Introduction
Pancreatic cancer is a highly malignant tumor, and
pancreaticoduodenectomy is currently the effective treatment for
pancreatic cancer (1). Based on
the GLOBOCAN 2012 estimates, pancreatic cancer ranked as the 7th
cause of cancer-associated mortality and the 11th most common type
of cancer in the world. The estimated 5-year survival rate for
pancreatic cancer is <5% (2).
Pancreatic head cancer can invade the portal vein, superior
mesenteric vein and other blood vessels. Sometimes it is difficult
to judge whether the tumor can be resected. Three-dimensional (3D)
reconstruction imaging of the surgical site prior to surgery plays
an important role in judging whether the pancreatic head cancer can
be resected. 3D laparoscopic pancreaticoduodenectomy has the
advantages of a stereoscopic view, accurate spatial positioning and
a clear view of the anatomy, which are beneficial to the dissection
of lymph nodes and the reconstruction of the digestive tract
(3). Intraoperative navigation can
accurately identify the blood vessels around the tumor, prevent
unnecessary damage, reduce bleeding and shorten the surgical
duration (4). Intraoperative
navigation has obvious advantages in 3D laparoscopic
pancreaticoduodenectomy. The current study discusses the use of
intraoperative navigation in the 3D laparoscopic
pancreaticoduodenectomy of a 64-year-old man.
The chief surgeon who performed the surgery in the
present case has experience of >100 cases of open
pancreaticoduodenectomy and 32 cases of laparoscopic
pancreaticoduodenectomy. The average duration of these laparoscopic
pancreaticoduodenectomy surgeries is ~9 h (range, 7 h and 22 min to
12 h and 37 min). The average blood loss is ~170 ml (range, 40-600
ml).
Case report
Patient information
A 64-year-old man with an ~10-year medical history
of type 2 diabetes mellitus was referred on January 11, 2022.to the
Department of Hepatobiliary and Pancreatic Surgery of Kanghua
Hospital (Dongguan, China) complaining of upper abdominal pain
without nausea or vomiting for 1 day.
Physical examination revealed that there was no
yellowing of the skin and sclera. The abdomen was flat, the
abdominal muscle was not tense, abdominal tenderness and rebound
tenderness were negative, and bowel sounds were heard three times
every minute. The results of the laboratory examinations are
presented in Table I; no
abnormality was revealed in the laboratory examination. For blood
routine testing, an automatic hemocytometer (model, BC 5000;
Mindray) was used, while an automatic biochemical analyzer (model,
ADVIA 2400; Siemens AG) was used for blood biochemical testing and
a blood coagulation analyzer (model, CS 5100; Sysmex Corporation)
was used for blood coagulation testing. An electrocardiogram and
chest X-ray showed no obvious abnormalities. Abdominal computed
tomography (CT) showed an oval low-density shadow on the head of
the pancreas, ~19x22 mm in size, with clear edges and the internal
texture was heterogeneous (Fig.
1). Enhanced CT showed that the edge of the shadow was
enhanced, with no enhancement internally (Fig. 2).
 | Table IResults of laboratory
examinations. |
Table I
Results of laboratory
examinations.
| Variable | Result | Reference
interval |
|---|
| Hemoglobin, g/l | 136 | 130-175 |
| Albumin, g/l | 39.6 | 40-55 |
| Aspartate
aminotransferase, U/l | 11 | 15-40 |
| Alanine
aminotransferase, U/l | 12 | 9-50 |
| Total bilirubin,
µmol/l | 8.4 | 0-26 |
| Direct bilirubin,
µmol/l | 3 | 0-8 |
| Plasma prothrombin
time, sec | 10 | 9.8-12.1 |
| Activated partial
thromboplastin time, sec | 25 | 25-31.3 |
| Blood amylase,
U/l | 289 | 25-100 |
| Blood lipase,
U/l | 422 | 13-60 |
| Sugar chain
antigen-199, U/ml | 25.59 | ≤34 |
| Blood sugar,
mmol/l | 5.73 | 4.1-5.9 |
The preoperative diagnosis was pancreatic head mass,
and pathological results were required for a definitive diagnosis.
The treatment plan, operative risks and possible postoperative
complications were explained to the patient and their family
members. The risks of surgery mainly included the injury of
important blood vessels during the operation, such as the inferior
mesenteric vein, portal vein, splenic vein and superior mesenteric
artery. Postoperative complications mainly included
pancreaticoenteric anastomotic leakage, biliary-enteric anastomotic
leakage, gastrointestinal anastomotic leakage, bleeding, abdominal
infection, intestinal obstruction, pulmonary infection,
atelectasis, lower extremity venous thrombosis and tumor
recurrence. Written informed consent was obtained for the
procedure.
Methods 3D reconstruction imaging of
the surgical site
High quality thin-section CT images of the patient
were acquired before surgery. Plain and enhanced CT examinations of
the upper abdomen were performed by 256-slice spiral CT (Philips
Healthcare). The images were input into the medical image 3D
visualization system (MI-3DVS; software copyright no., 2008SR18798;
Software School of South China University of Technology; Guangzhou,
China) for 3D reconstruction imaging to judge the relationships of
the tumor and the peripheral blood vessels, common bile duct and
pancreatic duct, and to evaluate whether the tumor was resectable
(Figs. 3 and 4).
3D laparoscopic navigation system
During the operation, the surgical image of from the
3D high-definition laparoscope (model, 22204011U114; Karl Storz SE
and Co. KG) was input into the video parser, and then output to the
3D laparoscope navigation system (software copyright no.,
2018SR840555; Software School of South China University of
Technology; Guangzhou, China) in the computer by the video
acquisition card in line-by-line format. The 3D reconstruction
image of the artery, vein, pancreas and pancreatic duct was
rendered and color-set in the 3D laparoscopic navigation system.
The transparency of the 3D reconstructed image was initially set to
0.5, and the transparency of the tumor, artery, vein, pancreas and
pancreatic duct could be adjusted according to the needs of the
operation during the navigation process. During the operation, the
3D reconstructed image was projected into the 3D laparoscopic
operation view to assist in the navigation during the
operation.
Navigation process during surgery
The operation was performed using the five-hole
method. After the establishment of a pneumoperitoneum, the
abdominal cavity was first examined to determine whether there was
tumor metastasis. The gastrocolic ligament was incised to expose
the pancreas, and then the pancreas was matched with the 3D
reconstructed pancreas image established before surgery in the 3D
laparoscopic navigation system according to the shape of the
pancreas. The course of the superior marginal artery of the
pancreas was shown, and the common hepatic artery, the proper
hepatic artery and the gastroduodenal artery were dissected by
navigation (Fig. 5). The lymph
nodes in group 8a were removed for rapid pathological examination
(Fig. 6). The proper hepatic
artery and gastroduodenal artery were used as markers to match with
the 3D reconstructed image to navigate the portal vein. The
transparency of the pancreas was adjusted to 0.2-0.3. The superior
mesenteric vein was navigated with the marker of the portal vein
(Fig. 7), and then the posterior
pancreatic tunnel was established (Fig. 8). The pancreatic duct, superior
mesenteric artery, inferior pancreaticoduodenal artery, and veins
of the pancreatic uncinate process draining back to the portal vein
were navigated (Fig. 9), and then
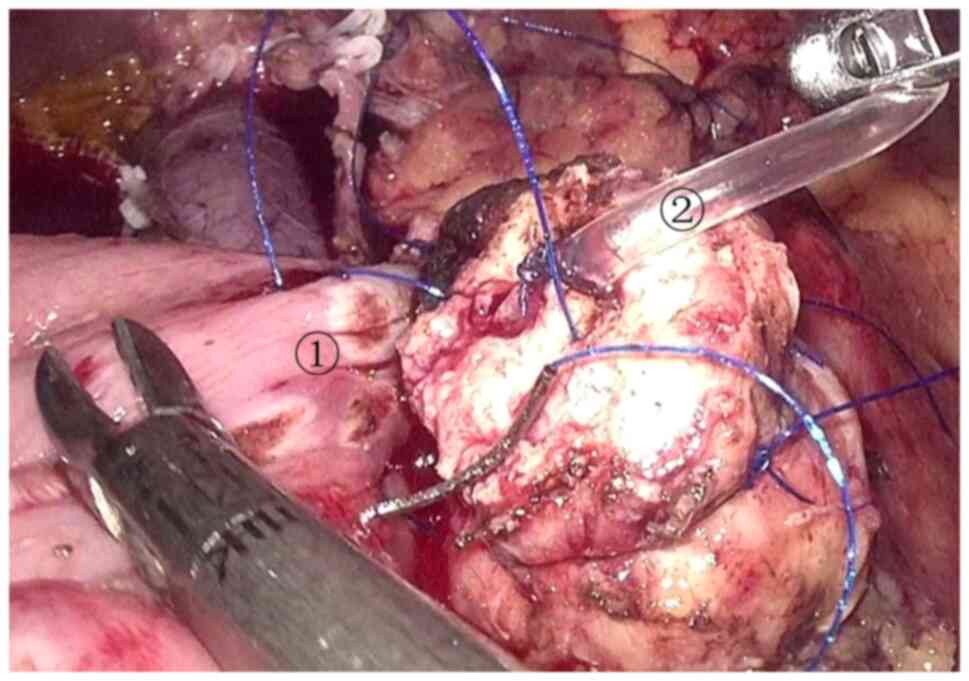
the uncinate process was excised (Fig. 10). After a tumor specimen was
removed (Fig. 11), the superior
mesenteric artery and superior mesenteric vein were used as markers
to navigate the lymph node dissection. An ~5-cm incision was made
in the transverse mesocolon, and the jejunum stump was sent to the
pancreas stump and the common hepatic duct stump through the
transverse mesocolon incision, and a pancreaticojejunostomy and a
cholangiojejunostomy were performed (Figs. 12 and 13). The gastrointestinal anastomosis was
performed at a distance of ~50 cm from the cholangiojejunostomy
(Fig. 14). A drainage tube was
placed near the pancreaticojejunostomy and another one was placed
near the cholangiojejunostomy.
Results
The operation took 520 min, the intraoperative blood
loss was ~50 ml, and antibiotics were used before and during the
operation. Intraoperative frozen rapid pathological examination of
lymph nodes in group 8a showed lymph node inflammatory reaction.
Somatostatin and albumin were used after surgery. The patient was
treated with 45 ml normal saline plus 0.3 mg somatostatin, pumped
intravenously, at 4 ml per hour for 3 days, in addition to
intravenous infusion of albumin at 20 g per day, for 6 days. An
insulin pump was used to control the blood glucose level under the
guidance of an endocrinologist after surgery. The post-surgery
drainage volumes obtained from the tubes near the
pancreaticojejunostomy and cholangiojejunostomy are shown in
Fig. 15. On the 1st day after the
operation, 65 ml of fluid was drained from near the
pancreaticojejunostomy, 70 ml of fluid was drained from near the
cholangiojejunostomy, and the amylase in the drainage fluid of the
pancreaticojejunostomy was measured at 610 U/l. On the 2nd day
after the operation, 10 ml was drained from near the
pancreaticojejunostomy, 15 ml was drained from near the
cholangiojejunostomy, and the amylase in the drainage fluid of the
pancreaticojejunostomy was measured at 1,436 U/l. On the 3rd day
after the operation, there was no liquid from the drainage tubes.
On the 5th day after the operation, the patient ate food. On the
10th day after the operation, no peritoneal effusion was found in
the abdominal cavity by B-ultrasound examination, and the abdominal
drainage tubes were removed. The increase of drainage volume cannot
be determined to be caused by eating, as it may also be caused by
the unobstructed drainage tube. The patient was discharged on the
12th day after the operation with no postoperative complications.
The patient was followed up in the 1st month and 3rd month after
the operation, and there was no abnormality. The patient will
follow up every year for 5 consecutive years. Pathological results
revealed chronic pancreatitis, pancreatic pseudocyst, focal
necrosis of duodenal mucosa and chronic cholecystitis.
Discussion
The anatomical structure of the pancreaticoduodenal
region is complex. Pancreaticoduodenectomy has the characteristics
of being a difficult operation, with a high risk and numerous
potential complications. At present, laparoscopic or robotic
pancreaticoduodenectomy is still one of the most challenging
operations in abdominal surgery (5).
The relationships between the tumor and surrounding
structures, such as the portal vein, superior mesenteric artery,
superior mesenteric vein, celiac trunk, common hepatic artery,
common bile duct and pancreatic duct, need to be accurately judged
before evaluating whether the tumor can be resected (6). At present, CT and MRI are the main
imaging examinations before surgery, and both techniques provide
two-dimensional images, which cannot display the relationship
between the tumor and important pipelines in a three-dimensional
manner. It is also difficult to differentiate between blood vessels
in the surgical area (7). The 3D
reconstructed image can stereoscopically display the relationship
between the tumor and the surrounding structures, and display the
variation in the blood vessels in the operative area, which is
helpful for selecting the surgical plan, for reducing accidental
injuries during the operation and for shortening the surgical
duration (8).
The main reason for the poor surgical outcome of
pancreatic cancer is tumor recurrence and metastasis. Lymph node
dissection and negative resection margins are of great significance
for improving the surgical effect and prolonging the survival time
of patients (9,10). There are fat, lymph, nerve and
other tissues behind the pancreatic head, which is the site where
tumors are prone to metastasize and recur. The 3D reconstructed
image plays an important role in the complete removal of fat,
lymph, nerves and other tissues behind the pancreatic head. The
diameter and position of the pancreatic duct can be displayed on
the 3D reconstructed image, which is helpful for revealing the
pancreatic duct during surgery. For patients who need vascular
resection and reconstruction, the 3D reconstructed image can
display information such as the position of tumor invasion, the
deformed length and angle of the involved blood vessel, which is
helpful for choosing the method of vascular resection and
reconstruction.
In laparoscopic or robotic pancreaticoduodenectomy,
the 3D reconstructed image is matched with the actual image by
image projection in order to aid navigation in the operation. The
shape of the navigation image is adjusted by computer software
according to the actual image, so that the actual image and the
navigation image are accurately matched. The tumor, pancreatic
duct, common bile duct and surrounding blood vessels can be made 3D
and visualized, so that surgeons can quickly identify important
anatomical structures, reduce accidental injuries, reduce
intraoperative bleeding and increase the speed of the operation.
The position of pancreatic dissection determined before surgery can
also be projected to the intraoperative image to guide the
dissection of the pancreas.
The difficulty and risk of the operation are
increased for the variant hepatic artery. If the variant hepatic
artery is damaged, the blood perfusion of the liver and biliary
tract in the corresponding area will be affected, and complications
such as liver dysfunction, bile leakage and bleeding may be
incurred after surgery (11,12).
Navigation can display the shape, course and adjacent relationship
of the variant hepatic artery, which is of great significance for
preventing the damage of variant blood vessels. There are small
branches of the pancreatic duct in pancreatic tissue, and
postoperative pancreatic leakage is hard to avoid. The 3D
reconstructed image can show the course of the pancreatic duct, as
well as the number and shape of the main pancreatic duct and the
auxiliary pancreatic duct. Knowledge of the exact location of the
pancreatic duct is helpful to prevent pancreatic leakage after
surgery.
There is currently rapid development of laparoscopic
and robotic pancreatic surgery techniques (13,14),
which provide the advantages of less trauma, faster recovery and
fewer complications (15,16). After surgery, patients can receive
comprehensive treatment earlier and thus the prognosis is improved.
Laparoscopic and robotic surgery can magnify the view of the
surgical field, but cannot see through important anatomical
structures in the surgical field and do not have the sense of touch
of the human hand. The risk of surgery is increased due to tumor
growth that deforms vital anatomical structures around the tumor
and deviates some important pipelines from their original
positions, and also due to the variation of anatomical structures,
especially ectopic important pipelines. In the operation of 3D
laparoscopic tumor resection, the use of navigation technology can
quickly identify important anatomical structures, identify the
deformation and ectopic anatomical structures, avoid unnecessary
injuries, and make the operation more convenient and safe.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD and ZL were involved in data analysis and
interpretation, conception and design of the study, and drafted the
manuscript. ML, SK, XL and JZ participated in the operation,
collected the data and confirm the authenticity of the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kanghua Hospital (Dongguan, China; approval no.
22015). Written informed consent was obtained from the patient.
Patient consent for publication
The patient provided written consent and agreed for
their data (shown in the figures) to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karim SAM, Abdulla KS, Abdulkarim QH and
Rahim FH: The outcomes and complications of pancreaticoduodenectomy
(Whipple procedure): Cross sectional study. Int J Surg. 52:383–387.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang H, Guo X, Xia J, Zhu F, Shen M, Wang
X, Wang M and Qin R: Comparison of Totally 3-Dimensional
laparoscopic pancreaticoduodenectomy and open
pancreaticoduodenectomy. Pancreas. 47:592–600. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sabri SA and York PJ: Preoperative
planning for intraoperative navigation guidance. Ann Transl Med.
9(87)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zimmerman AM, Roye DG and Charpentier KP:
A comparison of outcomes between open, laparoscopic and robotic
pancreaticoduodenectomy. HPB (Oxford). 20:364–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumar RP, Pelanis E, Bugge R, Brun H,
Palomar R, Aghayan DL, Fretland ÅA, Edwin B and Elle OJ: Use of
mixed reality for surgery planning: Assessment and development
workflow. J Biomed Inform. 112S(100077)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gabrielli ME, Saun TJ, Jung JJ and
Grantcharov TP: Assessment of 3-Dimensional vs 2-Dimensional
imaging and technical performance using a multiport intraoperative
data capture and analytic system for patients undergoing
laparoscopic Roux-en-Y gastric bypass surgery. JAMA Netw Open.
3(e1920084)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Simpfendörfer T, Li Z, Gasch C, Drosdzol
F, Fangerau M, Müller M, Maier-Hein L, Hohenfellner M and Teber D:
Three-Dimensional reconstruction of preoperative imaging improves
surgical success in laparoscopy. J Laparoendosc Adv Surg Tech A.
27:181–185. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aiolfi A, Lombardo F, Bonitta G, Danelli P
and Bona D: Systematic review and updated network meta-analysis
comparing open, laparoscopic, and robotic pancreaticoduodenectomy.
Updates Surg. 73:909–922. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bencini L, Urciuoli I, Trafeli M, Paolini
C, Moraldi L, Tribuzi A, Pacciani S and Coratti A: Robotic
pancreatic surgery: Minimally invasive approach to challenging
operations. Minerva Surg. 76:138–145. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Subar D, Gobardhan PD and Gayet B:
Laparoscopic pancreatic surgery: An overview of the literature and
experiences of a single center. Best Pract Res Clin Gastroenterol.
28:123–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kawaida H, Kono H, Hosomura N, Amemiya H,
Itakura J, Fujii H and Ichikawa D: Surgical techniques and
postoperative management to prevent postoperative pancreatic
fistula after pancreatic surgery. World J Gastroenterol.
25:3722–3737. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu DB, Zhao ZM, Xu Y and Liu R: Hybrid
pancreatoduodenectomy in laparoscopic and robotic surgery: A
single-center experience in China. Surg Endosc. 35:1703–1712.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nassour I, Paniccia A, Moser AJ and
Zureikat AH: Minimally invasive techniques for pancreatic
resection. Surg Oncol Clin N Am. 30:747–758. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chalikonda S, Aguilar-Saavedra JR and
Walsh RM: Laparoscopic robotic-assisted pancreaticoduodenectomy: A
case-matched comparison with open resection. Surg Endosc.
26:2397–2402. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kabir T, Tan HL, Syn NL, Wu EJ, Kam JH and
Goh BKP: Outcomes of laparoscopic, robotic, and open
pancreatoduodenectomy: A network meta-analysis of randomized
controlled trials and propensity-score matched studies. Surgery.
171:476–489. 2022.PubMed/NCBI View Article : Google Scholar
|