Leukaemia is a common cancer of the haematopoietic
system, whose pathogenesis has not been fully elucidated. Leukaemia
cells are stagnant in different stages of cell development due to
uncontrolled proliferation, impaired differentiation and blocked
regulation. They proliferate massively in the bone marrow and other
haematopoietic tissues and infiltrate other non-haematopoietic
tissues and organs, and these processes are multifactorial and
multistage. According to the French-American-British classification
standard, leukaemia is divided into four categories: Acute myeloid
leukaemia (AML), acute lymphoblastic leukaemia (ALL), chronic
lymphocytic leukaemia (CLL) and chronic myeloid leukaemia (CML). If
left untreated, these conditions may lead to bone marrow failure
and eventual death, and only a few patients are cured, even with
the best treatments such as intensive chemotherapy or
haematopoietic stem cell (HSC) transplantation (1). With the improvement of treatment
technology, especially the progress of HSC transplantation in
recent years, the remission rate of patients with leukaemia has
been improved, but the majority of patients still experience
relapse after remission according to the World Health Organization
(WHO) (2).
Extracellular vesicles (EV) are lipid bimolecular
membrane vesicles released by cells into the extracellular
microenvironment that play an important role in intercellular
communication, and also participate in various physiological and
pathological body processes. EVs are divided into exosomes,
apoptotic bodies, microvesicles and large oncosomes according to
their different formation methods. For example, exosomes can be
released by almost all cells, transferred to target cells through
cell-to-cell communication, and perform numerous biological
functions (10). Recently, there
has been an increase in research into exosomes, especially in the
medical field. The formation of exosomes is a continuous process
involving sorted endosomes, intraluminal vesicles (ILV) and
intracellular multivesicular bodies (MVB). The cell membrane is
invaginated to form a goblet structure when stimulated by microbial
attack or stress conditions. Extracellular material or membrane
proteins are internalized and form early-sorting endosomes (ESEs),
the extracellular cargo enters the cell by cell membrane
internalization (for example, via phagocytosis) (11). To participate in the assembly of
exosomes, some cargo may enter the cell more efficiently with the
help of receptors expressed on the cell membrane. Ubiquitinated
molecules such as proteins or RNAs bind to ESEs with the assistance
of sorting proteins, and the ESEs gradually mature into
late-sorting endosomes (LSEs). ILVs in LSEs are the precursors of
exosomes, and LSEs eventually evolve into MVBs. Occasionally, MVBs
are degraded after fusion with lysosomes. Or, MVBs fuse with the
plasma membrane to release ILVs into the extracellular environment,
which are exosomes. Exosomes affect the function of target cells
through endocytosis or ligand-receptor recognition (12). The formation process of exosomes is
presented in Fig. 1.
Exosome-mediated intercellular communication
regulates normal physiological activities between cells and is also
widely involved in the pathological process of numerous diseases,
including tumorigenesis. Generally, tumour cells can produce more
exosomes compared with normal cells, and these tumour-derived
exosomes manifest multiple abilities to change the local and
distant microenvironment, which contributes to tumorigenesis,
metastasis and immune escape (13). Common methods for collecting
exosomes from body fluids such as urine, bronchoalveolar lavage
fluid, serum and ascites of patients with tumours include
ultracentrifugation, ultrafiltration and commercial kits. Each
method has advantages and disadvantages. Only the extracted
exosomes with high purity, concentration and content while avoiding
heterologous protein interference can meet the requirements of
subsequent research (14). With
the rapid development of modern medical technology, advanced
technologies, such as biophysical techniques based on spectroscopy
(scanning electron microscopy, atomic force microscopy), and
antibody-based techniques (flow cytometry, transmission electron
microscopy) can be used to characterise and identified various
exosomes (15). A paper-based
isotachophoresis technique enables rapid identification of exosomes
from healthy cells or tumour cells, and this technique can
sensitively detect exosomes down to 2.0-3.0x10-18 M with
potential clinical application value (16).
The pathogenesis of AML involves a tumour cell
favourable BMM, which supports the formation and progression of
leukaemia cells, and for exosomes to inhibit the haematopoiesis
function of bone marrow through the targeted delivery of miRNAs to
haematopoietic progenitor cells (20). AML-derived exosomes that contain
coding and non-coding RNAs phagocytosed by bone marrow stromal
cells (BMSCs) can promote tumour cell growth by stimulating the
secretion of growth factors (21).
AML-derived exosomes containing miRNAs that downregulate the
expression of retention factors, such as stem cell factor and C-X-C
chemokine ligand 12 (CXCL12), in BMSCs may disrupt the
haematopoietic function of the bone marrow (22).
AML-derived exosomes inhibit the expression of
haematopoietic factor Dickkopf-related protein 1 (DKK1) and HSC
support factors, such as CXCL12, kit ligand and insulin-like growth
factor I, in the bone marrow. This forms a favourable tumour cell
microenvironment and impairs the haematopoiesis ability of bone
marrow (23). The loss of the
haematopoiesis ability in patients with AML is partly attributable
to AML-derived exosomes transporting miRNAs to HSPCs, which inhibit
the C-MYB gene function through miR-150 and miR-155, and inhibit
the clonogenic ability of myeloid cells (24). Exosomes containing miR-4532
released by AML cells interact with HSCs and impair the clonogenic
ability of HSCs through the JAK2 and STAT3 signalling pathways,
inhibiting bone marrow haematopoiesis by increasing the expression
of haematopoietic inhibitor DKK1(25). Exosomes containing miR-7977
released by AML cells can enhance the proliferation ability of
BMSCs through the Hippo-YAP signalling pathway, which may be
involved in the upregulation of leukaemia-supporting stroma growth
(26). A recent study confirmed
that the expression level of miR-425-5p is reduced in patients with
CD34+CD38- AML, and exosomes containing
miR-425-5p derived from the BMSCs of these patients can inhibit AML
cell proliferation, invasion and migration through the Wilms tumour
1 associated protein pathway, this indicates that miR-425-5p may be
a potential therapeutic target for patients with AML (27).
Tumour-derived exosomes are important messengers in
leukaemia that remodel the BMM into a malignant ecology to improve
support for ALL cell survival through inflammatory cytokines,
chemokines and adhesion molecules. Abnormal production of
inflammatory mediators in bone marrow may reduce the normal
function of HSCs during the tumorigenesis of ALL. Exosomes secreted
by tumour cells contain miRNAs, such as miR-146a-5p, miR-181b-5p
and miR-199b-3p, that bind to Toll-like receptor (TLR)8 on the
surface of bone marrow mesenchymal stromal cells (BM-MSCs). This
activates inflammatory pathways and remodels the tumour
microenvironment (TME) through TLR8 signalling, significantly
reducing the haematopoietic capacity of normal HSCs and promoting
the progression of ALL (28,29).
The pathogenesis of CLL is closely associated with
the BMM, which supports tumour cell growth and a dysfunctional
immune system. The interaction of BMSCs with CLL-derived exosomes
induces the expression of a cancer-associated fibroblast phenotype,
and exosome-stimulated BMSCs exhibit an enhanced proliferation and
inflammatory cytokine secretion ability. This is favourable for
creating a microenvironment that supports tumour cell survival
(30). Furthermore, the
endocytosis of exosomes by endothelial cells increases angiogenesis
in vitro and in vivo and promotes tumour cell
metastasis (31). A study
suggested that B cell receptor (BCR) signalling can promote CLL
pathogenesis and tumour cell survival. MiR-150 and miR-155
expression levels are significantly elevated in CLL-derived
exosomes by α-IgM stimulation, promoting disease progression;
however, the tyrosine kinase inhibitor (TKI) ibrutinib may offset
this effect (32). CLL-derived
exosomes regulate the dynamic interaction between tumour cells and
the bone marrow microenvironment, and regulate the TME through
NF-κB and the PI3K/AKT pathway, providing support for the
pathogenesis of CLL (33).
Exosomes released by CML cells stimulate BMSCs to
produce pro-inflammatory chemokines, such as IL-8, that promote the
expression of malignant phenotypes of CML cells through C-X-C
chemokine receptor signalling (34). Exosomes released from CML cells
contain miRNAs that promote the formation of the TME. Among the 124
miRNAs identified from the CML cell line LAMA84, miR-126 can
down-regulate the expression levels of chemokine CXCL-12 and
vascular cell adhesion molecule-1 (VCAM-1) in endothelial cells,
This impairs the adherence of tumour cells to endothelial cells,
which may facilitate tumour cell migration from the bone marrow and
dissemination in vivo (35).
A recent study showed that K562 cell-derived
exosomes transfer miR-711 into BMSCs, inhibiting the adhesion
ability of BMSCs by down-regulating CD44 molecule expression. There
is a significantly higher level of miR-711 in exosomes derived from
K562 cells compared with exosomes derived from parental cells
(36). The BM-MSCs co-cultured
with exosomes derived from K562 cells showed a lower adhesion rate,
which may be associated with tumour cell metastasis.
Hyperleukocytic acute leukaemia patient-derived exosomes contain
miR-125b, which can reduce colony forming units and the expression
of BM-MSC haematopoietic-related factors α-globulin, γ-globulin,
colony-stimulating factor 2, CRTX4 and CXCL12. These results
indicated that exosomes carrying miR-125b might affect the
haematopoietic differentiation function of HSCs and the
haematopoietic support for BM-MSCs (37).
CML-derived exosomes containing miR-320 can be
endocytosed by the adjacent BMSCs and then inhibit the function of
osteoblasts at least partially via β-catenin, which contributes to
CML progression. Notably, β-catenin is a key regulator of
osteogenesis that can promote the maturation of osteoblastic
precursor cells into mature osteoblasts through Wnt signalling, the
3'-UTR of β-catenin contains a binding site that miR-320 can
recognize (38). K562 cell-derived
exosomes induce T cell fate to evolve toward tumour-favourable
suppressor T cells instead of traditional killer T cells by
promoting the expression of NAD (P)H quinone oxidoreductase 1,
programmed death ligand 1 (PD-L1) and forkhead box protein P3
(FOXP3), while increasing the secretion of cytokines such as IL-10,
IL-6 and IL-17(39).
Cancer-associated cachexia (CAC) impacts the quality
of life of patients with CML, especially in the advanced stage. It
has been confirmed that mice significantly lost weight and body fat
percentage after being injected with CML-derived exosomes (40). Further research confirmed that the
adipogenic ability of adipose-derived stem cells are reduced by
exosomes containing miR-92a-3p, indicating that tumour-derived
exosomes may be involved in the occurrence of CAC (40). The regulatory effects of exosomes
on the bone marrow microenvironment are presented in Table I.
AML-derived miR-5195-3p can simulate tumour cell
proliferation by activating the cell cycle, and can also improve
the anti-apoptotic ability of tumour cells by down-regulating Bcl-2
and up-regulating Bax expression. However, AML-derived miR-23b-5p
can reduce tumour cell proliferation and induce apoptosis via the
PI3K/AKT pathway (41,42). BMSCs-derived miR-7-5p inhibits AML
cell proliferation and promotes apoptosis through PI3K/AKT/mTOR
signalling, indicating that these miRNAs may be potential
therapeutic targets for AML treatment (43). BM-MSCs derived exosomes that
contain miR-222-3p can inhibit AML cell line THP-1; however, these
effects of BM-MSCs exosomes are reduced when the IRF2/inositol
polyphosphate 4-phosphatase type II (INPP4B) signalling pathway is
blocked, which suggests that BM-MSCs regulate THP-1 cells
proliferation and apoptosis through IRF2/INPP4B signalling, which
may provide novel therapeutic strategies for AML (44).
A study confirmed that miRNA-181b-5p is highly
expressed in ALL cell lines, and exosomes carrying this miRNA can
promote the proliferation, migration and invasion ability of ALL
cells. MiRNA-181b-5p can also promote the progression of ALL by
inhibiting cell apoptosis (45).
CML-derived exosomes can promote the proliferation ability of
tumour cells through TGF-β1 signalling in a CML mouse xenograft
model while promoting the expression of BCL-w, BCL-xl and survivin
to resist apoptosis (46). Adult T
cell leukaemia/lymphoma (ATL) is a haematological disease in which
lymphocytes proliferate abnormally in the bone marrow, and human
T-lymphotropic virus type 1 (HTLV-1) is the causative agent.
Lymphocytes will release exosomes containing viral Tax proteins,
pro-inflammatory mediators and viral mRNA transcripts after being
infected by HTLV-1, which can improve the survival of leukaemia
cells by inhibiting Fas expression through AKT signalling, further
studies confirmed that this effect depends on the anti-apoptotic
protein cFLIP (47). Paediatric
acute lymphoblastic leukaemia (pALL) is a malignancy of the
lymphoid line of blood cells that accounts for a large percentage
of all childhood leukaemia cases. Exosomes isolated from patients
serum containing miR-181a, which can promote disease progression by
upregulating proliferation genes (e.g. PCNA, Ki-67) and survival
genes (e.g. MCL-1 and BCL2). Specific inhibition of miR-181a can
reverse pALL exosome-induced proliferation function of leukaemia
cells in vitro, suggesting miR-181a may be a therapeutic
target for pALL (48). These
findings highlight the effects of exosomes on the proliferation and
apoptosis of leukaemia cells which may provide novel ideas for the
treatment of leukaemia.
The formation of new blood vessels contributes to
the development of malignant tumours. Exosomes circulate freely in
body fluids, accumulate in the TME and promote tumour angiogenesis
(49). Exosomes deliver
angiogenesis-promoting molecules and genetic agents to recipient
cells, and are responsible for reprogramming the phenotype and
function of endothelial cells in the TME, promoting leukaemia
progression (50).
CML-derived exosomes stimulate vascular endothelial
cell proliferation and promote the formation of new blood vessels
through Src signalling in CML angiogenesis. Tyrosine kinase
inhibitors dasatinib and imatinib (IM) can significantly inhibit
the secretion of exosomes and the proliferation of the human
umbilical cord endothelium in vitro. A further study
confirmed dasatinib can attenuate angiogenesis induced by exosomes
in a Matrigel mouse embolization model, CML cell-derived exosomes
could induce angiogenic activity in human umbilical vein
endothelial cells (HUVECs), and dasatinib manifested an inhibitory
effect on exosome through Src signaling (51).
Exosomes isolated from the blood of patients with
CML have been reported to contain amphiregulin which can activate
the epidermal growth factor receptor signalling of BMSCs,
increasing the expression of MMP-9 and IL-8, promoting the adhesion
of leukaemia cells to stromal cells, supporting the development of
disease (52). The progression of
CML is often associated with increased angiogenesis, and
CML-derived exosomes can induce the proliferation and angiogenesis
of human umbilical vein endothelial cells via miR-92a (53). The exosomes secreted by K562 cells
can promote the expression of VEGFR and the formation of new blood
vessels, and anti-angiogenic gold nanoparticles can attenuate the
pro-angiogenic effect of exosomes, highlighting nanomedicine-based
potentiality to prevent the spread of leukaemia cells (54).
A hypoxic environment can regulate tumour
angiogenesis. Exosomes released from K562 cells cultured in a
hypoxic (1%) environment were more prone to induce tumour
angiogenesis via miR-210 compared with those cultured in normoxic
(20%) conditions by downregulating angiogenesis inhibitory factor
ephrin-A3, which is essential for tumour cell survival in hypoxic
environments (55). Exosomes
secreted by K562 cells that contain miR-92a can promote tumour
angiogenesis under hypoxic conditions through targeted inhibition
of hypoxia-inducible factor 1 by miR-135b (56). Exosomes released from CML cells
contain miR-21, which can increase expression levels of IL-8 and
VCAM-1 and promote tumour angiogenesis. A vascular network
formation assay confirmed that curcumin-treated exosomes induce the
secretion of proteins with antiangiogenic activity and attenuate
the angiogenic ability of exosomes (57). A previous study has indicated that
AML-derived exosomes contain miRNAs for VEGF and VEGFR expression,
promoting HUVEC cell proliferation and vascular remodelling while
resisting apoptosis by promoting HUVEC cell glycolysis (58). These findings may contribute to
developing novel therapeutic strategies for AML.
CLL-derived exosomes can activate the AKT signalling
pathway in BMSCs following activation of cyclin D1, which produces
vascular endothelial growth factor, providing a ‘homing and
nurturing’ microenvironment for tumour cells and promoting CLL
progress (59). Exosomes released
by ATL cells containing angiogenic factors act on vascular
endothelial cells to create a favourable angiogenesis
microenvironment for tumour cells. Tumour cells of patients with
ATL release exosomes containing miR-21, miR-155 and vascular
endothelial growth factor, which interact with MSCs and induce the
activation of the NF-κB signalling through Tax protein, this
activates leukaemia related genes and angiogenic genes that
regulate the properties of MSCs and promote leukaemia progression
(60). These findings may provide
novel ideas for treating haematological malignancies.
The immune escape of tumour cells is one of the
important reasons for the rapid progression of leukaemia. Leukaemia
cells evade the supervision of the immune system by down-regulating
the expression level of tumour antigens and releasing immune
inhibitory cytokines to inhibit the function of lymphocytes
(61).
NKG2D is a common activating receptor that is
abundantly expressed on NK cells, NKT cells and CD8+ CTL
cells, which plays an immune surveillance role for tumour cells,
and the abnormal loss of NKG2D may be an important reason for
tumour cell immune evasion (62).
Exosomes secreted by tumour cells can down-regulate NKG2D
expression in NK cells and CD8+ T cells while promoting
the expression of TGF-β1 in tumours, creating an inhibitory immune
microenvironment for tumour cell growth. Szczepanski et al
(63) have confirmed that
AML-derived exosomes can reduce the expression level of NKG2D and
weaken the killing ability of NK cells, sera from patients with
acute myeloid leukaemia contained elevated levels of TGF-β,
neutralizing anti-TGF-β antibodies inhibited exosome-mediated
suppression of natural killer cell activity and NKG2D
down-regulation. RNA hY4 is a highly abundant non-coding RNA that
is enriched in exosomes derived from the plasma of patients with
CLL (64). RNA sequencing and
proteomic analysis has revealed that hY4 is a driver for TLR7
signalling and promotes inflammatory cytokines such as CCL2, CCL4
and IL-6 release along with up-regulated PD-L1 expression. This
contributes to tumour-associated inflammatory responses and immune
escape, indicating that regulation of exosome-mediated inflammatory
response may provide novel ideas for the treatment of CLL.
Tumour-derived exosomes contain NKG2D ligands (MICA/B, ULBP1-6)
that can bind to NKG2D competitively, impairing the monitoring
ability of NK cells to tumour cells (65). AML-derived exosomes down-regulate
naive CD4+ T cell activation, mediate Fas/FasL-driven T
cell apoptosis, promote
CD4+CD25+FOXP3+Treg
cell activation and mediate tumour cell immune escape (66,67).
However, certain tumour-derived exosomes can induce
specific antitumour immune responses and are expected to develop as
a promising tumour vaccine. Dendritic cells (DCs) have numerous
dendritic or pseudopodia-like protrusions that stretch out when
they mature, and they have a powerful antigen-presenting function
in the body. DC cells can recognize invading pathogens effectively,
present antigens peptides to T cells and activate the adaptive
immune responses, resulting in anti-pathogen immune reactions
(68). Mature DCs produce exosomes
that elicit potent immune activation, resulting in tumour
eradication and bacterial or virus elimination. Notably, DC cells
are closely associated with the pathogenesis of tumours. The
CD8+ CTL-mediated immune response initiated by DCs is an
important component of antitumour immunity and the basis for DC
cell immunotherapy (69). In a
previous study, the autologous monocytes of the patient were
extracted and induced to mature DCs in vitro and loaded with
tumour antigens to generate tumour antigen-loaded DCs; these DCs
were then injected into the patient to activate the lymphocytes,
which stimulated an antitumour immune response (70,71).
The specific mechanism of DC cell immunotherapy is shown in
Fig. 2. Numerous DC-based
immunotherapies have been developed and have produced satisfactory
treatment effects in certain clinical trials (72,73).
Targeted delivery of antigens to DCs via exosomes represents a
potential candidate approach for DC vaccines, which demonstrate
convincing therapeutic effects in myeloma cells, liver cancer and
breast cancer cells in vitro (74-76).
Exosomes from TGF-β1-silenced L1210 cells (LEXTGF-β1si) can reduce
the expression level of TGF-β1 in DCs and promote the maturation of
DCs significantly. The matured DCs activate CD4+ T cells, and the
subsequent tumour-specific CTL responses include killing responses
and inflammatory responses, suggesting that targeting DCs via
LEXTGF-β1si may be a promising immunotherapy strategy (77).
A previous study down-regulated PD-L1 expression in
leukaemia cell exosomes using PD-L1 short hairpin RNA, and then
compared the capacity of exosomes derived from PD-L1-silenced acute
lymphocytic leukaemia-derived cells (LEXPD-L1si) and non-modified
exosomes to induce anti-leukaemia immunity. The results confirmed
that LEXPD-L1si improved DC maturation and subsequently Th cell
proliferation and cytokine release while influencing the killing
ability of CTL cells. The following in vivo experiments
confirmed that LEXPD-L1si vaccination inhibits tumour growth and
significantly prolongs the survival rate of mice (78). Exosomes can affect the antitumor
ability of the body by regulating PD-L1 expression and the
down-regulation of PD-L1 by exosomes induces anti-leukaemia
immunity, which demonstrates the potential application of this
therapy in leukaemia immunotherapy (79,80).
These findings provide evidence for a novel exosome-mediated
mechanism in leukaemia, and the specific effects of exosomes on
tumour cell immune escape require further in-depth research.
Leukaemia chemotherapy resistance means leukaemia
cells are insensitive or resistant to chemotherapy drugs. Drug
resistance may lead to the recurrence of leukaemia and cause
treatment failure, which are also the main points and difficulties
of treatment for leukaemia.
Although there had been a rapid development of novel
therapies for leukaemia, minimal residual disease (MRD) is still
responsible for relapse and drug resistance. MRD is an obstacle to
the treatment of leukaemia. Galectin-3 may play an important role
in the MRD process by promoting the anti-apoptotic, colony
formation and cell drug resistance abilities of tumour cells
(81). Studies have confirmed that
BM-MSCs can up-regulate the expression level of Galectin-3 in ALC
cells, activate the Wnt/β-catenin signalling pathway and promote
the progress of drug resistance in tumour cells (82). BMSCs of the B cell precursor from
patients with acute lymphoblastic leukaemia (pre-B ALL) can
encapsulate Galectin-3 in exosomes and deliver it to B-ALL cells,
inducing drug resistance to nilotinib and vincristine by activating
NF-κB signalling. Galectin-3 may be a potential target to
counteract the protective effect of BMSCs on tumour cells (83).
Exosomes secreted by AML cells contain heat shock
protein 70 and lysosome-associated membrane protein 3, which
contribute to the interaction between AML and BMSCs. These proteins
protect AML cells from apoptosis induced by the chemotherapeutic
drug, etoposide, while reducing the sensitivity of AML cells to
chemotherapeutic drugs by promoting the production of IL-8(84). The interaction between AML and
BMSCs contributes to a protective environment for tumour cell
development and resistance to chemotherapeutics (85), BMSC-secreted exosomes protect AML
cells from Ara-C-induced cytotoxicity, and this chemoresistance is
closely related to the decrease in nucleoside transporter activity
from the cell surface. The progression of patients with AML is
often accompanied by a FMS-like tyrosine kinase 3 (Flt3) mutation,
and several chemotherapeutic drugs targeting FLT3 mutations have
achieved satisfactory clinical treatment effects (86,87).
Exosomes released by BMSCs of AML patients protect tumour cells by
resistance to AC220 (a FLT3 kinase inhibitor) therapy, which is
associated with miRNA-155 and miRNA-375 carried by exosomes
(88). However, the specific
mechanism has not been clearly understood. Another study confirmed
that AML-derived exosomes containing miR-10a can target regulation
of nuclear pre-MRNA domain-containing 1A and activate Wnt/β-catenin
signalling to reduce the sensitivity to cytarabine of leukaemia
cells (89).
BMSC-derived exosomes reduce spontaneous apoptosis
of CLL cells and increase chemoresistance to fludarabine,
ibrutinib, idelalisib and vegetate; in addition, the migratory
capacity of CLL cells is also increased (90). Previous studies have confirmed
fibroblast growth factor 2 (FGF2) is highly expressed in BMSCs and
AML. Studies showed that exosomes are secreted by BMSCs containing
FGF2, which are endocytosed by leukaemia cells and protect tumour
cells from the chemotherapeutic agents TKIs, blocking the effects
of FGF2 on stromal cells can reduce exosome release, indicting
inhibition of FGF2 may be a therapeutic option for tumour
resistance to TKIs (91,92). IM is a common TKI that is widely
used in CML and can significantly improve the survival rate and
quality of life of patients with CML. However, it has been shown
that exosomes are closely associated with IM resistance; proteomic
analysis of exosomes showed that 151 proteins were up-regulated and
128 proteins were down-regulated in the exosomes of patients who
were IM-resistant compared with patients with CML who were
IM-sensitive (93). Further
bioinformatical analysis has shown that ribosomal protein RPL13 and
RPL14 are notably up-regulated in IM-resistant patients with CML,
and proteomic analysis of exosomes may provide novel ideas for
treating IM resistance (93).
IM serves an important role in CML treatment and IM
resistance is also an obstacle to CML treatment. A previous study
showed that ubiquitin-specific protease is significantly
up-regulated in IM-resistant clinical samples; miR-146a-5p
down-regulated the IM resistance of CML, human umbilical cord
mesenchymal stem cell-derived exosomes can promote IM-induced
apoptosis through miR-145a-5p/USP6,indicating the potential role of
miR-146a-5p in leukaemia chemoresistance (94). A study has confirmed that miR-328
is significantly decreased in IM-resistant patients, where
overexpression of miR-328 sensitizes drug-resistant tumour cells to
IM. By contrast, knockdown of miR-328 confers IM resistance in
tumour cells, and targeting delivery exosomes can increase
expression of miR-328 and increase tumour cell sensitivity to IM
(95). MiR-365 in exosomes of CML
cells is closely associated with IM resistance of tumour cells. CML
cells that receive an exosomal delivery of miR-365 show a lower
chemosensitivity and apoptosis rate, and miR-365 can induce
chemoresistance in tumour cells by inhibiting the expression of
pro-apoptotic proteins (96). An
in vitro study has confirmed that combining exosomes
secreted by human umbilical cord mesenchymal stromal cells with IM
in K562 cells can enhance the expression of IM-induced Bax
expression through caspase-3/9 signalling, which promotes tumour
cell apoptosis and increases the sensitivity of tumour cells to IM
(97). Therefore, combining IM
with exosomes may be a potential strategy for tumour cell drug
resistance. These findings highlight the role of exosomes in tumour
cell drug resistance, and regulating miRNA expression through
exosomes may provide new ideas for overcoming drug resistance in
leukaemia treatment. The roles of exosomes in the pathogenesis of
leukaemia are summarised in Fig.
3.
The potential function of exosomes is far beyond
immunology, neurobiology, stem cell science and oncology. Notably,
exosomes have a far-reaching impact on tumour cell pathogenesis,
immune escape, cell proliferation and chemoresistance. For a long
time, exosomes have been hypothesised to be involved in the whole
process of tumour metastasis although the specific effects are
still unknown and controversial (98).
In recent years, exosomes have received more
attention as a promising tumour screening, diagnostic and
prognostic biomarker. Bioactive molecules packaged in exosomes can
promote tumour cells remodelling the microenvironment, targeting
distant cells and promoting cancer metastasis. The biological
agents such as miRNA in exosomes are stably and abundantly present
in the body, and extracellular enzymes will not readily degrade
them, this makes exosomes potentially become tumour cell biomarkers
(99). Molecular and genetic
analysis of exosomes in leukaemia patients may potentially provide
a prognosis for patients and provide new strategies for clinical
treatment (100-102).
Exosomes from AML tumour cells can be used as a biomarker for AML
treatment, as patients who achieved remission and survived at
3-year follow-up had significantly lower exosome levels compared
with deceased patients. Therefore, specific proteins and miRNAs in
exosomes can be used to trace the presence of MRD and guide doctors
to adjust treatment strategies on time (103,104). Exosomes isolated from the plasma
of patients with AML contain TGF-β1, which inhibits the
cytotoxicity of NK cells (105).
Changes in exosomes may reflect the response of a patient with AML
to chemotherapy, and the exosomal profile may suggest the presence
of residual disease in patients considered to have achieved
complete remission. MiR-125b in AML-derived exosomes can target the
BAK1 gene, inhibit tumour cell apoptosis and promote the
pathogenesis of AML. The elevated miR-125b levels indicate patients
are at higher risk of relapse and death, and so miR-125b levels may
be a prognostic indicator for AML (106,107).
A study detected the content of exosomal miR-532 in
198 patients with AML and revealed that up-regulation of miR-532 is
negatively correlated with energy metabolism such as fructose and
glutamine in tumour cells. Patients with high expression of miR-532
had an improved overall survival rate, indicating that miR-532 may
be used as a predictor of survival in patients with AML (108). Non-coding RNAs in exosomes
support the pathogenesis of CLL, and miR-155 is overexpressed in
monoclonal B lymphocytosis (MBL), which may potentially promote the
transition of MBL to CLL (109).
MiR-155 can be used as a biomarker for the risk of progression in
individuals with MBL, as well as to identify patients with CLL who
may not respond well to therapy. MiR-150 is highly expressed in CLL
patients compared with healthy subjects, and the expression level
of miR-150 is associated with tumour burden, disease aggressiveness
and poor prognostic factors. Patients with low cellular miR-150 or
patients with a high serum miR-150 level have a shorter
treatment-free survival (TFS) and overall survival (OS); therefore,
miR-150 may be able to monitor for disease progression as well as
be an indicator for CLL prognosis (110). CML is a malignant proliferation
driven by a characteristic fusion gene called BCR-ABL. The U.S.
Food and Drug Administration has approved TKIs for treating CML
considering their satisfactory therapeutic effect in the majority
of patients (111). Monitoring
BCR-ABL-derived exosomes using digital PCR after TKI therapy can
identify active tumour cells and guide the follow-up treatment
strategies for CML patients (112,113). These results show that exosomes
can provide prognostic judgement on patients with leukaemia and new
strategies for clinical treatment as an important biomarker.
However, the specific mechanism remains to be further invested.
Exosomes are small intracellular membrane-based
vesicles similar to the cellular components of the body, and this
non-immunogenic drug delivery vehicle has advantages compared with
traditional drug delivery systems, such as liposomes or
nanoparticles (114). The unique
biocompatibility, tissue specificity, high stability, tumour homing
ability and tuneable targeting efficiency confer exosomes as an
attractive drug delivery system with potential application in
tumour therapy (115). BCR-ABL
fusion protein promotes CML pathogenesis and IM is a selective
inhibitor of BCR-ABL; exosomes loaded with IM or BCR-ABL siRNA can
target CML cells and inhibit cancer cell growth by interacting with
IL-3 receptors on tumour cell surface, alleviating drug resistance
and adverse reactions during treatment (116). Curcumin can inhibit the
expression of BCR-ABL in CML cells through exosome-derived miR-21
and inhibit the growth of tumour cells, suggesting that packaging
miR-21 in exosomes may contribute to the antileukemic effect of
curcumin in CML (117). Tumour
cell-derived exosomes carry tumour-associated antigens that can
stimulate DC cell activation and induce immune responses, CD11c,
MHCII and IL-12 are up-regulated in exosome-loaded DC cells and
activate CD4+ T cells effectively, prolonging the
survival time of WEHI-3B mice (myelomonocytic leukaemia mice)
significantly, exosomes enriched from patient sera are likely to
provide an optimized source of individual-specific antigens for DC
loading and vaccination, considering that exosomes are abundant in
the serum of tumour patients (118). High expression levels of
epidermal growth factor receptor (EGFR) contribute to the rapid
progression of tumours, and is involved in tumour invasion and
metastasis. A novel class of exosomes called synthetic multivalent
antibodies retargeted exosomes (SMART-Exos) is generated in this
situation. These SMART-Exos contain two different antibodies that
can simultaneously target EGFR on tumour cells and CD3 receptors on
T cells. Further in-depth study confirms SMART-Exos show tight
binding ability to both EGFR-expressing triple-negative breast
cancer MDA-MB-468 cells and T cells, which demonstrated
satisfactory antitumour activity by activating T cells to attack
breast cancer cells. SMART-Exo may likely be adapted for other
disease models by utilizing different types of functional
antibodies (119). Cancer
cell-derived exosomes were engineered to carry DOXIL (doxorubicin
HCl liposome injection) and injected into HT1080 tumour-bearing
nude mice. These drug-loaded exosomes showed an enhanced
therapeutic effect and more effective clearance of tumour cells in
mice compared with the DOXIL alone group (120). These studies provides new
strategies for exosome-based targeted delivery of antitumour
drugs.
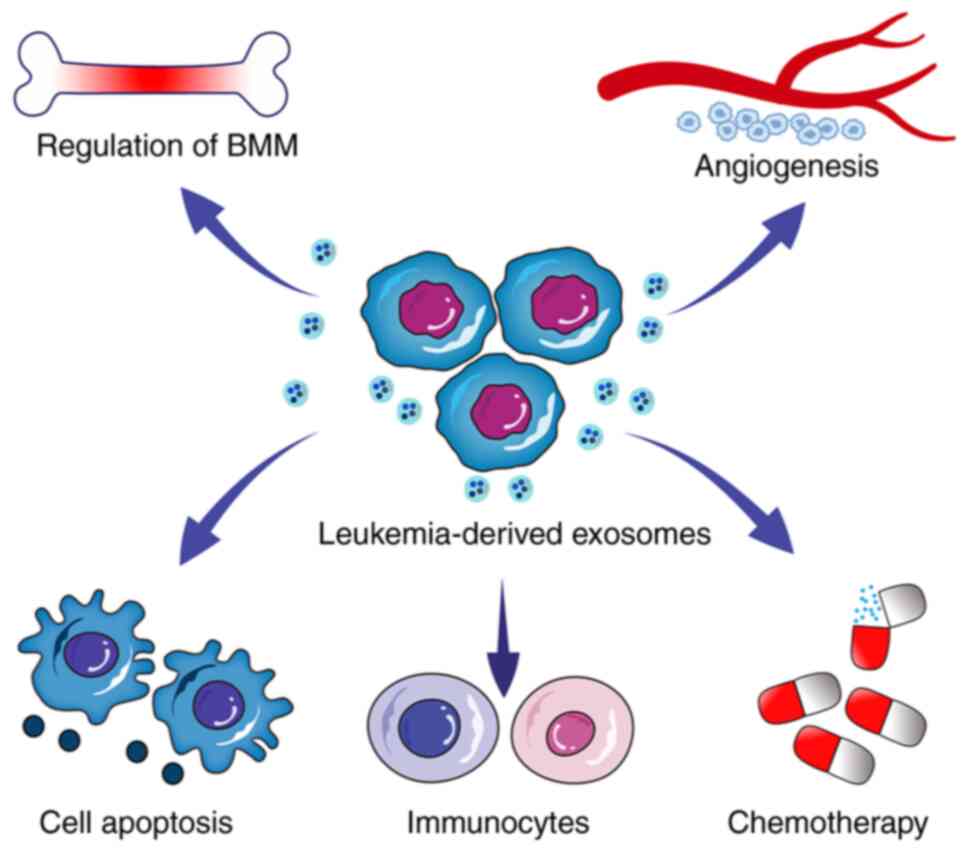
Increasing studies have confirmed that
tumour-derived exosomes can affect the pathogenesis and development
of leukaemia by affecting the bone marrow microenvironment,
apoptosis, angiogenesis, chemoradiotherapy resistance and immune
escape. Certain exosome-based therapies are now used for clinical
or research purposes with an in-depth understanding of the
physiological and pathological roles of exosomes, although
exosome-related technical and regulatory issues remain to be
resolved. Notably, exosomes can be used as a biomarker to monitor
leukaemia progress and as a carrier to transport chemotherapeutic
drugs to inhibit the development of leukaemia, demonstrating the
potential of exosome-based intervention ability in leukaemia.
Notably, there need to be unified standards regarding the
efficiency and drug delivery of exosomes, which are the challenges
exosomes face. However, in-depth research is needed on the specific
mechanism of exosomes in tumour cells, such as how obtaining
high-purity exosomes for treatment and accurate delivery is
particularly important, and correlational research which may impact
leukaemia treatment. The following study on exosomes should focus
on solving the problems of tumour cell drug resistance and
recurrence, considering a significant proportion of patients
relapse after treatment. In conclusion, exosomes demonstrate
potential value in diagnosing leukaemia and treating and monitoring
disease development. Therefore, a complete understanding of the
specific mechanism of exosomes in leukaemia may provide new ideas
and strategies for future clinical treatment.
Not applicable.
Funding: This research was supported by the Shandong Province
Health Department (grant nos. 2019WS589 and 2017WS407) and Shandong
Province Traditional Chinese Medicine Science and Technology
Development Plan (grant no. 2017-216).
Not applicable.
DLD conceived and designed this review. LC and TX
wrote the first draft. BW participated in writing of the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Juliusson G and Hough R: Leukemia. Prog
Tumor Res. 43:87–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alsobhi E, Farahat F, Daghistani M, Awad
K, Al-Zahran O, Al-Saiari A and Koshak F: Overall survival of adult
acute myeloid leukemia based on cytogenetic and molecular
abnormalities during 5 years in a single center study. Saudi Med J.
40:1171–1176. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schubert D: A brief history of adherons:
The discovery of brain exosomes. Int J Mol Sci.
21(7673)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
5
|
Keller S, Sanderson MP, Stoeck A and
Altevogt P: Exosomes: From biogenesis and secretion to biological
function. Immunol Lett. 107:102–108. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Simpson RJ, Jensen SS and Lim JW:
Proteomic profiling of exosomes: Current perspectives. Proteomics.
8:4083–4099. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Mardani R, Jafari Najaf Abadi MH, Motieian
M, Taghizadeh-Boroujeni S, Bayat A, Farsinezhad A, Gheibi HS,
Motieian M and Pourghadamyari H: MicroRNA in leukemia: Tumor
suppressors and oncogenes with prognostic potential. J Cell
Physiol. 234:8465–8486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y,
Fu W, Yi J, Wang J and Du G: The biology, function, and
applications of exosomes in cancer. Acta Pharm Sin B. 11:2783–2797.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng L and Hill AF: Therapeutically
harnessing extracellular vesicles. Nat Rev Drug Discov. 21:379–399.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
De Toro J, Herschlik L, Waldner C and
Mongini C: Emerging roles of exosomes in normal and pathological
conditions: New insights for diagnosis and therapeutic
applications. Front Immunol. 6(203)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang L and Yu D: Exosomes in cancer
development, metastasis, and immunity. Biochim Biophys Acta Rev
Cancer. 1871:455–468. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4(27031)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khatun Z, Bhat A, Sharma S and Sharma A:
Elucidating diversity of exosomes: Biophysical and molecular
characterization methods. Nanomedicine (Lond). 11:2359–2377.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo S, Xu J, Estell AP, Ivory CF, Du D,
Lin Y and Dong WJ: Paper-based ITP technology: An application to
specific cancer-derived exosome detection and analysis. Biosens
Bioelectron. 164(112292)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Agarwal P and Bhatia R: Influence of bone
marrow microenvironment on leukemic stem cells: Breaking up an
intimate relationship. Adv Cancer Res. 127:227–252. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharma A, Khatun Z and Shiras A: Tumor
exosomes: Cellular postmen of cancer diagnosis and personalized
therapy. Nanomedicine (Lond). 11:421–437. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tan Z, Kan C, Wong M, Sun M, Liu Y, Yang
F, Wang S and Zheng H: Regulation of malignant myeloid leukemia by
mesenchymal stem cells. Front Cell Dev Biol.
10(857045)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Boyiadzis M and Whiteside TL: Exosomes in
acute myeloid leukemia inhibit hematopoiesis. Curr Opin Hematol.
25:279–284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huan J, Hornick NI, Shurtleff MJ, Skinner
AM, Goloviznina NA, Roberts CT Jr and Kurre P: RNA trafficking by
acute myelogenous leukemia exosomes. Cancer Res. 73:918–929.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huan J, Hornick NI, Goloviznina NA,
Kamimae-Lanning AN, David LL, Wilmarth PA, Mori T, Chevillet JR,
Narla A, Roberts CT Jr, et al: Coordinate regulation of residual
bone marrow function by paracrine trafficking of AML exosomes.
Leukemia. 29:2285–2295. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kumar B, Garcia M, Weng L, Jung X,
Murakami JL, Hu X, McDonald T, Lin A, Kumar AR, DiGiusto DL, et al:
Acute myeloid leukemia transforms the bone marrow niche into a
leukemia-permissive microenvironment through exosome secretion.
Leukemia. 32:575–587. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hornick NI, Doron B, Abdelhamed S, Huan J,
Harrington CA, Shen R, Cambronne XA, Chakkaramakkil VS and Kurre P:
AML suppresses hematopoiesis by releasing exosomes that contain
microRNAs targeting c-MYB. Sci Signal. 9(ra88)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao C, Du F, Zhao Y, Wang S and Qi L:
Acute myeloid leukemia cells secrete microRNA-4532-containing
exosomes to mediate normal hematopoiesis in hematopoietic stem
cells by activating the LDOC1-dependent STAT3 signaling pathway.
Stem Cell Res Ther. 10(384)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoshida M, Horiguchi H, Kikuchi S, Iyama
S, Ikeda H, Goto A, Kawano Y, Murase K, Takada K, Miyanishi K, et
al: miR-7977 inhibits the Hippo-YAP signaling pathway in bone
marrow mesenchymal stromal cells. PLoS One.
14(e213220)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang L, Khadka B, Wu J, Feng Y, Long B,
Xiao R and Liu J: Bone marrow mesenchymal stem cells-derived
exosomal miR-425-5p inhibits acute myeloid leukemia cell
proliferation, apoptosis, invasion and migration by targeting WTAP.
Onco Targets Ther. 14:4901–4914. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chiarini F, Lonetti A, Evangelisti C,
Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM,
Mccubrey JA and Martelli AM: Advances in understanding the acute
lymphoblastic leukemia bone marrow microenvironment: From biology
to therapeutic targeting. Biochim Biophys Acta. 1863:449–463.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rios de Los Rios J, Enciso J,
Vilchis-Ordoñez A, Vázquez-Ramírez R, Ramirez-Ramirez D, Balandrán
JC, Rodríguez-Martínez A, Ruiz-Tachiquín M, Pompa-Mera E, Mendoza
L, et al: Acute lymphoblastic leukemia-secreted miRNAs induce a
proinflammatory microenvironment and promote the activation of
hematopoietic progenitors. J Leukoc Biol. 112:31–45.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang Y, Li J and Geng Y: Exosomes derived
from chronic lymphocytic leukaemia cells transfer miR-146a to
induce the transition of mesenchymal stromal cells into
cancer-associated fibroblasts. J Biochem. 168:491–498.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Paggetti J, Haderk F, Seiffert M, Janji B,
Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E, et al:
Exosomes released by chronic lymphocytic leukemia cells induce the
transition of stromal cells into cancer-associated fibroblasts.
Blood. 126:1106–1117. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yeh YY, Ozer HG, Lehman AM, Maddocks K, Yu
L, Johnson AJ and Byrd JC: Characterization of CLL exosomes reveals
a distinct microRNA signature and enhanced secretion by activation
of BCR signaling. Blood. 125:3297–3305. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Prieto D, Sotelo N, Seija N, Sernbo S,
Abreu C, Durán R, Gil M, Sicco E, Irigoin V, Oliver C, et al:
S100-A9 protein in exosomes from chronic lymphocytic leukemia cells
promotes NF-κB activity during disease progression. Blood.
130:777–788. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Corrado C, Raimondo S, Saieva L, Flugy AM,
De Leo G and Alessandro R: Exosome-mediated crosstalk between
chronic myelogenous leukemia cells and human bone marrow stromal
cells triggers an interleukin 8-dependent survival of leukemia
cells. Cancer Lett. 348:71–76. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Taverna S, Amodeo V, Saieva L, Russo A,
Giallombardo M, De Leo G and Alessandro R: Exosomal shuttling of
miR-126 in endothelial cells modulates adhesive and migratory
abilities of chronic myelogenous leukemia cells. Mol Cancer.
13(169)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang YH, Liu J, Lin J, Li SQ, Xu YM, Min
QH, Zhong QH, Sun F, Li J, You XH, et al: K562 cell-derived
exosomes suppress the adhesive function of bone marrow mesenchymal
stem cells via delivery of miR-711. Biochem Biophys Res Commun.
521:584–589. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang Y, He H, He J, Gu X, Hu P, Zuo R and
Sa Y: Hyperleukocytic acute leukemia circulating exosomes regulate
HSCs and BM-MSCs. J Healthc Eng. 2021(9457070)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gao X, Wan Z, Wei M, Dong Y, Zhao Y, Chen
X, Li Z, Qin W, Yang G and Liu L: Chronic myelogenous leukemia
cells remodel the bone marrow niche via exosome-mediated transfer
of miR-320. Theranostics. 9:5642–5656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jafarzadeh N, Gholampour MA, Alivand MR,
Kavousi S, Arzi L, Rad F, Sadeghizadeh M and Pornour M: CML derived
exosomes promote tumor favorable functional performance in T cells.
BMC Cancer. 21(1002)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wan Z, Chen X, Gao X, Dong Y, Zhao Y, Wei
M, Fan W, Yang G and Liu L: Chronic myeloid leukemia-derived
exosomes attenuate adipogenesis of adipose derived mesenchymal stem
cells via transporting miR-92a-3p. J Cell Physiol. 234:21274–21283.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang D, Ming X, Xu J and Xiao Y:
Circ_0009910 shuttled by exosomes regulates proliferation, cell
cycle and apoptosis of acute myeloid leukemia cells by regulating
miR-5195-3p/GRB10 axis. Hematol Oncol. 39:390–400. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cheng H, Ding J, Tang G, Huang A, Gao L,
Yang J and Chen L: Human mesenchymal stem cells derived exosomes
inhibit the growth of acute myeloid leukemia cells via regulating
miR-23b-5p/TRIM14 pathway. Mol Med. 27(128)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang D, Wu X, Sun X, Tan W, Dai X, Xie Y,
Du A and Zhao Q: Bone mesenchymal stem cell-derived exosomal
microRNA-7-5p inhibits progression of acute myeloid leukemia by
targeting OSBPL11. J Nanobiotechnology. 20(29)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang F, Lu Y, Wang M, Zhu J, Li J, Zhang
P, Yuan Y and Zhu F: Exosomes derived from human bone marrow
mesenchymal stem cells transfer miR-222-3p to suppress acute
myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol
Cell Probes. 51(101513)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yan W, Song L, Wang H, Yang W, Hu L and
Yang Y: Extracellular vesicles carrying miRNA-181b-5p affects the
malignant progression of acute lymphoblastic leukemia. J Transl
Med. 19(511)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Raimondo S, Saieva L, Corrado C, Fontana
S, Flugy A, Rizzo A, De Leo G and Alessandro R: Chronic myeloid
leukemia-derived exosomes promote tumor growth through an autocrine
mechanism. Cell Commun Signal. 13(8)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jaworski E, Narayanan A, Van Duyne R,
Shabbeer-Meyering S, Iordanskiy S, Saifuddin M, Das R, Afonso PV,
Sampey GC, Chung M, et al: Human T-lymphotropic virus type
1-infected cells secrete exosomes that contain Tax protein. J Biol
Chem. 289:22284–22305. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Haque S and Vaiselbuh SR: Silencing of
exosomal miR-181a reverses pediatric acute lymphocytic leukemia
cell proliferation. Pharmaceuticals (Basel). 13(241)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Aslan C, Maralbashi S, Salari F, Kahroba
H, Sigaroodi F, Kazemi T and Kharaziha P: Tumor-derived exosomes:
Implication in angiogenesis and antiangiogenesis cancer therapy. J
Cell Physiol. 234:16885–16903. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ludwig N and Whiteside TL: Potential roles
of tumor-derived exosomes in angiogenesis. Expert Opin Ther
Targets. 22:409–417. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mineo M, Garfield SH, Taverna S, Flugy A,
De Leo G, Alessandro R and Kohn EC: Exosomes released by K562
chronic myeloid leukemia cells promote angiogenesis in a
Src-dependent fashion. Angiogenesis. 15:33–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Corrado C, Saieva L, Raimondo S, Santoro
A, De Leo G and Alessandro R: Chronic myelogenous leukaemia
exosomes modulate bone marrow microenvironment through activation
of epidermal growth factor receptor. J Cell Mol Med. 20:1829–1839.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Umezu T, Ohyashiki K, Kuroda M and
Ohyashiki JH: Leukemia cell to endothelial cell communication via
exosomal miRNAs. Oncogene. 32:2747–2755. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Roma-Rodrigues C, Fernandes AR and
Baptista PV: Counteracting the effect of leukemia exosomes by
antiangiogenic gold nanoparticles. Int J Nanomedicine.
14:6843–6854. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tadokoro H, Umezu T, Ohyashiki K, Hirano T
and Ohyashiki JH: Exosomes derived from hypoxic leukemia cells
enhance tube formation in endothelial cells. J Biol Chem.
288:34343–34351. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ohyashiki JH, Umezu T and Ohyashiki K:
Exosomes promote bone marrow angiogenesis in hematologic neoplasia:
The role of hypoxia. Curr Opin Hematol. 23:268–273. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Taverna S, Fontana S, Monteleone F, Pucci
M, Saieva L, De Caro V, Cardinale VG, Giallombardo M, Vicario E,
Rolfo C, et al: Curcumin modulates chronic myelogenous leukemia
exosomes composition and affects angiogenic phenotype via exosomal
miR-21. Oncotarget. 7:30420–30439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang B, Wang X, Hou D, Huang Q, Zhan W,
Chen C, Liu J, You R, Xie J, Chen P and Huang H: Exosomes derived
from acute myeloid leukemia cells promote chemoresistance by
enhancing glycolysis-mediated vascular remodeling. J Cell Physiol.
234:10602–10614. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ghosh AK, Secreto CR, Knox TR, Ding W,
Mukhopadhyay D and Kay NE: Circulating microvesicles in B-cell
chronic lymphocytic leukemia can stimulate marrow stromal cells:
Implications for disease progression. Blood. 115:1755–1764.
2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
El-Saghir J, Nassar F, Tawil N and
El-Sabban M: ATL-derived exosomes modulate mesenchymal stem cells:
Potential role in leukemia progression. Retrovirology.
13(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Vago L and Gojo I: Immune escape and
immunotherapy of acute myeloid leukemia. J Clin Invest.
130:1552–1564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Clayton A, Mitchell JP, Court J, Linnane
S, Mason MD and Tabi Z: Human tumor-derived exosomes down-modulate
NKG2D expression. J Immunol. 180:7249–7258. 2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Szczepanski MJ, Szajnik M, Welsh A,
Whiteside TL and Boyiadzis M: Blast-derived microvesicles in sera
from patients with acute myeloid leukemia suppress natural killer
cell function via membrane-associated transforming growth
factor-beta1. Haematologica. 96:1302–1309. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Haderk F, Schulz R, Iskar M, Cid LL, Worst
T, Willmund KV, Schulz A, Warnken U, Seiler J, Benner A, et al:
Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci
Immunol. 2(eaah5509)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chitadze G, Bhat J, Lettau M, Janssen O
and Kabelitz D: Generation of soluble NKG2D ligands: Proteolytic
cleavage, exosome secretion and functional implications. Scand J
Immunol. 78:120–129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Whiteside TL: Immune modulation of T-cell
and NK (natural killer) cell activities by TEXs (tumour-derived
exosomes). Biochem Soc Trans. 41:245–251. 2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T lymphocytes. J
Immunol. 183:3720–3730. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Santos PM and Butterfield LH: Dendritic
cell-based cancer vaccines. J Immunol. 200:443–449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yin W, Ouyang S, Li Y, Xiao B and Yang H:
Immature dendritic cell-derived exosomes: A promise subcellular
vaccine for autoimmunity. Inflammation. 36:232–240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Van Acker HH, Versteven M, Lichtenegger
FS, Roex G, Campillo-Davo D, Lion E, Subklewe M, Van Tendeloo VF,
Berneman ZN and Anguille S: Dendritic cell-based immunotherapy of
acute myeloid leukemia. J Clin Med. 8(579)2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhou J, Wang S, Sun K and Chng WJ: The
emerging roles of exosomes in leukemogeneis. Oncotarget.
7:50698–50707. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC,
Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, et al: Dendritic cell
biology and its role in tumor immunotherapy. J Hematol Oncol.
13(107)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sabado RL, Balan S and Bhardwaj N:
Dendritic cell-based immunotherapy. Cell Res. 27:74–95.
2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Huang L, Rong Y, Tang X, Yi K, Qi P, Hou
J, Liu W, He Y, Gao X, Yuan C and Wang F: Engineered exosomes as an
in situ DC-primed vaccine to boost antitumor immunity in breast
cancer. Mol Cancer. 21(45)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z,
Qi H, Guo H and Yin H: Dendritic cell-derived exosomes elicit tumor
regression in autochthonous hepatocellular carcinoma mouse models.
J Hepatol. 67:739–748. 2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Wang X, He L, Huang X, Zhang S, Cao W, Che
F, Zhu Y and Dai J: Recent progress of exosomes in multiple
myeloma: Pathogenesis, diagnosis, prognosis and therapeutic
strategies. Cancers (Basel). 13(1635)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Huang F, Wan J, Hao S, Deng X, Chen L and
Ma L: TGF-β1-silenced leukemia cell-derived exosomes target
dendritic cells to induce potent anti-leukemic immunity in a mouse
model. Cancer Immunol Immunother. 66:1321–1331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Huang F, Li Z, Zhang W, Li J and Hao S:
Enhancing the anti-leukemia immunity of acute lymphocytic
leukemia-derived exosome-based vaccine by downregulation of PD-L1
expression. Cancer Immunol Immunother. 71:2197–2212.
2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Gabrusiewicz K, Li X, Wei J, Hashimoto Y,
Marisetty AL, Ott M, Wang F, Hawke D, Yu J, Healy LM, et al:
Glioblastoma stem cell-derived exosomes induce M2 macrophages and
PD-L1 expression on human monocytes. Oncoimmunology.
7(e1412909)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Hu K, Gu Y, Lou L, Liu L, Hu Y, Wang B,
Luo Y, Shi J, Yu X and Huang H: Galectin-3 mediates bone marrow
microenvironment-induced drug resistance in acute leukemia cells
via Wnt/β-catenin signaling pathway. J Hematol Oncol.
8(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Cheng YL, Huang WC, Chen CL, Tsai CC, Wang
CY, Chiu WH, Chen YL, Lin YS, Chang CF and Lin CF: Increased
Galectin-3 facilitates leukemia cell survival from apoptotic
stimuli. Biochem Biophys Res Commun. 412:334–340. 2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Fei F, Joo EJ, Tarighat SS, Schiffer I,
Paz H, Fabbri M, Abdel-Azim H, Groffen J and Heisterkamp N: B-cell
precursor acute lymphoblastic leukemia and stromal cells
communicate through Galectin-3. Oncotarget. 6:11378–11394.
2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Chen T, Zhang G, Kong L, Xu S, Wang Y and
Dong M: Leukemia-derived exosomes induced IL-8 production in bone
marrow stromal cells to protect the leukemia cells against
chemotherapy. Life Sci. 221:187–195. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Macanas-Pirard P, Broekhuizen R, González
A, Oyanadel C, Ernst D, García P, Montecinos VP, Court F, Ocqueteau
M, Ramirez P and Nervi B: Resistance of leukemia cells to
cytarabine chemotherapy is mediated by bone marrow stroma, involves
cell-surface equilibrative nucleoside transporter-1 removal and
correlates with patient outcome. Oncotarget. 8:23073–23086.
2017.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Daver N, Venugopal S and Ravandi F: FLT3
mutated acute myeloid leukemia: 2021 Treatment algorithm. Blood
Cancer J. 11(104)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Daver N, Schlenk RF, Russell NH and Levis
MJ: Targeting FLT3 mutations in AML: Review of current knowledge
and evidence. Leukemia. 33:299–312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Viola S, Traer E, Huan J, Hornick NI,
Tyner JW, Agarwal A, Loriaux M, Johnstone B and Kurre P:
Alterations in acute myeloid leukaemia bone marrow stromal cell
exosome content coincide with gains in tyrosine kinase inhibitor
resistance. Br J Haematol. 172:983–986. 2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Wu J, Zhang Y, Li X, Ren J, Chen L, Chen J
and Cao Y: Exosomes from bone marrow mesenchymal stem cells
decrease chemosensitivity of acute myeloid leukemia cells via
delivering miR-10a. Biochem Biophys Res Commun. 622:149–156.
2022.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Crompot E, Van Damme M, Pieters K,
Vermeersch M, Perez-Morga D, Mineur P, Maerevoet M, Meuleman N,
Bron D, Lagneaux L and Stamatopoulos B: Extracellular vesicles of
bone marrow stromal cells rescue chronic lymphocytic leukemia B
cells from apoptosis, enhance their migration and induce gene
expression modifications. Haematologica. 102:1594–1604.
2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Javidi-Sharifi N, Martinez J, English I,
Joshi SK, Scopim-Ribeiro R, Viola SK, Edwards DT V, Agarwal A,
Lopez C, Jorgens D, et al: FGF2-FGFR1 signaling regulates release
of leukemia-protective exosomes from bone marrow stromal cells.
Elife. 8(e40033)2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Shah CA, Bei L, Wang H, Platanias LC and
Eklund EA: The leukemia-associated Mll-Ell oncoprotein induces
fibroblast growth factor 2 (Fgf2)-dependent cytokine
hypersensitivity in myeloid progenitor cells. J Biol Chem.
288:32490–32505. 2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Li MY, Zhao C, Chen L, Yao FY, Zhong FM,
Chen Y, Xu S, Jiang JY, Yang YL, Min QH, et al: Quantitative
proteomic analysis of plasma exosomes to identify the candidate
biomarker of imatinib resistance in chronic myeloid leukemia
patients. Front Oncol. 11(779567)2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Chen X, Chen Y, Zhang M, Cheng H, Mai H,
Yi M, Xu H, Yuan X, Liu S and Wen F: HucMSC exosomes promoted
imatinib-induced apoptosis in K562-R cells via a
miR-145a-5p/USP6/GLS1 axis. Cell Death Dis. 13(92)2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Dong Y, Lin Y, Gao X, Zhao Y, Wan Z, Wang
H, Wei M, Chen X, Qin W, Yang G and Liu L: Targeted blocking of
miR328 lysosomal degradation with alkalized exosomes sensitizes the
chronic leukemia cells to imatinib. Appl Microbiol Biotechnol.
103:9569–9582. 2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Min QH, Wang XZ, Zhang J, Chen QG, Li SQ,
Liu XQ, Li J, Liu J, Yang WM, Jiang YH, et al: Exosomes derived
from imatinib-resistant chronic myeloid leukemia cells mediate a
horizontal transfer of drug-resistant trait by delivering miR-365.
Exp Cell Res. 362:386–393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Liu Y, Song B, Wei Y, Chen F, Chi Y, Fan
H, Liu N, Li Z, Han Z and Ma F: Exosomes from mesenchymal stromal
cells enhance imatinib-induced apoptosis in human leukemia cells
via activation of caspase signaling pathway. Cytotherapy.
20:181–188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Yu D, Li Y, Wang M, Gu J, Xu W, Cai H,
Fang X and Zhang X: Exosomes as a new frontier of cancer liquid
biopsy. Mol Cancer. 21(56)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo
X, Wei Q, Wang J, Xiong H, Chen C, Xu B, et al: Exosome: Emerging
biomarker in breast cancer. Oncotarget. 8:41717–41733.
2017.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Boyiadzis M and Whiteside TL:
Plasma-derived exosomes in acute myeloid leukemia for detection of
minimal residual disease: Are we ready? Expert Rev Mol Diagn.
16:623–629. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
An T, Qin S, Xu Y, Tang Y, Huang Y, Situ
B, Inal JM and Zheng L: Exosomes serve as tumour markers for
personalized diagnostics owing to their important role in cancer
metastasis. J Extracell Vesicles. 4(27522)2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Bobrie A, Colombo M, Raposo G and Théry C:
Exosome secretion: Molecular mechanisms and roles in immune
responses. Traffic. 12:1659–1668. 2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Tzoran I, Rebibo-Sabbah A, Brenner B and
Aharon A: Disease dynamics in patients with acute myeloid leukemia:
New biomarkers. Exp Hematol. 43:936–943. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Hornick NI, Huan J, Doron B, Goloviznina
NA, Lapidus J, Chang BH and Kurre P: Serum exosome MicroRNA as a
minimally-invasive early biomarker of AML. Sci Rep.
5(11295)2015.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Hong CS, Muller L, Whiteside TL and
Boyiadzis M: Plasma exosomes as markers of therapeutic response in
patients with acute myeloid leukemia. Front Immunol.
5(160)2014.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Li Q, Wu Y, Zhang Y, Sun H, Lu Z, Du K,
Fang S and Li W: miR-125b regulates cell progression in chronic
myeloid leukemia via targeting BAK1. Am J Transl Res. 8:447–459.
2016.PubMed/NCBI
|
|
107
|
Jiang L, Deng T, Wang D and Xiao Y:
Elevated serum exosomal miR-125b level as a potential marker for
poor prognosis in intermediate-risk acute myeloid leukemia. Acta
Haematol. 140:183–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lin X, Ling Q, Lv Y, Ye W, Huang J, Li X,
Guo Q, Wang J, Li Z and Jin J: Plasma exosome-derived microRNA-532
as a novel predictor for acute myeloid leukemia. Cancer Biomark.
28:151–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Ferrajoli A, Shanafelt TD, Ivan C, Shimizu
M, Rabe KG, Nouraee N, Ikuo M, Ghosh AK, Lerner S, Rassenti LZ, et
al: Prognostic value of miR-155 in individuals with monoclonal
B-cell lymphocytosis and patients with B chronic lymphocytic
leukemia. Blood. 122:1891–1899. 2013.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Stamatopoulos B, Van Damme M, Crompot E,
Dessars B, Housni HE, Mineur P, Meuleman N, Bron D and Lagneaux L:
Opposite prognostic significance of cellular and serum circulating
MicroRNA-150 in patients with chronic lymphocytic leukemia. Mol
Med. 21:123–133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Okimoto RA and Van Etten RA: Navigating
the road toward optimal initial therapy for chronic myeloid
leukemia. Curr Opin Hematol. 18:89–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2020 Update on diagnosis, therapy and monitoring.
Am J Hematol. 95:691–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Bernardi S, Foroni C, Zanaglio C, Re F,
Polverelli N, Turra A, Morello E, Farina M, Cattina F, Gandolfi L,
et al: Feasibility of tumor-derived exosome enrichment in the
onco-hematology leukemic model of chronic myeloid leukemia. Int J
Mol Med. 44:2133–2144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Kibria G, Ramos EK, Wan Y, Gius DR and Liu
H: Exosomes as a drug delivery system in cancer therapy: Potential
and challenges. Mol Pharm. 15:3625–3633. 2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Ha D, Yang N and Nadithe V: Exosomes as
therapeutic drug carriers and delivery vehicles across biological
membranes: Current perspectives and future challenges. Acta Pharm
Sin B. 6:287–296. 2016.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Bellavia D, Raimondo S, Calabrese G, Forte
S, Cristaldi M, Patinella A, Memeo L, Manno M, Raccosta S, Diana P,
et al: Interleukin 3-receptor targeted exosomes inhibit in vitro
and in vivo chronic myelogenous leukemia cell growth. Theranostics.
7:1333–1345. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Taverna S, Giallombardo M, Pucci M, Flugy
A, Manno M, Raccosta S, Rolfo C, De Leo G and Alessandro R:
Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia
cells growth: A possible role for exosomal disposal of miR-21.
Oncotarget. 6:21918–21933. 2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Gu X, Erb U, Büchler MW and Zöller M:
Improved vaccine efficacy of tumor exosome compared to tumor lysate
loaded dendritic cells in mice. Int J Cancer. 136:E74–E84.
2015.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Cheng Q, Shi X and Zhang Y: Reprogramming
exosomes for immunotherapy. Methods Mol Biol. 2097:197–209.
2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Qiao L, Hu S, Huang K, Su T, Li Z,
Vandergriff A, Cores J, Dinh PU, Allen T, Shen D, et al: Tumor
cell-derived exosomes home to their cells of origin and can be used
as Trojan horses to deliver cancer drugs. Theranostics.
10:3474–3487. 2020.PubMed/NCBI View Article : Google Scholar
|