Introduction
Benign fibrous histiocytoma (FH), also known as
dermatofibroma, is one of the most common benign tumors of the skin
worldwide (1). It includes a group
of mesenchymal lesions comprised of fibroblasts, histiocytes and
myofibroblasts (1-3).
It most often occurs on the extremities of middle-aged adults and
is slightly more common in females compared with males (4). There are numerous clinicopathologic
variants of FH, including benign cellular, aneurysmal, epithelioid
cell, atypical, deep penetrating, granular cell and lipidized FH
(3,4).
By definition, lipidized FHs consist of abundant
lipid-laden histiocytes and distinctive stromal hyalinization
(4). Lipidized FH most commonly
develops in the lower limbs; thus, it is previously referred to as
‘ankle-type’ FH. Clinically, in comparison with other FH variants,
it appears as a large exophytic yellow nodule surrounding the ankle
(3-5).
Lipidized FH is often misdiagnosed as other benign and malignant
tumors (Liu et al, unpublished data). In our three pathology
departments [Fenlan Laboratory (Hangzhou, China); Yexian First
People's Hospital (Pingdinghan, China); People's Liberation Army
989 Hospital (Pingdingshan, China)], cases with marked stromal
hyalinization and inconspicuous histiocytes are presented, which
could be mistaken for other tumor types, such as sclerosing
epithelioid fibrosarcoma. Thus, eight cases of lipidized FH were
collected for use in the present study, and the pathological and
clinical features were determined and the immunostaining were
carried out for subsequent differentiation from other similar
tumors.
Materials and methods
Patients
Clinical data were collected from the medical
records of eight patients diagnosed with lipidized FH from November
2019 to November 2021. The diagnosis was confirmed by Dr Zhao Ming
(Zhejiang Provincial People's Hospital, People's Hospital of
Hangzhou Medical College, Hangzhou, China). A total of three cases
originated from the tissue bank of the Department of Pathology of
the Peoples' Liberation Army 989 Hospital (Pingdingshan, China),
four cases originated from the tissue bank of Fenlan Medical
Laboratory (Hangzhou, China) and one case originated from the
tissue bank of Department of Pathology of Yexian People's Hospital
(Pingdingshan, China). The cohort included three male and five
female patients (male: female ratio, 1.7:1) with a mean age of 48
years (range: 38-62 years). The present study was approved by the
989 Hospital Medical Ethics Committee, Fenlan Lab Medical Ethics
Committee and Yexian First People's Hospital Committee. All
participants signed an informed consent form and all patient data
were anonymized. The inclusion criterion was a diagnosis in
accordance with lipidized FH.
Tissue preparation
Hematoxylin and eosin-stained slides were available
for all cases and this staining was conducted using a method
described by Sommer et al (6). The surgical specimens were fixed in
10% neutral buffered formalin in the room temperature, dehydrated
in graded alcohol solutions and embedded in paraffin. Specimens
were cut into 4-µm-thick sections for hematoxylin and eosin
staining and visualization was carried out using light
microscopy.
Immunohistochemistry
Sections (4-µm) from paraffin blocks were also
stained immunohistochemically using BOND-MAX Automated IHC/ISH
Stainer (Leica Microsystems GmbH). Sections were mounted onto
slides, air dried for 20 min and baked at 60˚C for 20 min. The
heat-induced antigen retrieval method was performed using Tris-EDTA
buffer (1X; cat. no. #K0071; Shanghai Jiehao Biotechnology Co.,
Ltd.) and endogenous peroxidase activity usually responsible for
background staining, was quenched with 3% peroxidase-blocking
reagent, (Henan Celnovte Biotechnology Co., Ltd.), which was
applied at 37˚C for 10 min. The slides were incubated with the
following commercially available antibodies: CD68 (cat. no.
CCR-0702; KP1; 1:1,000), smooth muscle actin (cat. no. CAM-0190;
IA4; 1:50), S-100 protein (cat. no. CSM-0101; polyclonal; 1:500),
CD34 (cat. no. CCM-0550; QBend10; 1:50), desmin (cat. no. CDM-0021;
D33; 1:50), MUC4 (cat. no. CMM-0270; 8G7; 1:50) and cytokeratin
(cat. no. CCM-0960; AE1/AE3; 1:50) at 37˚C for 30 min. All
antibodies were purchased from Henan Celnovte Biotechnology Co.,
Ltd. The sections were then examined using a light microscope.
Tissue sections were then washed (2x6 min) and incubated with
Microstacker™ + Linker in room temperature for 15 min for signal
amplification. After TBS washing (2x6 min), Microstacker™ Flex
HRP-polymer detection reagent (ready-to-use; cat. no. #SD5100;
mouse/rabbit linker; Celnovte Biotechnology Co., Ltd) was applied
for at 37˚C for 30 min. After incubation with the polymer reagent,
tissue sections were thoroughly washed with TBS buffer (3x6 min)
and incubated with Microstacker™ DAB + Chromogen at 37˚C for 6 min.
Slides were buffer washed (2x6 min), counterstained with
hematoxylin at 37˚C for 2 min and washed with TBS and
dH2O for 6 min respectively. Ultimately, dehydration
through graded ethanol solutions as well as 90% xylene was
performed and sections were mounted in synthetic resin and were
observed under a light microscope.
The IHC results were scored by two independent
observers according to the percentage of positively stained cells
(0+, 1-25% staining; 1+, 26-50% staining; 2+, 51-75% staining; 3+,
76-100% staining).
Results
Clinical findings
The patient cohort included three males and five
females with a mean age of 48 years (range, 38-62) at the time of
diagnosis (Table I). Clinical
symptoms were recorded in all eight cases. All patients reported
the presence of a mass with no other complications. The
preoperative duration ranged from 11 months to 6 years. All eight
cases were followed up. None of these tumors had recurred locally
at 6 to 24 months (median, 13.6 months) after the operation. No
cases exhibited a history of hypertension, diabetes or
hyperlipemia. A total of three tumors were located on the right
buttock, one on the left buttock, one on the left lower leg, one on
the right lower leg, one on the right shin and one on the left
forearm.
 | Table IClinical features of patients with
lipidized fibrous histiocytoma. |
Table I
Clinical features of patients with
lipidized fibrous histiocytoma.
| Patient | Age, y | Gender | Location | During | Size, mm | Follow up, mo |
|---|
| 1 | 38 | M | R lower leg | 3 y | 7 | 6 |
| 2 | 39 | M | L buttock | 5 y | 22 | 8 |
| 3 | 41 | F | R buttock | 6 y | 35 | 13 |
| 4 | 56 | F | R buttock | 3 y | 20 | 10 |
| 5 | 51 | M | R lower leg | 11 mo | 28 | 24 |
| 6 | 50 | M | R buttock | 1 y | 25 | 22 |
| 7 | 62 | F | L forearm | 2 y | 20 | 15 |
| 8 | 49 | M | R shin | 9 mo | 15 | 11 |
Macroscopic pathological features
Grossly, all cases demonstrated a well-demarcated
lesion, and lesions were elevated. The average tumor diameter was
21 mm (range, 7-35 mm). The cut surface of the lesions was yellow
with parts that were white (Fig.
1).
Microscopic pathological features
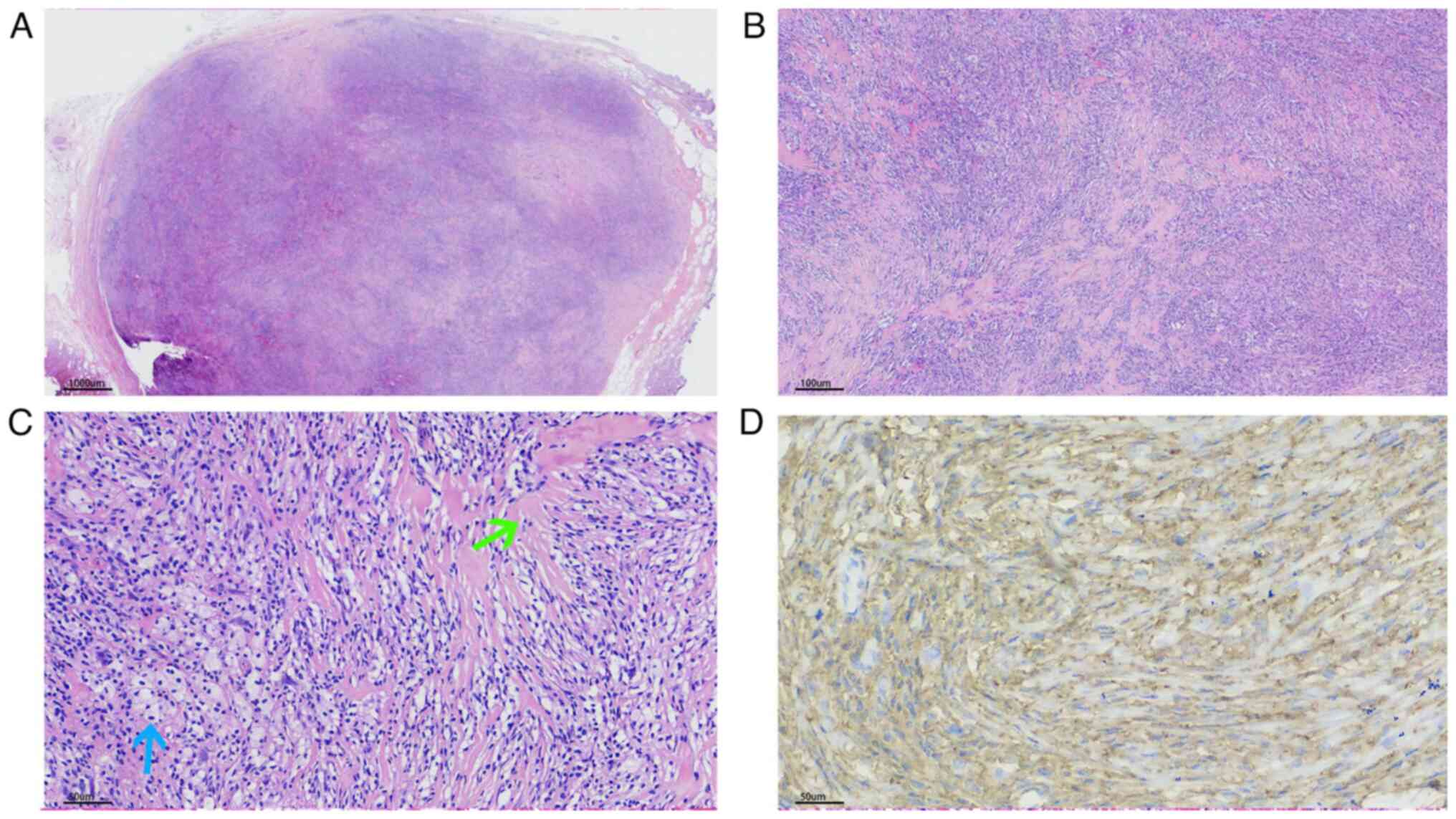
There were solitary nodules in the reticular dermis.
The nodules were well circumscribed; however, no fibrous or
capsule-like membrane was revealed (Figs. 2A, 3A and 4A). The nodules were comprised of two
markedly different components: Proliferating eosinophilic
fibroblasts and lipid-laden histiocytes. In the majority of areas,
the fibroblasts exhibited no cytologic atypia, with elongated
nuclei, fine chromatin and small basophilic nucleoli. However,
nuclear pleomorphism was present in the deep or peripheral area.
Notably, no pathological mitotic figures were observed. The
fibroblasts were arranged in a storiform pattern or with
intersecting fascicles forming a loose lattice pattern (Figs. 2B, 2C, 3B
and 4B). The histiocytes were oval
to polygonal in shape with large hypochromatic nuclei, prominent
nucleoli and abundantly vacuolated cytoplasm (Figs. 2D, 3C and 4C). In the majority of areas, histiocytes
were combined with proliferating eosinophilic fibroblasts. There
were also a number of binucleated or multinucleated Touton-type
giant cells (Fig. 3D). Notably,
there was marked stromal hyalinization, which was observed in
>90% of the tumor area (Figs.
2C, 3B and 4C). Hyalinized collagen fibers
transmigrated with normal collagen fibers (Fig. 2E), and the hyalinized materials
exhibited different colors in microscopy; notably, certain areas
presented as bright red and others presented as dull red (Figs. 2A, 3A and 4A). The bright red areas were rich in
lipid-laden histiocytes and the dull red areas possessed fewer
lipid-laden histiocytes. There were prominent hyalinized vessels in
some cases (Fig. 2E). The
epidermis exhibited hyperplasia, irregular elongation of the rete
ridge, and basal pigmentation (Figs.
2A and 3A).
 | Figure 2Patient 2. (A) A well-circumscribed
lesion located at the dermis (magnification, x1). The epidermis
exhibited hyperplasia and irregular elongation of the rete ridge
(black arrow). (B) Fibroblasts arranged in a storiform pattern
(magnification, x7). (C) Fibroblasts arranged in a storiform
pattern, and hyalinized collagen fibers transmigrated with normal
collagen fibers (magnification, x9). (D) Histiocytes were oval to
polygonal in shape with large hypochromatic nuclei, prominent
nucleoli and abundantly vacuolated cytoplasm (blue arrow). The
fibroblasts exhibited no cytologic atypia, with elongated nuclei,
fine chromatin and small basophilic nucleoli (green arrow). (E)
Prominent hyalinized vessels were documented (magnification, x40).
(F) The histiocytes were strongly positive for CD68 (4+,
magnification, x40). |
Immunohistochemical staining was positive for CD68
(Figs. 2F and 4D), focally positive for CD34,
particularly in the peripheral region of the lesion, and negative
for S-100 protein, smooth muscle actin, desmin, MUC4 and
cytokeratin. These histopathologic findings led to the diagnosis of
lipidized FH.
Discussion
Lipidized FH represents a small fraction of
dermatofibromas (2%) (5,7). Iwata and Fletcher (4) observed that patients with lipidized
FH have an increased age (mean, 54.8 years) compared with those
with ordinary FH (third to fifth decades). Wagamon et al
(8) reported that the ages in the
lipidized FH group ranges from 35 to 75 years with a mean value of
53 years, whereas ages in the non-lipidized FH group ranges from 27
to 72 years with a mean value of 48 years (8). In the cohort of the present study,
ages ranged from 38 to 62 years (mean, 48 years), which was
consistent with the results obtained by Zaballos et al
(7).
Moreover, Iwata and Fletcher (4) recommends the alternative name
‘ankle-type’ FH. However, Zaballos et al (7) observed five lesions located on the
back, four lesions on the legs, three lesions on the arms and one
lesion on the abdomen. Results of an alternate previous study
demonstrated that lipidized FH does not differ clinically from
non-lipidized FH in tumor location (8). In the present study, three tumors
were located on the right buttock, one on the left buttock, one on
the left lower leg, one on the right lower leg, one on the right
shin and one on the left forearm, indicating that lipidized FH was
not concentrated in the lower limbs.
Lipidized FH often presents with an increased size
compared with common FH (4,7,8).
The size of lipidized FH, with a median of 25 mm (and a range up to
80 mm) at the greatest dimension, is notably larger compared with
ordinary FH (4). Results of the
present study indicated that the average diameter of the tumor was
21 mm (range, 7-35 mm). Following sectioning, the majority of the
cases in the present study were yellow in color, which is
indicative of the presence of abundant foamy histiocytes with few
fibroblasts or stromal hyalinization. In certain areas, the cut
surface was yellow mixed with white. Moreover, under microscopic
examination, the white area presented with abundant
hyalinization.
Histological diagnostic criteria for lipidized FH
are as follows: Over 75% of the area is occupied by foamy cells and
stromal hyalinization (3,5), the majority of lesions are moderately
vascularized and exhibit perivascular hyalinization and the
fibroblasts are arranged in a storiform pattern (4,5). In
the cohort of the present study, the tumor exhibited two
extremities. In case 2 and case 3, the most dominant features were
prominent stromal hyalinization, hyalinized vessels and lipid-laden
histiocytes. The hyalinized materials exhibited two different
colors under the microscope; certain areas presented as bright red
and other areas presented as dull red. The bright red areas were
rich in lipid-laden histiocytes and the dull red areas exhibited
fewer lipid-laden histiocytes. This phenomenon may be associated
with the different processes of the disease, such as myositis
ossificans. Hyalinized collagen fibers transmigrated with normal
collagen fibers in certain areas. These results indicated that the
hyalinized materials may have taken place of the previous normal
collagen fibers. In these cases, lipidized FH should therefore be
distinguished from other malignant tumors, such as sclerosing
epithelioid fibrosarcoma (SEF), particularly with marked stromal
hyalinization and inconspicuous histiocytes (9). SEF is a rare, malignant mesenchymal
tumor with unique features consisting of cords, nests or sheets of
monotonous epithelioid cells within a dense collagenous background
(9). SEF also has prominent
hyalinized sclerotic collagenous stroma indicative of osteoids or
cartilage, as in lipidized FH (9).
However, in patients 5 and 8, the tumors exhibited
scant stromal hyalinization and prominent lipid-laden histiocytes.
In these cases, lipidized FH should also be differentiated from
other benign tumors, such as xanthoma (1,3,4).
Xanthoma is a histiocytic proliferation that frequently occurs in
association with hyperlipidemia, and occurs in tendons or bursae
(10). It often contains
cholesterol crystals and lacks a fibroblastic myofibroblastic
neoplastic component (10).
In conclusion, lipidized have a wide spectrum. Some
cases show the prominent stromal hyalinization, hyalinized vessels
and lipid-laden histiocytes, and should be differentiated from the
malignant tumors, such as sclerosing epithelioid fibrosarcoma.
However, some cases exhibit the prominent lipid-laden histiocytes
and scant stromal hyalinization and should be differentiated from
the xanthoma. The present study was limited by relative infrequency
of lipidized FH and limiting the number of patients in this cohort.
Future studies should focus on the microenvironment in different
areas of the lipidized FH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYL designed the study. FYL and HJW recruited the
cases. CYL, FYL, HJW and GYW analyzed the experimental data and
composed all figures and tables. CYL wrote the manuscript. FYL and
HJW confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the 989 Hospital
Medical Ethics Committee, Fenlan Lab Medical Ethics Committee and
Yexian First People's Hospital Committee. Written informed consent
was obtained at the time of the initial data collection for
participation.
Patient consent for publication
All patients consented for publication in written
form.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meister P, Konrad E and Krauss F: Fibrous
histiocytoma: A histological and statistical analysis of 155 cases.
Pathol Res Pract. 162:361–379. 1978.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gonzalez S and Duarte I: Benign fibrous
histiocytoma of the skin. A morphologic study of 290 cases. Pathol
Res Pract. 174:379–391. 1982.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Seo JK, Shin EJ, Jeong KH and Shin MK:
Lipidized fibrous histiocytoma: Differential diagnosis from
juvenile xanthogranuloma. Ann Dermatol. 31:254–256. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iwata J and Fletcher CD: Lipidized fibrous
histiocytoma: Clinicopathologic analysis of 22 cases. Am J
Dermatopathol. 22:126–134. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alves JV, Matos DM, Barreiros HF and
Bártolo EA: Variants of dermatofibroma-a histopathological study.
An Bras Dermatol. 89:472–477. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sommer W, Knöfel AK, Izykowski N, Oldhafer
F, Avsar M, Jonigk D, Warnecke G and Haverich A: Physical exercise
reduces transplant arteriosclerosis in a mouse aorta
transplantation model. J Thorac Cardiovasc Surg. 149:330–337.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zaballos P, Mir-Bonafé JF, Avilés JA and
Bañuls J: Dermoscopy of lipidised dermatofibroma: A morphological
study of 13 cases. Aus J Dermatopathol. 60:e127–e131.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wagamon K, Somach SC, Bass J, Sigel JE,
Xue W, Schluchter M and Jaworsky C: Lipidized dermatofibromas and
their relationship to serum lipids. J Am Acad Dermatol. 54:494–498.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kosemehmetoglu K, Ardic F, Kilpatrick SE,
Aydingoz U, Sumathi VP and Michal M: Sclerosing epithelioid
fibrosarcoma of bone: morphological, immunophenotypical, and
molecular findings of 9 cases. Virchows Arch. 478:767–777.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wilkinson PE, Merkourea S, Gopalakrishnan
R and Argyris PP: Primary intraosseous xanthomas of the jaws: A
series of six cases including an example with formation of
apoptosis-related hyaline globules, so-called ‘thanatosomes’. Head
Neck Pathol. 14:859–868. 2020.PubMed/NCBI View Article : Google Scholar
|