (group C) (Fig.
5). Nude mouse models of IH were constructed successfully.
H&E staining showed that, in the celecoxib group, a large
number of adipocytes appeared in the plugs on week 4, while these
fat-like tissues were rare in the other groups (Fig. 6). On weeks 1 and 2, no lipid
droplet was observed in any of the xenografts, while lipid droplets
could only be observed in the celecoxib group on week 4. This
suggests that celecoxib induced the adipogenic differentiation of
Hem-MSCs. In addition, since part of the mass was adipose tissue,
fewer collagen fibers were observed in the xenograft tumors.
Celecoxib reduces the number of
fibroblasts and collagen fibers
As revealed by H&E staining, the transplanted
tissue in all groups developed an increased number of collagen
fibers surrounding the microvasculature. In the b-FGF group, the
expression of collagen was greater than that of the control and
celecoxib groups on the same week (Fig. 6).
Ammoniacal silver staining revealed the presence of
fibers of various sizes. On week 1, the reticular fibers stained
dark, and collagenous fibers, appearing yellow or brown in color,
were present in all the groups. The number of fibers increased over
time, reaching its peak on week 4. Compared with the findings in
other groups, the b-FGF group had the largest number of fibers,
particularly collagenous fibers, while the number of collagenous
fibers in the other two groups showed no apparent difference
(Fig. 7).
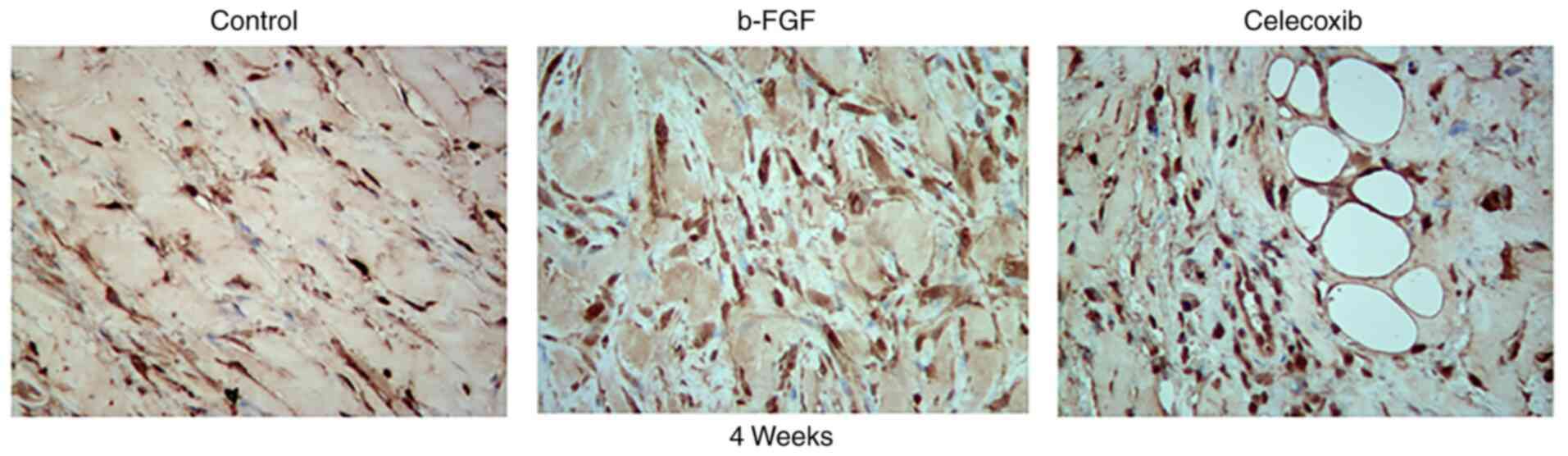
The number of fibroblasts was the largest in the
b-FGF group compared with that in the other two groups according to
the results of immunohistochemical analysis (Fig. 8). This is consistent with the
presence of fibers in the different groups.
Discussion
The present results showed that celecoxib can
inhibit the proliferation and induce the adipogenic differentiation
of hemangioma-derived mesenchymal stem cells (Hem-MSCs) in
vitro and in vivo. There may be two events involved in
the self-regression of infantile hemangioma (IH), including
apoptosis (19) and adipogenesis
(20). This process is accompanied
by tissue repair mediated by fibroblasts. Finally, the lesion may
be replaced by fibro-fatty tissue. The present study focused on
adipogenesis and cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2)
signaling, and investigated the effect of celecoxib on the
involution of IH in an IH model constructed by Hem-MSCs. When
treated with celecoxib, a COX-2 inhibitor, the IH model exhibited a
characteristic pathological evolution.
Adipogenesis is the most essential pathological
process during the involution of IH. Adipogenesis, lipid metabolism
and associated factors play an important role in the survival,
metastasis and invasion of numerous tumors. It has been reported
that trans-differentiation of breast cancer cells into adipocytes
can repress cancer metastasis, and overcome therapy resistance and
cancer relapse (21). Fatty acid
transport protein (FATP) is associated with tumor
immunosuppression. Inhibition of FATP2 suppresses the progression
of tumors through the reduction in arachidonic acid and PGE2 levels
(22). The effect of a COX-2
inhibitor on adipogenesis and expression of associated factors has
been studied in non-alcoholic steatohepatitis (23), diabetes (24), breast cancer (21) and melanoma (25). Previous results showed that a COX-2
inhibitor can induce peroxisome proliferator-activated receptor-γ
(PPAR-γ) expression and adipogenesis. Elevated PPAR-γ level can
inhibit the production of 15-deoxy-δ 12,14-prostaglandin
J2(26), a PPAR-γ agonist, by
inhibiting COX-2 and preventing inflammation. Based on these
studies and the characteristics of IH, it was hypothesized that
effective treatment of IH may rely on inducing adipogenesis.
The fibro-fatty accumulation after involution of IH
indicates adipogenesis during this period (27). Previous research has focused on
this process with the aim of identifying improved treatments for IH
(28). Propranolol, a widely used
drug for treating IH, was reported to promote adipogenesis, which
was associated with the PPAR-γ and CEBPα pathways (29). Furthermore, rosiglitazone, one of
the agonists of PPAR-γ, can promote the adipogenic differentiation
of Hem-MSCs and activate the PPAR-γ signaling pathway of
endothelial cells in hemangioma (12). The present study showed that
celecoxib can induce the adipogenic differentiation of Hem-MSCs and
increase the expression of associated genes in vitro. In
vivo, apparent adipose tissue was only present in the celecoxib
group, and could not be observed in the other groups. It was
hypothesized that COX-2 inhibitors have the potential to induce
adipogenesis, which may be associated with the PPAR-γ/CEBP
signaling pathway.
The potential mechanism behind the effect of
celecoxib in IH involution is more complex than simply inducing
adipogenesis. It was reported that PPAR-γ agonists exert an
anti-angiogenic effect by inhibiting vascular endothelial growth
factor (VEGF)-induced endothelial COX-2 expression and
prostaglandin production (23).
Celecoxib can be used to inhibit angiogenesis through blocking
VEGF. It has been reported that COX-2 promoted the angiogenesis of
several cancer types by upregulating angiogenic factors, including
VEGF (30), which is also the most
important factor in the pathogenesis of hemangioma. Celecoxib can
reduce the microvessel density within xenografts and the growth of
several tumors (31). The present
results showed that celecoxib can inhibit the proliferation of
Hem-MSCs in vitro. However, the microvessel density of the
xenografts showed no difference between the celecoxib and control
groups. This may be caused by the indirect oral mechanism of action
of celecoxib on IH xenografts.
Other essential components of the resident tissue in
involuted IH lesions are collagen and extracellular matrix (ECM).
Scars appear when the repaired tissue contains excessive
fibroblasts with high expression of ECM, particularly excessive
synthesis and deposition of collagen (32). There are numerous factors involved
in this process, including basic fibroblast growth factor (b-FGF),
which has multiple functions in mitogenesis, migration,
morphogenesis, angiogenesis, organ development, organ regeneration
and wound healing (33). b-FGF is
able to enhance cell adhesion, cell proliferation and secretion of
ECM (34). The present results
showed that b-FGF enhances the proliferation of Hem-MSCs in
vitro, while, in vivo, b-FGF promotes collagen
deposition in xenografts.
By contrast, as a non-steroidal anti-inflammatory
drug, celecoxib can reduce fibrogenesis and expression of collagen
(35), thus delaying all types of
wound healing (36), and reducing
scar formation and adhesion (37).
The results of histological analysis of the present IH model showed
that there was a lower number of collagen fibers in the celecoxib
group. This indicated that celecoxib accentuated adipogenesis while
simultaneously reducing collagen production. Celecoxib could have a
potential effect on IH regression by inhibiting the differentiation
and proliferation of fibroblasts. The IH model constructed by
Hem-MSCs has the potential to generate fibers (including reticular
and collagenous fibers) by fibroblasts differentiated from
Hem-MSCs, according to the results of ammoniacal silver and
immunohistochemical staining. It was previously reported that
celecoxib can inhibit the proliferation and collagen expression of
fibroblasts (38), which is
consistent with the present results. Cell proliferation and
collagen production play important roles in cancer formation and
tissue fibrosis, which is associated with IH proliferation and the
residual fibro-adipose tissue. Treatment with celecoxib may reduce
the possibility of a remaining scar.
In conclusion, based on the aforementioned findings,
it may be hypothesized that celecoxib may be used to induce the
regression of IH by promoting adipogenesis with formation of fewer
fibers, which may lead to a remaining scar of the tumor. In order
to verify the mechanism behind the acceleration of adipogenesis,
the expression of adipogenesis-related factors and proteins
following treatment with celecoxib should be evaluated in future
studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW conducted the experimental operations and
manuscript writing. LK was responsible for the research design. BS
conducted the experimental operations. JC was involved in drafting
the work and revising it critically for important intellectual
content, and made substantial contributions to the conception of
the research design. WS made substantial contributions to the
conception of the research design and findings. JC and WS confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work (as well as the data provided) are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics Board
of The Children's Hospital of Nanjing Medical University (China)
(approval no. 201705097-1). Written informed consent was obtained
regarding the handling of samples from the parents according to the
Declaration of Helsinki. The experimental animal protocol was
reviewed and approved by the Animal Ethics and Welfare Committee of
Nanjing Medical University (China) (approval no.
IACUC-1902023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
You Y, Li Y, Xiao Y and Zhang J:
Propranolol vs. steroids in the treatment of infantile hemangiomas:
A meta-analysis. Mol Clin Oncol. 15(156)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Q, Xiang B, Chen S and Ji Y: Efficacy
and safety of oral atenolol for the treatment of infantile
haemangioma: A systematic review. Australas J Dermatol. 60:181–185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Satterfield KR and Chambers CB: Current
treatment and management of infantile hemangiomas. Surv Ophthalmol.
64:608–618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Itinteang T, Vishvanath A, Day DJ and Tan
ST: Mesenchymal stem cells in infantile haemangioma. J Clin Pathol.
64:232–236. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ji Y, Chen S, Li K, Li L, Xu C and Xiang
B: Signaling pathways in the development of infantile hemangioma. J
Hematol Oncol. 7(13)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koki AT and Masferrer JL: Celecoxib: A
specific COX-2 inhibitor with anticancer properties. Cancer
Control. 9:28–35. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xie C, Xu X, Wang X, Wei S, Shao L, Chen
J, Cai J and Jia L: Cyclooxygenase-2 induces angiogenesis in
pancreatic cancer mediated by prostaglandin E2. Oncol
Lett. 16:940–948. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li J, Hao Q, Cao W, Vadgama JV and Wu Y:
Celecoxib in breast cancer prevention and therapy. Cancer Manag
Res. 10:4653–4667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li F, Fan C, Zeng B, Zhang C, Chai Y, Liu
S and Ouyang Y: Celecoxib suppresses fibroblast proliferation and
collagen expression by inhibiting ERK1/2 and SMAD2/3
phosphorylation. Mol Med Rep. 5:827–831. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Esbona K, Inman D, Saha S, Jeffery J,
Schedin P, Wilke L and Keely P: COX-2 modulates mammary tumor
progression in response to collagen density. Breast Cancer Res.
18(35)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oak NR, Gumucio JP, Flood MD, Saripalli
AL, Davis ME, Harning JA, Lynch EB, Roche SM, Bedi A and Mendias
CL: Inhibition of 5-LOX, COX-1, and COX-2 increases tendon healing
and reduces muscle fibrosis and lipid accumulation after rotator
cuff repair. Am J Sports Med. 42:2860–2868. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuan SM, Guo Y, Zhou XJ, Shen WM and Chen
HN: Rosiglitazone accentuates the adipogenesis of
hemangioma-derived mesenchymal stem cells induced by adipogenic
media. Int J Clin Exp Med. 7:1741–1746. 2014.PubMed/NCBI
|
|
13
|
Yu XW, Wei D, Gao YS, Du HZ, Yu BY, Li RM,
Qian CM, Luo XJ, Yuan ST, Wang JS and Sun L: Synergistic
combination of DT-13 and Topotecan inhibits aerobic glycolysis in
human gastric carcinoma BGC-823 cells via NM IIA/EGFR/HK II axis. J
Cell Mol Med. 23:6622–6634. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yuan SM, Guo Y, Wang Q, Xu Y, Wang M, Chen
HN and Shen WM: Over-expression of PPAR-γ2 gene enhances the
adipogenic differentiation of hemangioma-derived mesenchymal stem
cells in vitro and in vivo. Oncotarget. 8:115817–115828.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chu TH, Chan HH, Kuo HM, Liu LF, Hu TH,
Sun CK, Kung ML, Lin SW, Wang EM, Ma YL, et al: Celecoxib
suppresses hepatoma stemness and progression by up-regulating PTEN.
Oncotarget. 5:1475–1490. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Atiakshin D, Buchwalow I and Tiemann M:
Mast cells and collagen fibrillogenesis. Histochem Cell Biol.
154:21–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen L, Li J, Zhang J, Dai C, Liu X, Wang
J, Gao Z, Guo H, Wang R, Lu S, et al: S100A4 promotes liver
fibrosis via activation of hepatic stellate cells. J Hepatol.
62:156–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wnęk A, Andrzejewska E, Kobos J, Taran K
and Przewratil P: Molecular and immunohistochemical expression of
apoptotic proteins Bax, Bcl-2 and Caspase 3 in infantile hemangioma
tissues as an effect of propranolol treatment. Immunol Lett.
185:27–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yuan SM, Chen RL, Chen HN, Shen WM and
Zhou XJ: The role of the expression of PPAR-gamma gene in the
adipogenesis in hemangioma evolution. Zhonghua Zheng Xing Wai Ke Za
Zhi. 29:45–48. 2013.PubMed/NCBI(In Chinese).
|
|
21
|
Ishay-Ronen D, Diepenbruck M, Kalathur
RKR, Sugiyama N, Tiede S, Ivanek R, Bantug G, Morini MF, Wang J,
Hess C and Christofori G: Gain fat-lose metastasis: Converting
invasive breast cancer cells into adipocytes inhibits cancer
metastasis. Cancer Cell. 35:17–32.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Veglia F, Tyurin VA, Blasi M, De Leo A,
Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et
al: Fatty acid transport protein 2 reprograms neutrophils in
cancer. Nature. 569:73–78. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tian F, Zhang YJ and Wang YH: Effects of
celecoxib on expression of PPARγ and NF-κB in type 2 diabetes rats
with non-alcoholic steatohepatitis. Zhonghua Gan Zang Bing Za Zhi.
24:590–595. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
24
|
Lima LH, Farah ME, Gum G, Ko P and de
Carvalho RAP: Sustained and targeted episcleral delivery of
celecoxib in a rabbit model of retinal and choroidal
neovascularization. Int J Retina Vitreous. 4(31)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bundscherer A, Hafner C, Maisch T, Becker
B, Landthaler M and Vogt T: Antiproliferative and proapoptotic
effects of rapamycin and celecoxib in malignant melanoma cell
lines. Oncol Rep. 19:547–553. 2008.PubMed/NCBI
|
|
26
|
Maggi LB Jr, Sadeghi H, Weigand C, Scarim
AL, Heitmeier MR and Corbett JA: Anti-inflammatory actions of
15-deoxy-delta 12,14-prostaglandin J2 and troglitazone: Evidence
for heat shock-dependent and -independent inhibition of
cytokine-induced inducible nitric oxide synthase expression.
Diabetes. 49:346–355. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Harbi S, Wang R, Gregory M, Hanson N,
Kobylarz K, Ryan K, Deng Y, Lopez P, Chiriboga L and Mignatti P:
Infantile hemangioma originates from a dysregulated but not fully
transformed multipotent stem cell. Sci Rep. 6(35811)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li HH, Lou Y, Zhang RR, Xie J and Cao DS:
Propranolol accelerats hemangioma stem cell transformation into
adipocyte. Ann Plast Surg. 83:e5–e13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
England RW, Hardy KL, Kitajewski AM, Wong
A, Kitajewski JK, Shawber CJ and Wu JK: Propranolol promotes
accelerated and dysregulated adipogenesis in hemangioma stem cells.
Ann Plast Surg. 73 (Suppl 1):S119–S124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese
JL and Xie K: Celecoxib inhibits vascular endothelial growth factor
expression in and reduces angiogenesis and metastasis of human
pancreatic cancer via suppression of Sp1 transcription factor
activity. Cancer Res. 64:2030–2038. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Knopfová L and Smarda J: The use of Cox-2
and PPARγ signaling in anti-cancer therapies. Exp Ther Med.
1:257–264. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mingyuan X, Qianqian P, Shengquan X,
Chenyi Y, Rui L, Yichen S and Jinghong X: Hypoxia-inducible
factor-1α activates transforming growth factor-β1/Smad signaling
and increases collagen deposition in dermal fibroblasts.
Oncotarget. 9:3188–3197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ma Y, Kakudo N, Morimoto N, Lai F,
Taketani S and Kusumoto K: Fibroblast growth factor-2 stimulates
proliferation of human adipose-derived stem cells via Src
activation. Stem Cell Res Ther. 10(350)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang
C and Li X: Promotion of skin regeneration in diabetic rats by
electrospun core-sheath fibers loaded with basic fibroblast growth
factor. Biomaterials. 32:4243–4254. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li F, Liu S, Ouyang Y, Fan C, Wang T,
Zhang C, Zeng B, Chai Y and Wang X: Effect of celecoxib on
proliferation, collagen expression, ERK1/2 and SMAD2/3
phosphorylation in NIH/3T3 fibroblasts. Eur J Pharmacol. 678:1–5.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fairweather M, Heit YI, Buie J, Rosenberg
LM, Briggs A, Orgill DP and Bertagnolli MM: Celecoxib inhibits
early cutaneous wound healing. J Surg Res. 194:717–724.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Olson JM, Haas AW, Lor J, McKee HS and
Cook ME: A comparison of the anti-inflammatory effects of Cis-9,
trans-11 conjugated linoleic acid to celecoxib in the
collagen-induced arthritis model. Lipids. 52:151–159.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Salib CG, Reina N, Trousdale WH, Limberg
AK, Tibbo ME, Jay AG, Robin JX, Turner TW, Jones CR, Paradise CR,
et al: Inhibition of COX-2 pathway as a potential prophylaxis
against arthrofibrogenesis in a rabbit model of joint contracture.
J Orthop Res. 37:2609–2620. 2019.PubMed/NCBI View Article : Google Scholar
|