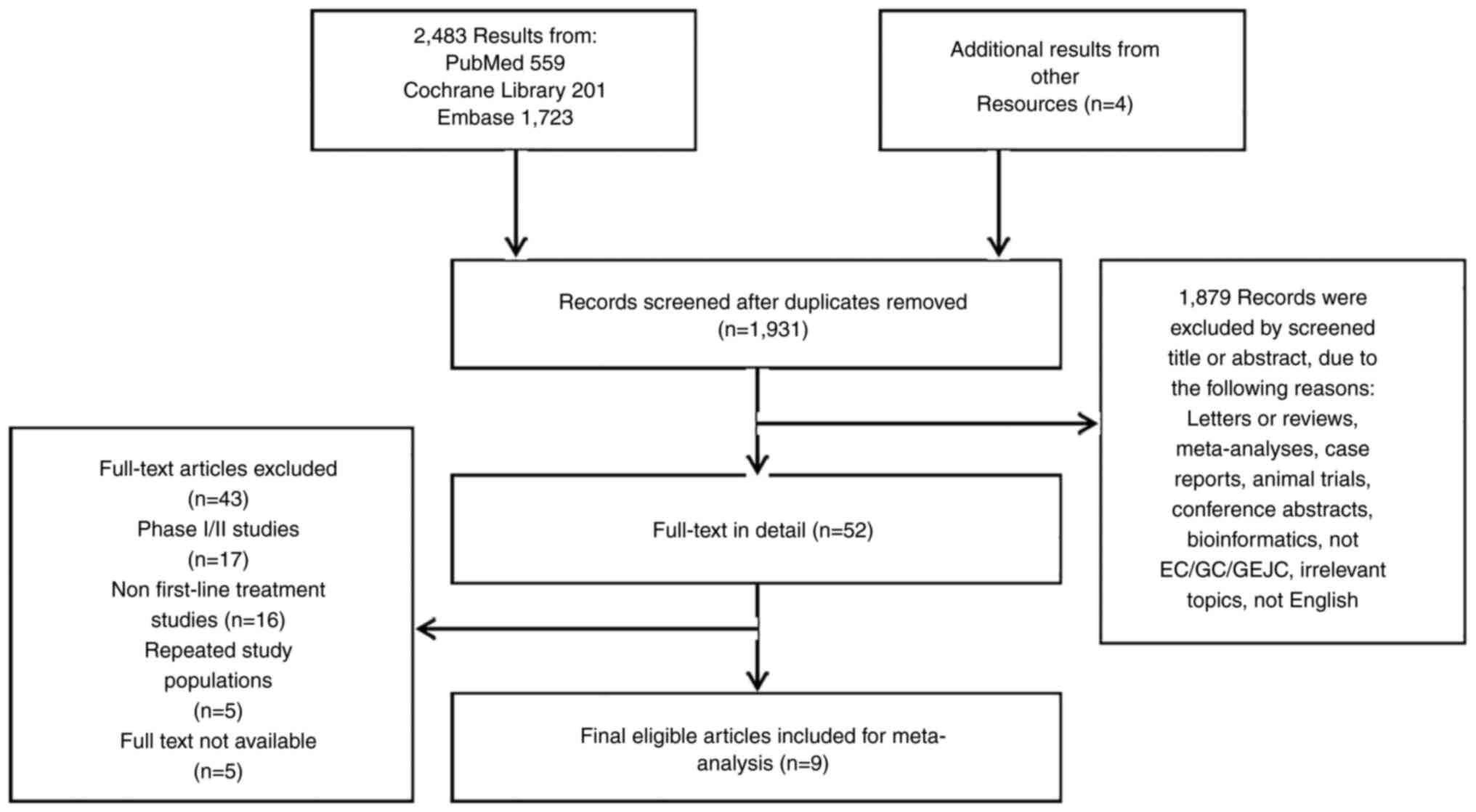
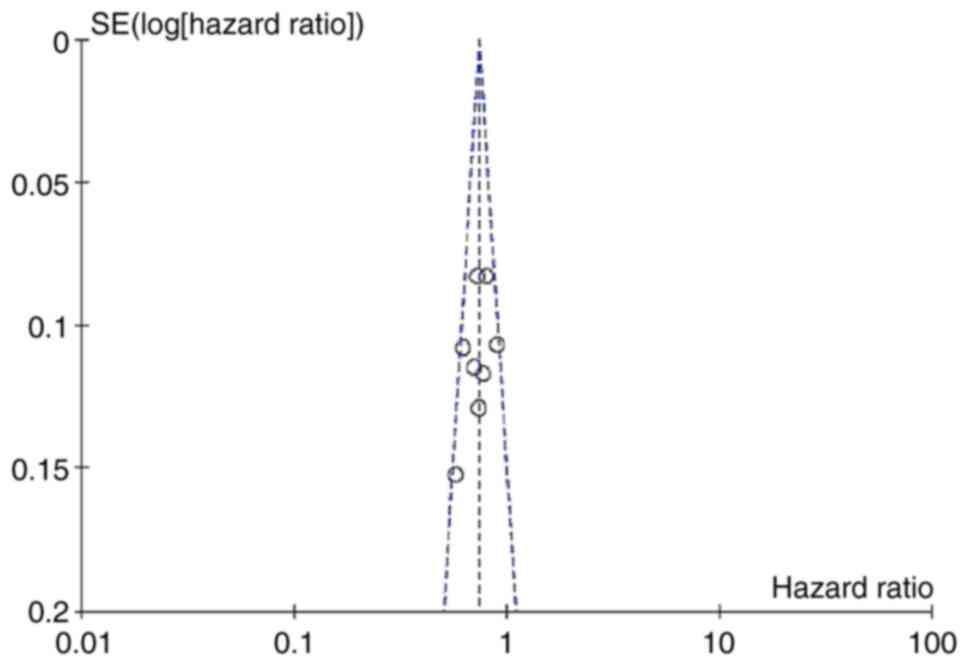
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baumgartner R, Taghizadeh H, Jomrich G,
Schoppmann SF, Preusser M and Ilhan-Mutlu A: Utilization and
efficacy of palliative chemotherapy for locally advanced or
metastatic gastroesophageal carcinoma. Anticancer Res. 40:965–975.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van den Ende T, Smyth E, Hulshof MCCM and
van Laarhoven HWM: Chemotherapy and novel targeted therapies for
operable esophageal and gastroesophageal junctional cancer. Best
Pract Res Clin Gastroenterol. 36-37:45–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen K, Wang X, Yang L and Chen Z: The
anti-PD-1/PD-L1 immunotherapy for gastric esophageal cancer: A
systematic review and meta-analysis and literature review. Cancer
Control. 28(1073274821997430)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seidel JA, Otsuka A and Kabashima K:
Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of
action, efficacy, and limitations. Front Oncol.
8(86)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vargas FA, Furness AJS, Litchfield K,
Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N,
Roddie C, et al: Fc effector function contributes to the activity
of human anti- CTLA-4 antibodies. Cancer Cell. 33:649–663.e4.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie anti-CTLA-4 and
anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kamphorst AO, Pillai RN, Yang S, Nasti TH,
Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al:
Proliferation of PD-1+ CD8 T cells in peripheral blood after
PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci
USA. 114:4993–4998. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin EM, Gong J, Klempner SJ and Chao J:
Advances in immuno-oncology biomarkers for gastroesophageal cancer:
Programmed death ligand 1, microsatellite instability, and beyond.
World J Gastroenterol. 24:2686–2697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tocheva AS and Mor A: Checkpoint
inhibitors: Applications for autoimmunity. Curr Allergy Asthma Rep.
17(72)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ribas A, Kirkwood JM and Flaherty KT:
Anti-PD-1 antibody treatment for melanoma. Lancet Oncol.
19(e219)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang MY, Jiang XM, Wang BL, Sun Y and Lu
JJ: Combination therapy with PD-1 PD-L1 blockade in non-small cell
lung cancer strategies and mechanisms. Pharmacol Ther.
219(107694)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bear AS, Vonderheide RH and O'Hara MH:
Challenges and opportunities for pancreatic cancer immunotherapy.
Cancer Cell. 38:788–802. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle
S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC,
et al: Signatures of tumour immunity distinguish Asian and
non-Asian gastric adenocarcinomas. Gut. 64:1721–1731.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leone AG, Petrelli F, Ghidini A, Raimondi
A, Smyth EC and Pietrantonio F: Efficacy and activity of PD-1
blockade in patients with advanced esophageal squamous cell
carcinoma: A systematic review and meta-analysis with focus on the
value of PD-L1 combined positive score. ESMO Open.
7(100380)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Watanabe H, Okada M, Kaji Y, Satouchi M,
Sato Y, Yamabe Y, Onaya H, Endo M, Sone M and Arai Y: New response
evaluation criteria in solid tumours-revised RECIST guideline
(version 1.1). Gan To Kagaku Ryoho. 36:2495–2501. 2009.PubMed/NCBI(Article in Japanese).
|
|
18
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
combination therapy in advanced esophageal squamous-cell carcinoma.
N Engl J Med. 386:449–462. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer. Jama Oncol. 6:1571–1580. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos B, Bragagnoli A, et al: First-line nivolumab plus
chemotherapy versus chemotherapy alone for advanced gastric,
gastro-oesophageal junction, and oesophageal adenocarcinoma
(CheckMate 649): A randomised, open-label, phase 3 trial. Lancet.
398:27–40. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janjigian YY, Kawazoe A, Yañez P, Li N,
Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al: The
KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive
gastric cancer. Nature. 600:727–730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): Multicentre, randomised, double blind, phase 3 trial.
BMJ. 377(e68714)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng
J, Yang S, Fan Y, Shi J, Zhang X, et al: Toripalimab plus
chemotherapy in treatment-naïve, advanced esophageal squamous cell
carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell.
40:277–288.e3. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lei M, Siemers NO, Pandya D, Chang H,
Sanchez T, Harbison C, Szabo PM, Janjigian Y, Ott PA, Sharma P, et
al: Analyses of PD-L1 and inflammatory gene expression association
with efficacy of nivolumab ± ipilimumab in gastric
cancer/gastroesophageal junction cancer. Clin Cancer Res.
27:3926–3935. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Z, Liu Z and Chen Z: Comparison of
treatment efficacy and survival outcomes between Asian and western
patients with unresectable gastric or gastro-esophageal
adenocarcinoma: A systematic review and meta-analysis. Front Oncol.
12(831207)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noori M, Mahjoubfar A, Azizi S, Fayyaz F
and Rezaei N: Immune checkpoint inhibitors plus chemotherapy versus
chemotherapy alone as first-line therapy for advanced gastric and
esophageal cancers: A systematic review and meta-analysis. Int
Immunopharmacol. 113(109317)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Patel MA, Kratz JD, Lubner SJ, Loconte NK
and Uboha NV: Esophagogastric cancers: Integrating immunotherapy
therapy into current practice. J Clin Oncol. 40:2751–2762.
2022.PubMed/NCBI View Article : Google Scholar
|