Introduction
Clinically, myasthenia gravis (MG) and Lambert-Eaton
syndrome are characterized by muscle weakness and easy
fatigability. These symptoms are brought about by an impairment of
nerve and muscle synaptic transmission (1). MG is often caused by autoantibodies
to the acetylcholine receptors on the postsynaptic membrane
(2), whereas, Lambert-Eaton
syndrome develops mainly due to autoantibodies to calcium channels
in the presynaptic membrane (3).
Numerous patients with MG also develop thymic abnormalities such as
thymoma and thymus hyperplasia (2). In contrast, Lambert-Eaton syndrome
frequently occurs concomitantly with lung cancer, especially small
cell lung cancer (3). Patients
with lung cancer who develop MG are extremely rare (4-17).
Furthermore, there have been no reports of MG developing several
years after the lung cancer was completely resected. We herein
report a rare case of MG that occurred seven years after a surgical
resection of lung cancer. This patient developed myasthenic crisis,
a critical condition in which rapidly occurred severe weakness of
the pharyngeal and respiratory muscles. After successful treatment
with mechanical ventilation, plasma exchange and
methylprednisolone, the patient has been followed up for more than
6 years.
Case report
A 58-year-old man underwent upper lobectomy and
mediastinal lymph node dissection due to T2aN0M0, stage IB lung
adenocarcinoma (Fig. 1) at Ibaraki
Medical Center, Tokyo Medical University (Ami, Ibaraki, Japan) in
January 2008. He had neither thyroid nor other autoimmune
disorders, nor a family history of neuromuscular or autoimmune
disorder. He did not have any postoperative recovery difficulties
that might have suggested pre-existing neuromuscular transmission
disorder. Fifteen months after resection, there was swelling of the
left lower tracheal bronchial lymph node, which was irradiated with
60 Gy. Six years after the end of irradiation, he presented at Mito
Medical Center, University of Tsukuba (Mito, Ibaraki, Japan) with a
chief complaint of double vision, bilateral eyelid drooping, and
difficulty swallowing for one month. He was not taking any
antiarrhythmic agents or other drugs impairing neuromuscular
transmission. Development of thymic disease and recurrence of lung
cancer were ruled out on imaging studies. Neurologic examination
showed ptosis and gaze palsy with facial muscle weakness and
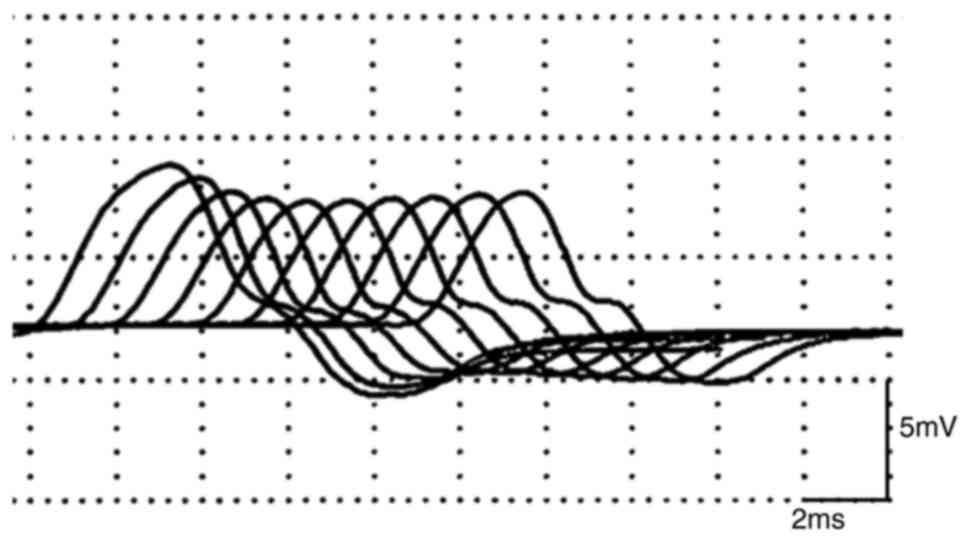
reduced gag reflex. The repetitive nerve stimulation test (RNS)
showed abnormal (>10%) decrement in facial, accessory, median
nerve (Fig. 2; median nerve,
stimulation frequency 10 Hz). Waning was observed in the repeated
stimulation test, and the serum anti-acetylcholine receptor (AchR)
antibody level was 67.0 nmol/l (normal: up to 0.2 nmol/l). With
these findings, we diagnosed generalized MG of Ossermann II type.
In addition to cholinesterase inhibitors (180 mg/day, oral), we
started plasma exchange and treatment with prednisolone (2.5
mg/day, oral) which was gradually increased. On day 26 after
hospitalization, he had acute respiratory failure and needed
mechanical ventilation for myasthenic crisis. He had pulse therapy
with methylprednisolone at 1,000 mg/day (three days) and plasma
exchange six times within a month, and was successfully discharged
from the hospital. Prednisolone (60 mg/day) gradually decreased to
10 mg/day, and pyridostigmine (180 mg/day, oral) continued at the
same dose for 16 weeks. However, the symptoms of MG worsened 17 and
25 months after the start of treatment for MG, and the dose of
prednisolone was increased up to 30 mg/day and plasma exchange was
performed again. Symptoms of MG were improved, and these drugs
gradually decreased. Six years have passed since the end of
treatment, but neither recurrence of lung cancer nor development of
thymoma has been confirmed. AchR antibody level in recent years has
been 6.5-8.2 nmol/l, which are higher than the normal value, but
the symptoms associated with MG are controlled. At present,
prednisolone has been tapered down to 60 mg/day and 13 mg/day,
respectively. Fig. 3 shows a
timeline with relevant data from the episode of care.
Discussion
When symptoms such as double vision, eyelid
drooping, and difficulty in swallowing occur in patients with a
history of thymic disease, a patient may have developed MG and
should be diagnosed and treated accordingly. When such myasthenic
symptoms manifest in patients with lung cancer, Eaton-Lambert
syndrome should be included in the differential diagnosis. In our
patient, whole-body imaging studies, including of the chest, showed
no recurrence and no development of thymoma for 14 years after the
diagnosis of lung cancer. MG was diagnosed on the basis of elevated
serum AchR level, a typical decreasing response to repeated
stimulation of EMG, and positive findings on the tensilon test.
The first case with MG associated with lung cancer
was reported by Hazard et al in 1986(4). Since then, to our best knowledge, 14
lung cancer patients with MG have been reported (4-17).
Of these 14 cases, six were small cell lung cancer, one was LCNEC,
and seven were non-small cell lung cancer. Previous reports have
reported that MG onset was more common in patients with small cell
lung cancer (4-6,13,16,17),
but there were cases of non-small cell lung cancer that also
developed MG (7-12,14,15).
Regarding the onset time of lung cancer and MG, nine patients were
diagnosed with lung cancer at the time of MG onset (4,5,9,10,12-16).
In three cases, however, the onset of MG preceded the diagnosis of
lung cancer by eight months to six years (6,8,17).
On the other hand, in two cases, the onset of MG anteceded the
diagnosis of lung cancer by two to three years (7,11).
One patient developed MG when lung cancer recurred (11). In this patient, chemotherapy,
radiation therapy, and surgical resection were performed, but two
years later, lung cancer recurred with pleural effusion and MG
developed (11). Interestingly, in
one case, as in our patient, the diagnosis of lung cancer preceded
the onset of MG (7). This patient
had developed MG three years after the diagnosis of lung cancer.
Due to invasion of the vertebral body at the time of his diagnosis
of lung cancer, complete resection was not performed. The
pathological diagnosis of lung cancer in this patient was poorly
differentiated anaplastic malignancy with a number of atypical
cells and multiple mitoses consistent with large-cell carcinoma of
the lung (7). One year later, the
patient developed adenocarcinoma of the other lung and he died of
systemic complications of MG ~2 years after the initial diagnosis
of MG (7). Considering this
course, the possibility of residual lung cancer and involvement of
new lung cancer in the onset of MG could not be ruled out in this
patient. In our patient with completely resected stage IB lung
cancer, there had been no lung cancer recurrence and no development
of thymoma until the onset of MG six years after irradiation.
Although the mechanism was unknown, considering that AchR antibody
levels were higher than normal even in recent years, we speculated
that the memory of immunocompetent cells was involved in the onset
of MG (18), not the residual
lesions of lung cancer.
Treatment of advanced lung cancer has made great
strides in the last decade; particularly, treatment with immune
checkpoint inhibitors (ICPIs) has significantly improved prognosis.
Alongside, the immune-related side effects (irAEs) caused by ICPI
have attracted attention, including ICPI-related MG (19,20).
Safa et al reported that MG is a life-threatening irAE of
acute onset and rapid progression after ICPI initiation (19). In a previous review of the 23
reported cases of ICPI-associated MG, 72.7% were de novo
presentations, 18.2% were exacerbations of pre-existing MG and 9.1%
were exacerbations of subclinical MG (20). Our patient did not receive any ICPI
treatment, therefore, the possibility of developing ICPI-related MG
can be completely ruled out. However, ICPI-related MG is an
interesting irAE from the viewpoint of pathogenesis and treatment
method, and future research will be expected.
Survival time was described in 6 lung cancer
patients with MG (7,8,10,15-17).
Three of them died within two years after initiation of therapy for
these diseases (7,16,17).
Only one patient was followed up for more than 3 years (10). This patient had lung cancer and MG
found at the same time, and the symptoms of MG disappeared after
lung cancer resection, and he had been taking pyridostigmine
bromide for 4 years and 2 months (10). In our patient, recurrence occurred
at the mediastinal lymph nodes 15 months after the resection, and
radical irradiation was performed on the mediastinum. MG developed
6 years after the irradiation. No additional treatment for lung
cancer was subsequently given, but there has been no recurrence of
lung cancer for 6 years. To the best of our knowledge, this was the
longest survived patient, and is interesting in terms of treatment
of lung cancer and MG.
In our patient, recurrence of lung cancer or
development of thymic disease could be ruled out on imaging studies
for more than six years after MG was controlled. We must carefully
follow up the patient not to overlook the recurrence of lung cancer
and the development of thymic disease, even if MG is controlled.
This case indicates that physicians should be aware that MG as a
potential comorbid disease can develop in patients with a history
of lung cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO, AS, KI, KF and HS contributed to the planning,
conduct, reporting, conception, design and acquisition of data and
drafting the manuscript. All authors read and approved the final
manuscript. SO and HS confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of the Mito Medical Center, University of Tsukuba-Mito Kyodo
General Hospital (approval no. 16-39).
Patient consent for publication
Written informed consent from the patient for the
publication of their data was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rodríguez Cruz PM, Cossins J, Beeson D and
Vincent A: The neuromuscular junction in health and disease:
Molecular mechanisms governing synaptic formation and homeostasis.
Front Mol Neurosci. 13(610964)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dresser L, Wlodarski R, Rezania K and
Soliven B: Myasthenia gravis: Epidemiology, pathophysiology and
clinical manifestations. J Clin Med. 10(2235)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang K, Luo YB and Yang H: Autoimmune
channelopathies at neuromuscular junction. Front Neurol.
10(516)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hazard PB, Bertorini TE and Griffin JP:
Myasthenia gravis associated with small cell carcinoma of the lung.
J Tenn Med Assoc. 79:273–276. 1986.PubMed/NCBI
|
|
5
|
Fujita J, Yamadori I, Yamaji Y, Yamagishi
Y, Takigawa K and Takahara J: Myasthenia gravis associated with
small-cell carcinoma of the lung. Chest. 105:624–625.
1994.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miyoshi R, Yamaji Y, Shima S, Fujita J,
Okada H and Takahara J: A case of small cell lung cancer that
developed during therapy for myasthenia gravis. Nihon Kyobu Shikkan
Gakkai Zasshi. 33:456–462. 1995.PubMed/NCBI(In Japanese).
|
|
7
|
Leavitt JA: Myasthenia gravis with a
paraneoplastic marker. J Neuroophthalmol. 20:102–105.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sakamaki Y, Yoon HE and Oda N:
Non-small-cell lung cancer associated with non-thymomatous
myasthenia gravis. Jpn J Thorac Cardiovasc Surg. 54:207–211.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takagi M and Akiba T: A case of surgically
treated primary lung cancer with myasthenia gravis. Nihon Kokyuki
Gakkai Zasshi. 45:198–201. 2007.PubMed/NCBI(In Japanese).
|
|
10
|
Kataoka K, Fujiwara T, Matsuura M and Seno
N: A case of simultaneously operated primary multiple lung cancers
with nonthymomatous myasthenia gravis. Jpn J Lung Cancer.
49:273–277. 2009.(In Japanese).
|
|
11
|
Peltier AC, Black BK, Raj SR, Donofrio P,
Robertson D and Biaggioni I: Coexistent autoimmune autonomic
ganglionopathy and myasthenia gravis associated with non-small-cell
lung cancer. Muscle Nerve. 41:416–419. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaygannejad V, Ghasemi M and Rajaee Z:
Myasthenia gravis as a presenting feature in a patient with lung
cancer: A case report. J Res Med Sci. 16:229–232. 2011.PubMed/NCBI
|
|
13
|
Ohira M, Jeong D and Oh SJ: Seropositive
myasthenia gravis associated with small-cell lung carcinoma. J Clin
Neurol. 7:43–46. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Takizawa M, Oda M, Matsumoto I, Waseda R,
Tanaka N and Watanabe G: Myasthenia gravis complicated with lung
cancer and middle mediastinal thymoma. Asian Cardiovasc Thorac Ann.
20:486–488. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Niimi K, Nagata E, Murata N, Sato M,
Tanaka J, Horio Y, Takiguchi H, Tomomatsu H, Tomomatsu K, Hayama N,
et al: Lung cancer associated with seronegative myasthenia gravis.
Intern Med. 54:1381–1384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yamasaki M, Funaishi K, Saito N, Yonekawa
T, Yamawaki T, Ihara D, Daido W, Ishiyama S, Deguchi N, Taniwaki M
and Hattori N: Acetylcholine receptor antibody-positive myasthenia
gravis associated with small-cell lung cancer: A case report.
Medicine (Baltimore). 97(e0541)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jia R, Chen J, Ge R, Zheng Q, Chen F and
Zhao Z: Coexistence of myasthenia gravis and Lambert-Eaton
myasthenic syndrome in a small cell lung cancer patient: A case
report. Medicine (Baltimore). 97(e10976)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stathopoulos P, Kumar A, Heiden JA,
Pascual-Goñi E, Nowak RJ and O'Connor KC: Mechanisms underlying B
cell immune dysregulation and autoantibody production in MuSK
myasthenia gravis. Ann NY Acad Sci. 1412:154–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Safa H, Johnson DH, Trinh VA, Rodgers TE,
Lin H, Suarez-Almazor ME, Fa'ak F, Saberian C, Yee C, Davies MA, et
al: Immune checkpoint inhibitor related myasthenia gravis: Single
center experience and systematic review of the literature. J
Immunother Cancer. 7(319)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Makarious D, Horwood K and Coward JI:
Myasthenia gravis: An emerging toxicity of immune checkpoint
inhibitors. Eur J Cancer. 82:128–136. 2017.PubMed/NCBI View Article : Google Scholar
|