Introduction
Breast cancer is one of the commonest female
malignancy worldwide, accounting for 25% of female cancer cases
(1). The morbidity of breast
cancer is up to 11.6%, with mortality of 6.6% across the world
(2). Patients with high tumor
stage have rather poor prognosis. In recent years, more and more
patients with breast cancer in early stage have been diagnosed by
breast cancer screening (3).
Consequently, surgery can be one of the options for them.
Mastectomy is the commonest surgical procedure for
patients with breast cancer in China (4). A variety of complications, such as
skin flap necrosis, wound dehiscence, inferior quality scarring,
and infection, may be triggered by tissue resection with high
tension wound closure, excessive wound gap and defects in surgery.
In some cases, a skin graft or flap mobilization can be used if the
surgical wound cannot be primarily closed (5). TopClosure® tension-relief
system (TRS) is a simple and practical system for skin stretching
and wound closure-secure. For patient undergoing valgus cystectomy,
TRS is a useful method to repair the large abdominal defect in
abdominal reconstruction with shorter operative duration and
hospital stay. TRS can clearly improve wound aesthetics (6). TRS shows high efficacy for the
closure of moderate and large scalp defects and serves as a topical
tension-relief platform for tension sutures (6). TRS can reduce local complications,
shorten hospital stay and reduce donor site morbidity (7). However, few studies report the effect
of TRS application on patients with breast cancer undergoing
mastectomy.
The present study was a randomized controlled study
to investigate the effect of TRS on clinical outcomes and prognosis
for patients with breast cancer undergoing mastectomy. The results
showed that application of TRS could significantly improve the
clinical outcomes, attenuated inflammation and improve wound
aesthetics.
Materials and methods
Patients
The present study enrolled 402 female patients
diagnosed with breast cancer, who underwent mastectomy without
reconstruction between March 2014 and June 2018. The inclusion
criteria were: i) All the patients were diagnosed as breast cancer
by histopathological examination; ii) breast cancer was diagnosed
for the first time and identified as primary breast cancer; iii)
none of the patients received any chemotherapy or radio-therapy
before the study and iv) patients were without serious
cardiovascular diseases, renal, or liver dysfunctions. The
following patients were excluded: i) Patients with other primary
malignant tumors or metastatic breast cancer and ii) patients who
had received prior breast surgery. Cancer stage was evaluated
according to the 8th edition of American Joint Committee on Cancer
(AJCC) Cancer Staging Manual (8).
The present study was approved by the Ethics Committee of Renmin
Hospital of Wuhan University (approval number WHRMH-214-019A).
Written informed consent was obtained from all participants.
All patients received mastectomy and were randomly
divided into two groups, TRS group and control group (n=201 for
each group) using a computer-generated list by Rv. Uniform formula
using SPSS software (SPSS Inc.). Patients in TRS group used a
Tension-relief System (TopClosure®, IVT Medical Ltd.)
for wound closure and patients in control group received primary
suture closure after mastectomy.
TopClosure® TRS
After mastectomy, attachment plates were placed ~3-5
cm away from wound edges, rendered firmly adherent to the skin on
both sides and secured to the skin-by-skin staples (Weck; Teleflex
Incorporated.). Wound edges were approximated by stress relaxation
through tension sutures (Ethicon, Inc.) as previously described
elsewhere (9). A strap was then
tightened gradually to finalize immediate primary closure of the
wound. Drains were placed in the axilla or under the flap. Definite
need for closure with skin graft or flap was considered technically
as a failure.
All patients were managed according to a standard
protocol. Drains were removed once daily with drainage <50 ml.
Patients were discharged if drains were removed without signs of
wound or systemic complication requiring in-hospital treatment.
Measurement of inflammatory factors
and white blood cell count
Serum levels of high-sensitivity C-reactive protein
(hs-CRP), TNF-α, IL-6 and procalcitonin (PTC) were detected using
ELISA. Commercial ELISA kits used were as follows: hs-CRP (cat. no.
MBS3800421; range ~1-16 mg/l; MyBioSource, Inc.), TNF-α (cat. no.
ab181421; range ~15.63-1,000 pg/ml), IL-6 (cat. no. ab178013; range
~7.8-500 pg/ml) and PTC (cat. no. ab221828; range ~6.25-400 pg/ml;
all from Abcam). White blood cell count was detected using a
Coulter automatic blood cell analyzer DxH800 (Beckman Coulter,
Inc.).
Vancouver scar scale (VSS) and 36-item
health survey scales
The present study used VSS to measure the scar
conditions of the patients. The 36-Item Health Survey Scales
(10) were used for measurement of
quality of life.
The VSS provides a numerical score of the worst
portion of a scar to describe scar quality, rating characteristics
of pigmentation, vascularity, pliability and height (11). Higher score of VSS generally
indicates worse condition for scaring. The 36-Item Health Survey
Scales is usually used for evaluating quality of life (12). The 36-Item Health Survey Scales
contains eight parameters, including physical function,
role-physical, pain, general health, emotional well-being,
role-emotional, social function and energy/fatigue. Higher score in
each parameter suggests improved quality of life.
Data collection
Demographic and clinical data included age, body
mass index, tumor stage, complications, side of operation,
pathological type, tension of skin flap closure, hospital length of
stay, flap necrosis, total volume of aspirate and the incidence of
infection and subcutaneous liquid accumulation. VSS score was
recorded at 2 weeks and 1-3 and 6 months following surgery,
respectively. The 36-Item Health Survey Scales were performed for
all patients at 1 month following surgery. Once mastectomy was
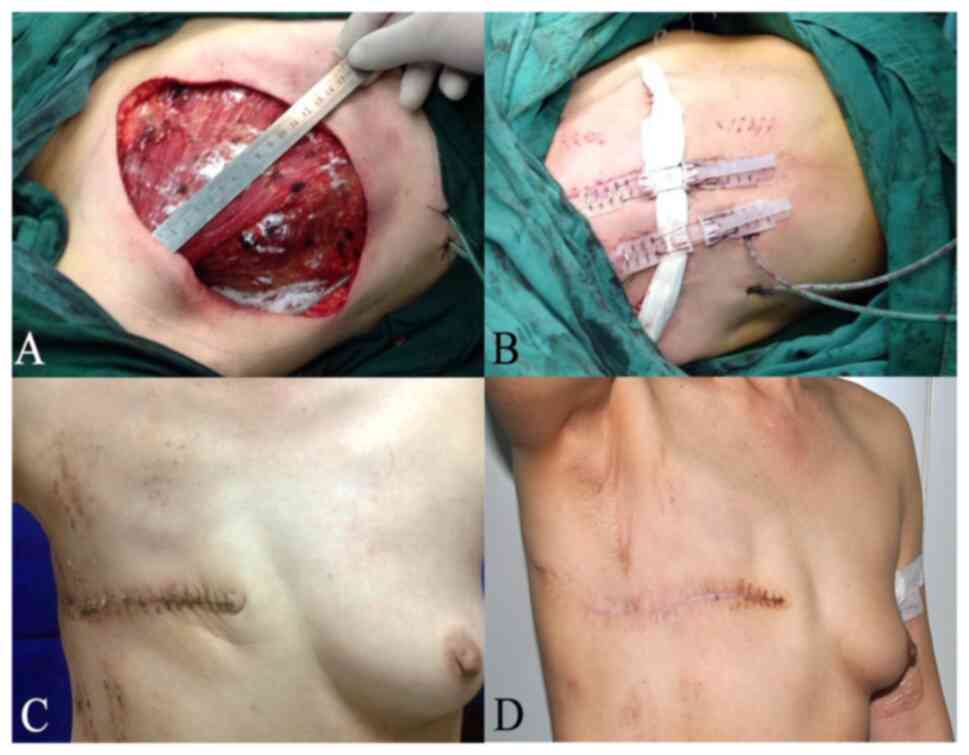
completed, the margin gap created was assessed intraoperatively
(Fig. 1A-D). Patients with margin
gaps <4 cm were considered as having a low-tension closure wound
closure whereas patients with margin gaps >4 cm were considered
as having a high-tension wound closure. All the patients were
followed up for 6 months.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc.). Normally distributed data were expressed by mean ±
standard deviation, while non-normally distributed data were
expressed by median (range). Comparison between two groups was made
using the Student's t-test or Mann-Whitney U test. Comparison among
three or more groups was conducted using one-way analysis of
variance followed by Tukey post hoc test. The rates were compared
by χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
The baseline characteristics of the
two groups
The present study enrolled 402 patients with breast
cancer undergoing mastectomy. The mean age was 43.7±6.89 years. The
baseline characteristics of all patients are listed in Table I. Patients in the TRS group had
higher ratio of skin flap closure with low-tension than the control
group. There were no significant differences for the other basic
characteristics between the two groups. Fig. 1A-D showed the process of wound
healing for a 46-year-old patient undergoing modified radical
mastectomy with TopClosure® TRS application.
 | Table IBaseline characteristics of all
patients. |
Table I
Baseline characteristics of all
patients.
| Characteristic | TRS group n=201 | Control group
n=201 | P-value |
|---|
| Age, year | 45.22±8.67 | 44.65±7.69 | 0.488 |
| BMI,
kg/m2 | 24.05±2.49 | 23.88±1.94 | 0.422 |
| Hypertension, n
(%) | 28 (13.93) | 26 (12.94) | 0.770 |
| Diabetes, n (%) | 18 (8.96) | 20 (9.95) | 0.865 |
| Side of
operation | | | |
|
Left, n
(%) | 113 (56.22) | 96 (47.76) | 0.110 |
|
Right, n
(%) | 88 (43.78) | 105 (52.24) | |
| TNM stage, n (%) | | | |
|
I | 35 (17.41) | 40 (19.90) | 0.792 |
|
II | 126 (62.69) | 124 (61.69) | |
|
III | 40 (19.90) | 37 (18.41) | |
| Pathological type, n
(%) | | | |
|
Invasive
ductal carcinoma | 151 (75.12) | 159 (79.10) | 0.598 |
|
Invasive
lobular carcinoma | 34 (16.92) | 30 (14.93) | |
| Mucinous
adenocarcinoma Tumor diameter, n (%) | 16 (7.96) | 12 (5.97) | |
|
>2
cm | 155 (77.11) | 141 (70.15) | |
|
≤2 cm | 46 (22.89) | 60 (29.85) | |
| Skin flap
closure | | | |
|
LTW, n
(%) | 109 (54.23) | 121 (60.20) | 0.267 |
|
HTW, n
(%) | 92 (45.77) | 80 (39.80) | |
TopClosure® TRS attenuates
inflammatory response in postoperative patients with breast
cancer
Serum levels of hs-CRP, TNF-α, IL-6 and PTC and
white blood cells (WBC) were detected 2 weeks following surgery.
The results showed that the levels of WBC, hs-CRP, TNF-α and IL-6
in TRS group were evidently lower compared with the control group
(Table II). No significant
difference was observed for serum PTC contents between the two
groups. This suggested that TRS application clearly attenuated
inflammation in patients with breast cancer following surgery.
 | Table IIComparison of inflammatory factors
between the two groups. |
Table II
Comparison of inflammatory factors
between the two groups.
| Inflammatory
factor | TRS group, n=201 | Control group,
n=201 | P-value |
|---|
| WBC,
x109/l | 8.96±2.35 | 10.32±2.51 | <0.001 |
| hs-CRP, mg/l | 1.05±0.16 | 1.13±0.25 | <0.001 |
| TNF-α, pg/ml | 103.97±20.79 | 128.75±34.74 | <0.001 |
| IL-6, pg/ml | 294.05±61.54 | 354.02±91.43 | <0.001 |
| PTC, ng/ml | 163.73±80.79 | 168.37±65.24 | 0.526 |
TopClosure® TRS improves
the clinical outcomes of postoperative patients with breast
cancer
The clinical outcomes were analyzed for patients
with breast cancer following surgery. As shown in Table III, compared with the controls,
TRS application significantly reduced the incidence of flap
necrosis (0/201; 0%) and infection (8/201; 3.98%) as well as the
duration of hospital stay (10.62±1.52 days). The findings indicated
TRS application markedly improved clinical outcomes for patients
with breast cancer following surgery.
 | Table IIIComparison of clinic outcomes between
two groups. |
Table III
Comparison of clinic outcomes between
two groups.
| | TRS group
n=201 | Control group
n=201 | P-value |
|---|
| Flap necrosis, n
(%) | 0 (0) | 28 (13.93) | <0.001 |
| Seroma formation, n
(%) | 38 (18.91) | 43 (21.39) | 0.619 |
| Total volume of
aspirate, ml | 378±72.87 | 384.69±77.87 | 0.485 |
| Infection, n
(%) | 8 (3.98) | 30 (14.92) | <0.001 |
| Hospital length of
stay, days | 11.17±2.05 | 13.22±3.18 | <0.001 |
TopClosure® TRS improves
quality of life and decreases severity of scarring
In order to evaluate the quality of life, a survey
of 36-Item Health Survey Scales was conducted for all patients at 1
month following surgery. The result revealed that patients
receiving TRS treatment had higher scores of physical function,
emotional well-being and general health (Table IV). The VSS scores were recorded
at 2 weeks and 1-3 and 6 months following surgery. As shown in
Fig. 2, VSS score of patients in
TRS group was significantly lower than the controls, suggesting
improved wound healing. These results suggested that TRS
application notably improved qulaity life and the scar
outcomes.
 | Table IVComparison for 36-Item Health Survey
Scales between two groups. |
Table IV
Comparison for 36-Item Health Survey
Scales between two groups.
| Parameters | TRS group
n=201 | Control group
n=201 | P-value |
|---|
| Physical
function | 55.12±18.07 | 49.58±13.47 | 0.001 |
| Role-physical | 40.55±26.88 | 38.06±27.73 | 0.362 |
| Pain | 44.84±15.93 | 47.30±18.24 | 0.149 |
| General health | 37.75±12.60 | 34.05±14.93 | 0.008 |
| Emotional
well-being | 50.31±19.11 | 44.22±15.10 | <0.001 |
| Role-emotional | 33.67±27.69 | 34.49±26.95 | 0.761 |
| Social
function | 39.30±25.04 | 35.88±24.18 | 0.164 |
| Energy/fatigue | 68.81±18.52 | 65.60±18.59 | 0.084 |
Discussion
Skin flap necrosis is a common complication of
mastectomy affecting ~3-32% of patients (13-15).
In order to avoid skin flap necrosis, accelerate wound healing and
to shorten hospital stay, the application of skin-stretching
devices has been described in various studies (16-18).
In the present study, TRS skin stretching together with secure
wound closure device were used to treat the surgical wounds of
mastectomy. It was observed that TRS notably improved the clinical
outcomes and quality of life as well as decreasing the severity of
scarring and inflamation. Consequently, TRS might be a potential
skin-stretching device for patients with breast cancer during
mastectomy.
In the present study, no skin flap necrosis was
observed in TRS group. This might be attributed to the novel
characteristics of the TRS. Reportedly, two malleable attachment
plates (APs) in TRS can be flexibly fixed to wound margins. Primary
wound closure can be achieved by stress relaxation through tension
sutures without injuring the underlying skin. The plates serve as a
tension-relief platform, shielding the skin from direct damage of
the tension sutures, reducing modified radical mastectomy flap
tension, especially in cases of high-tension flaps. Moreover, the
flap is tightly fixed to the chest after fixation and stretch of
the APs. Thus, eliminating dead space and promoting angiogenesis
(19-21).
Hence, incidence of flap necrosis is reduced. Furthermore, the
device can also be used for immediate or delayed primary closure of
large wounds by stress relaxation during surgery (7,22).
By contrast, conventional (relaxation-suturing) tension suturing
induces high and non-uniform tension on the flap margins, resulting
in local ischemia, necrosis, scarring and wound dehiscence. Due to
the expected high-tension closure and high rate of recurrence,
primary closure is not suitable for large skin defects. Therefore,
TRS has become a promising option for skin stretching and wound
closure-secure for trauma and oncologic surgery.
Emerging evidence illustrates that TRS serves as
substitute for skin grafts, tissue expanders and flap (23). The TRS significantly restrained
blood loss and reduced donor site morbidity, as well as improving
wound aesthetics and minimizing the risk for future reconstructive
procedure in a 3-day-old female infant undergoing surgical
resection of the giant scalp hemangioma (24). The same results can be found in
some other cases. For instance, a case study on a 36-year-old man
receiving surgical resection of the keloid showed that primary
closure with TRS contributed to simplified surgical procedures and
reduced the operative time (25).
Meanwhile, TRS contributes to cosmetic improvements of scarring and
decreases the probability of future reconstructive procedures of
anterior chest wall (25).
Ashkenazi et al (26) found
that a large skin defect treated with TRS could avoid extending the
scope of surgery with skin grafting or flaps, as well as reducing
possibility of donor site morbidity. In addition, TRS exhibits the
potential for immediate primary closure of high-tension mastectomy
wounds (26). For the closure of
extensive wounds, TRS reduces the incidence of major complications,
such as wound dehiscence or infections; moreover, all healed wounds
were stable in one-year follow-up (27).
However, the application of TRS is only illustrated
in few case reports. The present study conducted a randomized
controlled study to investigate the effect of TRS on breast surgery
for patients with breast cancer. The results showed that TRS
reduced the incidence of flap necrosis and infection, shortened
hospital stay, improved wound aesthetics and the quality of life.
These findings were consistent with the above previous studies
(24-27).
Surgery may trigger acute inflammatory response, which shows close
association with the clinical outcomes. Lee et al (28) reveals that postoperative
inflammation was an important risk factor for the mortality of
patients with breast cancer. The present study noted that TRS
application decreased inflammation following surgery. Thus, it was
hypothesized that it might affect the prognosis in a long term,
which needs further investigation in the future.
The present study also had some limitations. One
concern about the findings is that the sample size was too small.
In addition, the data were collected from one single center instead
of multicenter.
In conclusion, skin stretching and secure wound
closure was effectively achieved by the TRS with primary closure.
TRS significantly improved clinical outcomes and the quality of
life for patients with breast cancer, as well as suppressing
postoperative inflammation and infections. TRS might be a novel
potential skin-stretching device for mastectomy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and HF conducted most of the experiments and HF
wrote the manuscript; HJ and ZZ conducted the experiments and YZ
analyzed the data, MY designed the study and revised the
manuscript. MY and HF confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University (approval number
WHRMH-214-019A).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Bray F, Ferlay J,
Lortet-Tieulent J, Anderson BO and Jemal A: International variation
in female breast cancer incidence and mortality rates. Cancer
Epidemiol Biomarkers Prev. 24:1495–1506. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17 (S3):S43–S46. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bleyer A, Baines C and Miller AB: Impact
of screening mammography on breast cancer mortality. Int J Cancer.
138:2003–2012. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He ZY, Tong Q, Wu SG, Li FY, Lin HX and
Guan XX: A comparison of quality of life and satisfaction of women
with early-stage breast cancer treated with breast conserving
therapy vs. mastectomy in southern China. Support Care Cancer.
20:2441–2449. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jagsi R, Jiang J, Momoh AO, Alderman A,
Giordano SH, Buchholz TA, Pierce LJ, Kronowitz SJ and Smith BD:
Complications after mastectomy and immediate breast reconstruction
for breast cancer: A claims-based analysis. Ann Surg. 263:219–227.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Olshinka A, Ad-El D, Kalish E, Shay T and
Yaacobi DS: Closure of challenging pediatric scalp wounds by a
tension-relief system. J Craniofac Surg. 32:e650–e652.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Topaz M, Carmel NN, Topaz G and Zilinsky
I: A substitute for skin grafts, flaps, or internal tissue
expanders in scalp defects following tumor ablative surgery. J
Drugs Dermatol. 13:48–55. 2014.PubMed/NCBI
|
|
8
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Topaz M, Carmel NN, Silberman A, Li MS and
Li YZ: The TopClosure® 3S system, for skin stretching
and a secure wound closure. Eur J Plast Surg. 35:533–543.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao H, Wu Y, Tao Y, Zhou C, De Vrieze T,
Li X and Chen L: Psychometric validation of the Chinese version of
the lymphedema functioning, disability, and health questionnaire
for upper limb lymphedema in patients with breast cancer-related
lymphedema. Cancer Nurs. 45:70–82. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Finlay V, Burrows S, Burmaz M, Yawary H,
Lee J, Edgar DW and Wood FM: Increased burn healing time is
associated with higher Vancouver scar scale score. Scars Burn Heal.
3(2059513117696324)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lins-Kusterer L, Valdelamar J, Aguiar CVN,
Menezes MS, Netto EM and Brites C: Validity and reliability of the
36-item short form health survey questionnaire version 2 among
people living with HIV in Brazil. Braz J Infect Dis. 23:313–321.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou DX and Duan GX: Causes of skin flap
necrosis after breast cancer surgery and preventive measures. Chin
J Gen Pract. 9:1718–1719. 2011.(In Chinese).
|
|
14
|
Reintgen C, Leavitt A, Pace E,
Molas-Pierson J and Mast BA: Risk factor analysis for mastectomy
skin flap necrosis: Implications for intraoperative vascular
analysis. Ann Plast Surg. 76 (Suppl 4):S336–S339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Larson DL, Basir Z and Bruce T: Is
oncologic safety compatible with a predictably viable mastectomy
skin flap? Plast Reconstr Surg. 127:27–33. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Santiago GF, Bograd B, Basile PL, Howard
RT, Fleming M and Valerio IL: Soft tissue injury management with a
continuous external tissue expander. Ann Plast Surg. 69:418–421.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Laurence VG, Martin JB and Wirth GA:
External tissue expanders as adjunct therapy in closing difficult
wounds. J Plast Reconstr Aesthet Surg. 65:e297–e299.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Senchenkov A: The use of continuous
external tissue expander for direct closure of anterolateral thigh
free flap donor sites. J Plast Reconstr Aesthet Surg. 67:1766–1767.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shyu KG, Chang ML, Wang BW, Kuan P and
Chang H: Cyclical mechanical stretching increases the expression of
vascular endothelial growth factor in rat vascular smooth muscle
cells. J Formos Med Assoc. 100:741–747. 2001.PubMed/NCBI
|
|
20
|
Chang H, Shyu KG, Wang BW and Kuan P:
Regulation of hypoxia-inducible factor-1alpha by cyclical
mechanical stretch in rat vascular smooth muscle cells. Clin Sci
(Lond). 105:447–456. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Komorowski AL, Zanini V, Regolo L, Carolei
A, Wysocki WM and Costa A: Necrotic complications after nipple- and
areola-sparing mastectomy. World J Surg. 30:1410–1413.
2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Topaz M, Carmel NN, Topaz G, Li M and Li
YZ: Stress-relaxation and tension relief system for immediate
primary closure of large and huge soft tissue defects: An old-new
concept: New concept for direct closure of large defects. Medicine
(Baltimore). 93(e234)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu Z, Tong Y, Wu T, Zhao Y, Yu M and
Topaz M: TopClosure® tension-relief system for immediate
primary abdominal defect repair in an adult patient with bladder
exstrophy. J Int Med Res. 48(300060519891266)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu Z, Yang X, Zhao Y, Fan H, Yu M and
Topaz M: Early surgical management of large scalp infantile
hemangioma using the TopClosure® tension-relief system.
Medicine (Baltimore). 94(e2128)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu Z, Zhao Y, Yu M and Topaz M: A skin
stretch system for the immediately closing of the large skin
defects of the anterior chest wall following large keloid excision.
Eur J Plast Surg. 41:609–612. 2018.
|
|
26
|
Ashkenazi I, Olsha O and Topaz M:
Relaxation systems of the skin for the closure of large mammary
defects. Cir Esp (Engl Ed). 98:154–157. 2020.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
27
|
Choke A, Goh TL, Kang GC and Tan BK:
Delayed primary closure of extensive wounds using the TopClosure
system and topical negative pressure therapy. J Plast Reconstr
Aesthet Surg. 70:968–970. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee SK, Choi MY, Bae SY, Lee JH, Lee HC,
Kil WH, Lee JE, Kim SW and Nam SJ: Immediate postoperative
inflammation is an important prognostic factor in breast cancer.
Oncology. 88:337–344. 2015.PubMed/NCBI View Article : Google Scholar
|