ND is a type of disease in which cells and neurons
of the brain and spinal cord are lost, which is caused by the loss
of neurons or their myelin sheaths (18), eventually leading to dysfunction.
NDs include chronic diseases, such as Alzheimer's disease (AD),
Parkinson's disease (PD) and multiple sclerosis (MS), as well as
acute diseases, such as cerebral ischemia (CI) and brain injury
(BI). NDs may be related to oxidative stress (OS), protein
aggregate deposition, neuroinflammation, impaired mitochondrial
function, apoptosis induction and autophagy changes (19,20).
Extensive research on the mechanism and treatment of NDs has been
conducted; however, the pathogenesis of these diseases remains to
be fully elucidated. As there is currently no definite cure for
NDs, most cases require conservative treatment, but conservative
treatment with medications may be associated with gastrointestinal
responses (such as nausea and vomiting), cardiovascular reactions
(including tachycardia and arrhythmia) and mental disorders (such
as anxiety and depression) (21,22).
Saffron and its active constituents (mainly CR,
safranal and crocetin) have potent antioxidant and
anti-inflammatory effects on brain cells, prevent amyloid β (Aβ)
aggregation and regulate the steady-state concentration of metal
ions in the brain (23,24). Thus, saffron has therapeutic
potential for AD (25,26), PD (27) MS (28) and CI (29), and may reverse neurotoxicity caused
by toxic substances, thereby protecting neurons (30,31).
Furthermore, ND is usually accompanied by depressive symptoms. A
previous study indicated that 36.23% of patients with PD suffer
from depression, whereas 68.42% suffer from anxiety (32). Thus, due to its anti-depressant and
anxiolytic properties, saffron may effectively treat depressive
symptoms. Based on these pharmacological effects, saffron has a
reasonable prospect as an auxiliary drug for ND treatment.
In the last decade, the pharmacological action of
saffron for ND treatment has been extensively studied in animal
models. However, the available reviews do not provide comprehensive
summaries on the use and mechanism of action of saffron for the
treatment of ND. The pathogenesis of ND is relatively complex
(33,34). To achieve a better therapeutic
effect, the pathway of saffron action must be further clarified.
The present article reviewed the research progress on the use of
saffron extract and its active constituents for ND treatment,
particularly the pharmacological experiments performed in animal
models of AD, PD, MS and CI, to comprehensively summarize the
research results and administration methods of saffron in this
field, focusing on its pharmacological effects during treatment.
Up-to-date information on the potential mechanism by which saffron
exerts neuroprotection through pharmacological activities and its
therapeutic prospects in NDs is presented.
The literature was screened by WY and XQ. The
Chinese National Knowledge Infrastructure, PubMed, ScienceDirect,
ACS publication, Scopus and Medline databases, as well as Wiley
Online Library, were searched for articles published in the Chinese
and English languages, mainly referring to the literature from 1987
to 2022. The search terms mainly included crocin, safranal and
other active constituents of saffron, as well as neurodegenerative
disease,s such as AD and PD, which are terms that are commonly used
in the pharmaceutical industry. The pharmacological research
related to saffron was primarily distributed over the last decade.
The final list of included studies was approved by MZ and JP.
OS is caused by free radicals, atoms or groups with
unpaired electrons, such as hydroxyl, superoxide and nitric
monoxide (35). OS may cause
damage to the cells and tissues of the body, such as the DNA, RNA,
protein and lipid bilayer of nerve cells (36-38).
Oxidative damage to nerve tissue has been found in NDs, such as AD,
PD and amyotrophic lateral sclerosis. Studies have indicated a
close association between Aβ, Tau protein and OS in neurons. For
instance, Aβ aggregation may damage the mitochondria, leading to
mitochondrial dysfunction and the release of numerous reactive
oxygen species (ROS) and OS; the generation of ROS may also
increase the production of Aβ (39,40).
Free radicals, such as oxidized Fe3+, may promote the
phosphorylation and aggregation of Tau (41). Furthermore, hyperphosphorylation
and accumulation of Tau may also damage mitochondrial function,
thereby generating a substantial amount of ROS. Chronic OS and the
resultant peroxides, such as 4-hydroxynonenal, may also provoke Tau
hyperphosphorylation, causing conformational changes and Tau
aggregation (42). Aβ aggregation
and Tau hyperphosphorylation have crucial roles in the pathogenesis
of AD (43-46).
In addition, OS is also associated with the free iron content of
cells and elevated levels of iron ions may be found in the brains
of patients with PD or AD (47).
Therefore, it may be concluded that OS is intertwined with ND.
One of the main pathological features of AD is the
formation of senile plaques by the accumulating Aβ outside the
brain nerve cells. CR, the major active component of saffron, has
been indicated to increase the tightness of a cell-based
blood-brain barrier model, increase recombinant low-density
lipoprotein receptor-related protein 1 and P-glycoprotein
expression, improve Aβ clearance, reduce Aβ aggregation and inhibit
the formation of senile plaques (48). Both CR and the extract of saffron
(water/methanol, 50:50 v/v) was reported to inhibit the
accumulation of Aβ in the human brain through antioxidant effects
(49). Furthermore, saffron
extract was able to antagonize aluminum oxide-induced neurotoxicity
by elevating the activity of antioxidant enzymes, such as
superoxide dismutase (SOD), catalase and glutathione peroxidase
(GSH-Px) (50). Streptozotocin
(STZ) may cause OS by increasing the production of oxygen-free
radicals, thereby inducing cognitive impairment (51). In addition, CR may elevate GSH-Px
activity and the total thiol content and reduce malondialdehyde
(MDA) levels and OS damage, producing an antagonistic effect on
STZ-induced cognitive impairment in rats (52-54).
CR may also significantly decrease the Bax/Bcl-2 ratio and cleaved
caspase-3 levels by reducing ROS production and inhibiting
Aβ-induced apoptosis (55,56). Mitogen-activated protein kinases
(MAPKs) are serine-threonine kinases that mediate intracellular
signaling related to various cellular activities, including cell
proliferation, differentiation and transformation (57). When OS is triggered, ROS activates
downstream apoptosis pathways through the MAPK pathway, such as
NF-κB and p53, triggering cell death (58-60).
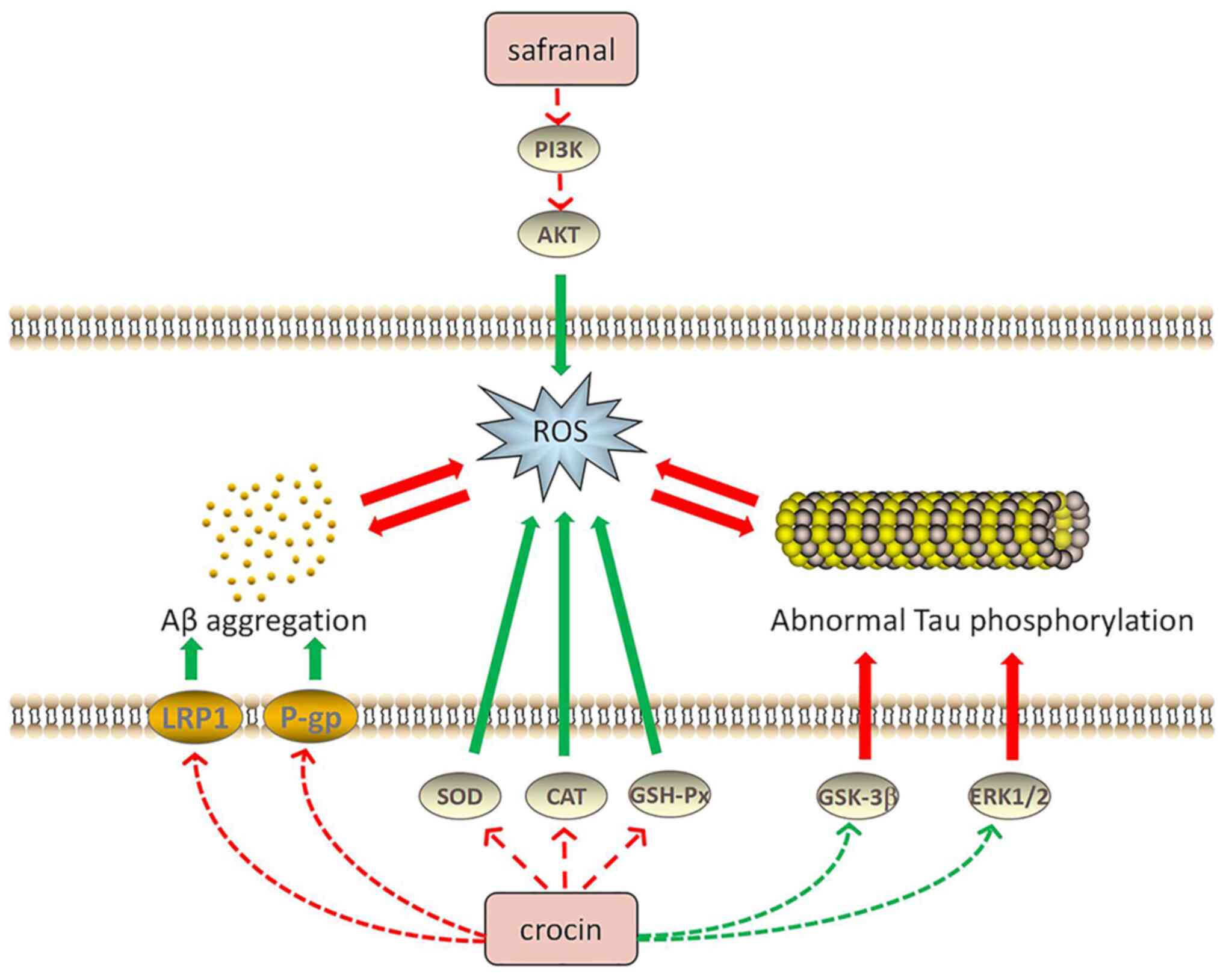
Safranal may reduce the ROS levels in cells, thereby reducing
Aβ-induced apoptosis through the PI3K/AKT and MAPK/extracellular
regulated protein kinase (ERK)1/2 pathways (61). Following treatment of AD rats with
safranal, the hippocampal levels of MDA, ROS and protein carbonyl
were observed to be reduced, while the activity of SOD and
myeloperoxidase was increased in the hippocampal tissue (62).
Abnormal Tau phosphorylation is also one of the
leading causes of AD. In the brain of patients with AD, abnormally
phosphorylated tau protein has been observed, which, unlike normal
tau protein, does not bind to microtubule proteins; instead, its
presence also prevents the latter from promoting the assembly of
tubulin into microtubules, leading to neurofibrillary tangles
(63). Therefore, decreasing Tau
hyperphosphorylation is an effective way to treat AD. Trans-CR 4
and trans-crocetin were selected to treat two AD neuronal cell
culture models and the results demonstrated that these two
compounds did not affect the viability of neuron-like cells. Both
trans-CR 4 and trans-crocetin exerted a crucial effect to inhibit
amyloidogenic pathways and were influential in suppressing the
active forms of ERK1/2 kinases and glycogen synthase kinase-3β, as
well as markedly reducing Tau phosphorylation (64). Furthermore, CR was able to
significantly decrease MDA, Aβ and phosphorylated Tau levels by
modulating the MAPK signaling pathway (65). AD is also characterized by abnormal
Tau aggregation, whereas CR is able to inhibit Tau aggregation and
suppress the formation of Tau protein filaments (66). Fig.
2 illustrates the relationship between saffron components, Aβ
aggregation and Tau abnormal phosphorylation in AD.
In addition to AD, saffron also improves PD symptoms
through its antioxidant effects. CR has a protective effect in
terms of reducing mitochondrial permeability transition
pore-induced dopaminergic neuron damage and PD complications, in
addition to ameliorating
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD
complications and reducing substantia nigra cell death (67). It was reported that the antioxidant
capacity of saffron contributes to PD treatment. After validating
the neuroprotective efficacy of methanolic saffron extract and its
active constituent, CR, in a drosophila model of Parkinson's
disease, studies have proposed that saffron may be used as a
complementary therapeutic agent for PD-mediated NDs (68,69).
While common NDs, such as AD and PD, have been found to cause
oxidative damage in neuronal tissues, saffron and its active
compounds may reduce oxidative stress by inhibiting Aβ aggregation,
tau protein phosphorylation or ROS production, proving that saffron
has considerable therapeutic potential for NDs. Table I presents the relevant research
progress regarding the use of saffron to treat NDs through its
antioxidant effects.
OS has an essential role in the pathological changes
of NDs, whereas neuroinflammation is also crucial for ND
pathogenesis (70).
Neuroinflammation is a defense mechanism that protects the central
nervous system (CNS) from tissue damage or viral attack (71). However, a continuous inflammatory
process in the CNS may inflict severe damage to the nervous system
and eventually lead to CNS damage (72). Inflammation-derived ND is a
specific CNS damage disease. The primary hallmark of inflammation
in the brain is the activation of glial cells, particularly
microglia, and ROS is involved in microglial activation (73). Microglia cells influence the NF-κB
signaling pathway. Tumor necrosis factor-α (TNFα) and interleukin
(IL)-1β are common cytokines secreted by activated glial cells
(74).
Studies have demonstrated the therapeutic potential
of saffron and its active constituents for
neuroinflammation-mediated NDs. Saffron extracts were able to
upregulate the synaptic proteins in the brains of 5XFAD mice,
transgenic mice transfected with five Familial Alzheimer's disease
mutations, and reduce Aβ pathology-related neuroinflammation
(48). In AD mice, safranal not
only reduced the expression of NF-κB and its downstream TNFα, IL-6,
apoptosis markers and glial fibrillary acidic protein, but also
elevated the mitochondrial membrane potential (∆ψm) (62). Safranal exerts anti-inflammatory
effects by inhibiting the classic NF-κB inflammatory pathway,
thereby improving AD (75).
Safranal also reduces the hyperactivity of acetylcholinesterase
(AChE) and inhibits cholinesterase overexpression. Furthermore,
crocetin may suppress NF-κB activation and P53 expression in the
hippocampus, significantly decreasing the pro-inflammatory cytokine
secretion and increasing anti-inflammatory cytokine levels in
plasma, while inhibiting apoptosis and decreasing Aβ in various
brain areas (76).
Acute NDs, such as CI, are also affected by
neuroinflammation, a crucial pathological process in the later
stage of CI (77). It has been
indicated that inflammation mediates CI-reperfusion injury.
Ischemic stroke may cause depression, which is a severe disease
inflicted by CI, and post-stroke depression (PSD) is a severe
complication of stroke (78). It
has been demonstrated that persistent CI leads to PSD (79). Thus, PSD is closely associated with
inflammation. CR was able to inhibit the inflammatory response by
inhibiting the activation of the Toll-like receptor 4/myeloid
differentiation factor 88/NF-κB signaling pathway in the
hippocampal tissue, thereby preventing the occurrence of PSD
(80). Table II displays the relevant research
progress regarding the use of saffron for treating NDs through its
anti-inflammatory effects. Fig. 3
presents the anti-inflammatory mechanisms of saffron components in
ND treatment.
Mitochondria are organelles with a double membrane
structure found in the cytoplasm of eukaryotes containing
extranuclear genetic material. Their internal membranes are the
aggregation sites of respiratory chain complexes. Mitochondria, the
main site of the body's energy metabolism, regulated the oxidative
phosphorylation process and synthesize ATP, which may also be
produced via glycolysis in microglial cells and astrocytes
(81). Mitochondria generate
energy and control the storage and release of Ca2+ to
maintain the dynamic balance of the intracellular Ca2+
concentration. Furthermore, Ca2+ may participate in
multiple cell activities, such as cell-matrix metabolism, cell
apoptosis and initiation of signal transduction pathways (82). Mitochondrial dysfunction may
prevent the aforementioned functions and is a major risk factor for
neurodegeneration (83).
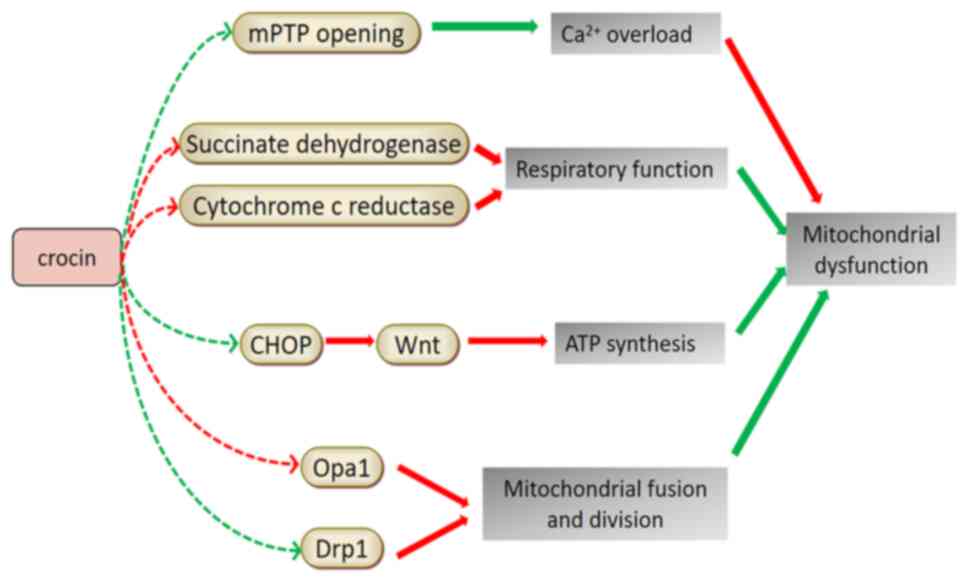
PD was the first ND associated with mitochondrial
dysfunction. In the compact part of the substantia nigra, the
nigrostriatal dopaminergic system and platelets of patients with
PD, a 30% reduction in the activity of the mitochondrial
respiratory chain complex I and a decrease in rate-limiting enzymes
of the tricarboxylic acid cycle-ketoglutarate dehydrogenase complex
were observed (84). In the
rotenone-induced PD model in Drosophila, the level of
mitochondrial enzyme activity in the Drosophila head was
significantly reduced. Following treatment with saffron extract and
CR, mitochondrial enzymes, succinate dehydrogenase and cytochrome
c reductase returned to normal levels, indicating that
saffron and its active constituents may improve mitochondrial
dysfunction (68). Experiments
using specific targeted small interfering RNA to knock down the
expression of the C/EBP homologous protein (CHOP) revealed that
CR-induced protection and inhibition of ER stress is mediated by
inverting the 1-methyl-4-phenylpyridinium (ion)-induced decrease of
Wnt protein through the CHOP pathway, thereby reducing cell damage
and apoptosis, inhibiting mitochondrial dysfunction and maintaining
ATP synthesis and ∆ψm (85).
Transient CI is responsible for sudden, temporary
and reversible neurological dysfunction. It has been demonstrated
that mitochondrial dysfunction may occur after CI reperfusion
(86). After pre-treatment of BI
rats with CR, it was observed that CR increased the mitochondrial
membrane fluidity, membrane phospholipid content, ∆ψm,
mitochondrial respiratory function, respiratory enzyme activity and
ATP content. CR also reduced MPTP opening and the free
Ca2+ concentration and protected the hippocampal
mitochondrial structure and function in rats with ischemic BI by
significantly ameliorating the hippocampal mitochondrial pathology
(87). In addition, CR can improve
the energy metabolism of cells after oxygen-glucose deprivation,
restore ∆ψm, reduce the intracellular Ca2+
concentration, upregulate optic atrophy 1 (Opa1) expression,
downregulate dynamin-related protein 1 (Drp1) expression and
restore the normal mitochondrial fusion and fission (88). Given the effects of the active
constituents of saffron on improving mitochondrial dysfunction in
cells, saffron extract was used to treat rats with focal brain
ischemia/reperfusion injury. The results suggested that saffron
extract significantly inhibited rat neuronal necrosis and astrocyte
proliferation, upregulated the expression of Opa1, downregulated
the expression of Drp1 and restored normal mitochondrial fusion and
fission (89). Table III presents the relevant research
progress of studies using saffron in treating NDs by improving
mitochondrial dysfunction. Fig. 4
illustrates the mechanisms of saffron components in improving
mitochondrial dysfunction for ND treatment.
Although the pathophysiological mechanisms remain to
be fully elucidated, patients with AD frequently exhibit symptoms
of reduced cognitive and memory functions, indicating that AD is
closely related to memory impairment. Aβ deposition, synaptic loss,
Tau phosphorylation and cholinergic system disorders are all
possible factors responsible for neuronal damage (3,90).
It has been reported that CR may increase the expression of
brain-derived neurotrophic factor (BDNF) and tropomyosin receptor
kinase B (TrkB) in the prefrontal cortex, thereby activating the
BDNF-TrkB signaling pathway and increasing the expression of the
memory-related protein postsynaptic density-95, which improves the
learning and memory ability in AD rats (91). A bilateral frontal-cortex Aβ
injection trial in rats demonstrated that CR significantly reduced
the number of TUNEL-positive cells in the cortical area 1 and
decreased c-Fos expression in the hippocampus, thereby alleviating
memory impairment due to Aβ deposition (92). Furthermore, electric shock
experiments conducted in mice demonstrated that saffron extract
prevented and improved the memory impairment of morphine-treated
mice (93). Similarly,
pentylenetetrazol-induced learning and memory deficits in rats were
significantly alleviated by CR (94), although the mechanism of action
requires further investigation.
Important neurotransmitters in the CNS, including
dopamine, norepinephrine, acetylcholine and serotonin, act on the
corresponding neurons and participate in short- and long-term
memory (95).
Acetylcholine-decomposing inhibitors of AChE are considered the
primary treatment for AD because of their ability to improve
cognitive impairment and the learning disabilities of AD. Studies
have reported that the loss of cholinergic neurons in PD is higher
than that in AD (96,97). Certain cholinergic fibers come from
basal forebrain cholinergic neurons. Patients with non-dementia PD
lose 30% of those neurons, whereas patients with PD dementia lose
54-70% (98). Saffron was found to
be a source of novel AChE inhibitors for treating AD using in
vitro enzymology and molecular docking methods (99). Furthermore, saffron extract and CR
may act on muscarinic choline receptors to improve learning and
memory ability (100,101). The accumulation and aggregation
of lead (Pb) in the food chain may poison the nervous system.
Experiments have demonstrated that Pb exposure may cause PD,
resulting in memory and cognitive impairment, symptoms similar to
dementia in AD (102,103). Saffron extract was able to
improve Pb-induced dopamine and noradrenergic injuries by restoring
tyrosine hydroxylase levels within the substantia nigra compacta,
ventral tegmental area, locus coeruleus, dorsal striatum and medial
forebrain bundle (104,105). Patients with MS usually suffer
from cognitive impairment (106),
with memory impairment and spatial perception disorders being the
most common cognitive deficits (107,108). Saffron extract has a positive
effect in improving learning and memory impairment and alleviate
impaired hippocampal stress parameters in rats with ethidium
bromide-induced MS (109).
Table IV presents the relevant
research progress on the use of saffron for treating NDs by
improving cognition and learning ability. Fig. 5 illustrates the mechanisms of
saffron components to improve cognition and learning ability for ND
treatment.
Depression is one of the psychological symptoms of
NDs. Studies have demonstrated that patients with NDs, including AD
and PD, exhibit depressive symptoms. Furthermore, elevated levels
of pro-inflammatory factors, such as IL-1β, IL-6 and TNFα, are
frequently found in the cerebrospinal fluid of patients with
depression, indicating the relationship between depression and the
occurrence of neuroinflammation (110-115).
The antidepressant mechanism of saffron has only been studied in
the last decades. CR had a significant antidepressant effect in a
chronic corticosterone-induced depression model in rats, as
evidenced by a substantial reduction in IL-1β and SOD levels in the
hippocampus, suggesting that the inhibition of inflammation and OS
is associated with the antidepressant effect (116). Patients with depression usually
have higher plasma corticosterone than normal individuals, it has
been demonstrated that saffron water extract and CR were able to
reduce the plasma levels of corticosterone in a rat model of
depression (117,118). Increasing the transcriptional
level of BDNF in the hippocampus may also have an antidepressant
effect (119,120), demonstrating that saffron may
alleviate the depressive symptoms of NDs. Therefore, saffron may
not only relieve the main symptoms of NDs, such as nervous
disorders and limb and cognitive dysfunction, but also have an
antidepressant effect treating its potential complications, which
is an advantage that other drugs do not possess.
Epilepsy is a chronic disease characterized by
sudden abnormal discharges of nerve cells in the brain, leading to
temporary brain dysfunction. NDs are characterized by cell death
and destruction of brain structures, which may increase the risk of
epileptic seizures (121). The
appearance and increase in the levels of inflammatory cytokines,
such as TNF, IL-1 and IL-6, is closely linked to the onset of
epilepsy (122). CR and crocetin,
the active constituents of saffron, inhibit the increase in the
inflammatory cytokines to varying degrees (75). This finding suggests that the
anti-epileptic action of saffron may be mediated by its
anti-inflammatory properties.
Saffron has always been widely used for food
coloring and flavoring. There has been a growing interest in using
special diets with saffron, and scientists have been paying
increasing attention to its safety while maintaining its taste
(15-17).
Furthermore, researchers have widely explored its nutritional
quality and medicinal effect. Numerous phytochemicals in Crocus
sativus have been proven to be the bioactive, therapeutic
constituents of saffron. Saffron has been used in the clinical
setting to treat cardiovascular and cerebrovascular diseases,
mental disorders and abnormal blood lipid and glucose levels
(128,129). Researchers have recently paid
considerable attention to treating NDs, focusing mainly on diseases
such as AD, PD, MS and CI (130).
CR and crocetin, the active constituents of saffron with
antioxidant effects, inhibit free radical formation and excitotoxic
damage, thereby protecting neurons. These constituents may also
reduce Aβ deposition, inhibit the abnormal aggregation of Tau
protein, reduce the secretion of inflammatory factors, improve
cognition and memory, improve mitochondrial dysfunction and
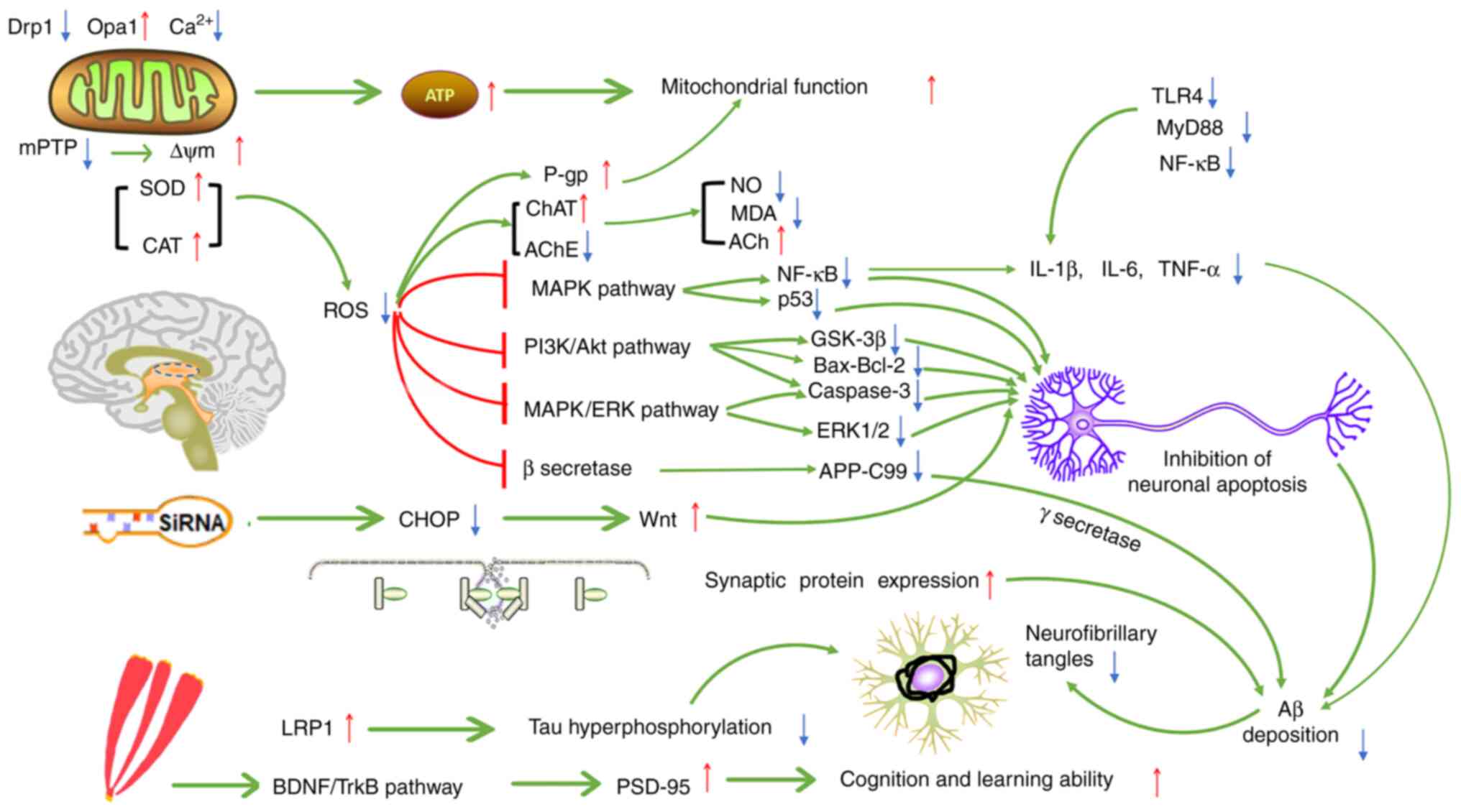
regulate the homeostasis of metal ions in ND models. Furthermore,
the pathways of the anti-inflammatory and antioxidant effects of
saffron and its capacity to improve mitochondrial function and
cognitive impairment are not independent; the interaction with one
another may affect ND pathogenesis, thereby protecting nerve cells
and preventing the further development of NDs. Fig. 6 illustrates the possible mechanisms
of saffron for ND treatment.
The therapeutic potential of saffron has been proved
in ND models. Its action on classic signaling pathways, such as
NF-κB and MAPK, and its antioxidant and anti-inflammatory
mechanisms, are also well known (65,76).
Several beneficial pharmacological effects of saffron or its
constituents have been confirmed in animal studies, but these
effects have rarely been demonstrated in clinical trials. The
results of a clinical trial suggested that saffron had similar
effects to improve cognitive function of patients with AD as
donepezil, while AChE levels were similar to those in the
donepezil-treated group, indicating that saffron may inhibit the
accumulation of senile plaques through its antioxidant effects,
which is consistent with the results of a preclinical
pharmacological study (131).
Furthermore, the therapeutic effect of saffron on cerebral ischemia
is similar to that of statins. Another clinical trial involving
patients with AD demonstrated that oral saffron extract increased
serum BDNF levels, inhibited inflammation and prevented neuronal
apoptosis (132). Saffron has
been clinically available to treat moderate AD, and antioxidant,
anti-inflammatory and other mechanisms of its active compounds have
been observed (133,134); it is thought that saffron has
excellent potential for future application in treating NDs.
Furthermore, saffron has only been used as a
traditional Chinese medicine to treat ND with limited efficacy. Its
use in treating NDs is still experimental and further clinical
studies are still required. The specific safety and effective dose
for the human body are unknown and numerous factors are involved in
determining its clinical efficacy. Accordingly, the following may
be suggested: i) The use of saffron in the treatment of AD and CI
may be investigated in subsequent clinical trials or drug
developmental stages, whereas the pharmacological effect for
treating other NDs requires further assessment in clinical trials;
ii) saffron may be used to prevent the side effects (including
anxiety, among others) of commonly used ND drugs, such as levodopa
and memantine; and iii) clinical trials and safety evaluation
should be conducted to assess the clinical value and effective dose
of saffron and to elucidate the dose-response and dose-toxicity
relationships of the active constituents of saffron.
Not applicable.
Funding: The preparation of the article was supported by the
National Interdisciplinary Innovation Team of Traditional Chinese
Medicine (grant no. ZYYCXTD-D-202209) and Xinglin Talent Program of
Chengdu University of Traditional Chinese Medicine (grant no.
BSH2020019).
Not applicable.
WY and XQ performed the literature search and wrote
the first draft of the manuscript. QW, TZ, MZ and JP obtained
funding, designed and conceived the study, supervised the
preparation of the article and revised the manuscript. FC
contributed to translation and data collection as part of the
manuscript preparation. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
China Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. 2020 edition, Part
1. Chinese medicines and Technology Press, Beijing, 2020.
|
|
2
|
Dai RC, Nabil WNN and Xu HX: The history
of saffron in China: From its origin to applications. Chin Med
Cult. 4:228–234. 2021.
|
|
3
|
Huang WJ and Long CL: The medicinal
history and recent study of Crocus sativus. J Minzu Univ
China (Natural Sciences Edition). 24:55–58. 2015.
|
|
4
|
Samarghandian S and Borji A:
Anticarcinogenic effect of saffron (Crocus sativus L.) and
its ingredients. Pharmacognosy Res. 6:99–107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Valle García-Rodríguez M, Serrano-Díaz J,
Tarantilis PA, López-Córcoles H, Carmona M and Alonso GL:
Determination of saffron quality by high-performance liquid
chromatography. J Agric Food Chem. 62:8068–8074. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hatziagapiou K and Lambrou GI: The
protective role of Crocus sativus L. (saffron) against
ischemia-reperfusion injury, hyperlipidemia and atherosclerosis:
Nature opposing cardiovascular diseases. Curr Cardiol Rev.
14:272–289. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Thushara RM, Hemshekhar M, Santhosh MS,
Jnaneshwari S, Nayaka SC, Naveen S, Kemparaju K and Girish KS:
Crocin, a dietary additive protects platelets from oxidative
stress-induced apoptosis and inhibits platelet aggregation. Mol
Cell Biochem. 373:73–83. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rahmani J, Manzari N, Thompson J, Clark
CCT, Villanueva G, Varkaneh HK and Mirmiran P: The effect of
saffron on weight and lipid profile: A systematic review,
meta-analysis, and dose-response of randomized clinical trials.
Phytother Res. 33:2244–2255. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Zhang A, Shen Y, Cen M, Hong X, Shao QS,
Chen Y and Zheng B: Polysaccharide and crocin contents, and
antioxidant activity of saffron from different origins. Ind Crop
Prod. 133:111–117. 2019.
|
|
10
|
Xue Y, Jin W, Xue Y, Zhang Y, Wang H,
Zhang Y, Guan S, Chu X and Zhang J: Safranal, an active constituent
of saffron, ameliorates myocardial ischemia via reduction of
oxidative stress and regulation of Ca2+ homeostasis. J
Pharmacol Sci. 143:156–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Poma A, Fontecchio G, Carlucci G and
Chichiriccò G: Anti-inflammatory properties of drugs from saffron
crocus. Antiinflamm Antiallergy Agents Med Chem. 11:37–51.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Singh G, Haileselassie Y, Ji AR, Maecker
HT, Sinha SR, Brim H, Habtezion A and Ashktorab H: Protective
effect of saffron in mouse colitis models through immune
modulation. Digest Dis Sci. 67:2922–2935. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lambrianidou A, Koutsougianni F,
Papapostolou I and Dimas K: Recent advances on the anticancer
properties of saffron (Crocus sativus L.) and its major
constituents. Molecules. 26(86)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Siddiqui SA, Ali Redha A, Snoeck ER, Singh
S, Simal-Gandara J, Ibrahim SA and Jafari SM: Anti-depressant
properties of crocin molecules in saffron. Molecules.
27(2076)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
José Bagur M, Alonso Salinas GL,
Jiménez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M and
Alonso GL: Saffron: An old medicinal plant and a potential novel
functional food. Molecules. 23(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Howes MR, Perry NSL, Vásquez-Londoño C and
Perry EK: Role of phytochemicals as nutraceuticals for cognitive
functions affected in ageing. Br J Pharmacol. 177:1294–1315.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bathaie SZ: Saffron as a functional food
and a nutraceutical using saffron and its constituents as the
nutraceutics to protect against chronic diseases. Acta Hortic.
1200:201–204. 2018.
|
|
18
|
Hardy J: Pathways to primary
neurodegenerative disease. Ann N Y Acad Sci. 924:29–34.
2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Höglund K and Salter H: Molecular
biomarkers of neurodegeneration. Expert Rev Mol Diagn. 13:845–861.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sheikh S, Safia Haque E and Mir SS:
Neurodegenerative diseases: Multifactorial conformational diseases
and their therapeutic interventions. J Neurodegener Dis.
2013(563481)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Di Stefano A, Sozio P and Cerasa LS:
Antiparkinson prodrugs. Molecules. 13:46–68. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Klietz M, Greten S, Wegner F and Höglinger
GU: Safety and tolerability of pharmacotherapies for Parkinson's
disease in geriatric patients. Drugs Aging. 36:511–530.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hosseinzadeh H and Sadeghnia HR: Safranal,
a constituent of Crocus sativus (saffron), attenuated
cerebral ischemia induced oxidative damage in rat hippocampus. J
Pharm Pharm Sci. 8:394–399. 2005.PubMed/NCBI
|
|
24
|
Salem M, Shaheen M, Tabbara A and Borjac
J: Saffron extract and crocin exert anti-inflammatory and
anti-oxidative effects in a repetitive mild traumatic brain injury
mouse model. Sci Rep. 12(5004)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saeedi M and Rashidy-Pour A: Association
between chronic stress and Alzheimer's disease: Therapeutic effects
of Saffron. Biomed Pharmacother. 133(110995)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gregory J, Vengalasetti YV, Bredesen DE
and Rao RV: Neuroprotective herbs for the management of Alzheimer's
disease. Biomolecules. 11(543)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hatziagapiou K, Kakouri E, Lambrou GI,
Bethanis K and Tarantilis PA: Antioxidant properties of Crocus
Sativus L. and its constituents and relevance to
neurodegenerative diseases; Focus on Alzheimer's and Parkinson's
disease. Curr Neuropharmacol. 17:377–402. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ghasemi Sakha F, Azimi Saeen A, Moazzeni
SM, Etesam F and Vaezi G: A randomized, triple-blind
placebo-controlled trial to determine the effect of saffron on the
serum levels of MMP-9 and TIMP-1 in patients with multiple
sclerosis. Iran J Allergy Asthma Immunol. 19:297–304.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yuan Y, Shan X, Men W, Zhai H, Qiao X,
Geng L and Li C: The effect of crocin on memory, hippocampal
acetylcholine level, and apoptosis in a rat model of cerebral
ischemia. Biomed Pharmacother. 130(110543)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jahromi GP, Khodadadi H, Fasihi-Ramandi M,
Esmaeili M and Shahriary A: Neuroprotective and antiapoptotic
effects of N-acetylcystein and Crocus sativus aqueous
extract on arsenic-induced neurotoxicity in SH-SY5Y human
dopaminergic neuroblastoma cells. Indian J Pharm Educ Res.
53:695–702. 2019.
|
|
31
|
Razavi BM and Hosseinzadeh H: Saffron as
an antidote or a protective agent against natural or chemical
toxicities. Daru. 23(31)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chuquilín-Arista F, Álvarez-Avellón T and
Menéndez-González M: Prevalence of depression and anxiety in
Parkinson disease and impact on quality of life: A community-based
study in Spain. J Geriatr Psychiatry Neurol. 33:207–213.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farkhondeh T, Samarghandian S, Shaterzadeh
Yazdi H and Samini F: The protective effects of crocin in the
management of neurodegenerative diseases: A review. Am J
Neurodegener Dis. 7:1–10. 2018.PubMed/NCBI
|
|
34
|
Bian Y, Zhao C and Lee SMY:
Neuroprotective potency of saffron against neuropsychiatric
diseases, neurodegenerative diseases, and other brain disorders:
From bench to bedside. Front Pharmacol. 11(579052)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Halliwell B: Oxidative stress and
neurodegeneration: Where are we now? J Neurochem. 97:1634–1658.
2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Praticò D: Oxidative stress hypothesis in
Alzheimer's disease: A reappraisal. Trends Pharmacol Sci.
29:609–615. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Reynolds A, Laurie C, Mosley RL and
Gendelman HE: Oxidative stress and the pathogenesis of
neurodegenerative disorders. Int Rev Neurobiol. 82:297–325.
2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wojsiat J, Zoltowska KM, Laskowska-Kaszub
K and Wojda U: Oxidant/antioxidant imbalance in Alzheimer's
disease: Therapeutic and diagnostic prospects. Oxid Med Cell
Longev. 2018(6435861)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Danielson SR and Andersen JK: Oxidative
and nitrative protein modifications in Parkinson's disease. Free
Radical Bio Med. 44:1787–1794. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yamamoto A, Shin RW, Hasegawa K, Naiki H,
Sato H, Yoshimasu F and Kitamoto T: Iron (III) induces aggregation
of hyperphosphorylated tau and its reduction to iron (II) reverses
the aggregation: Implications in the formation of neurofibrillary
tangles of Alzheimer's disease. J Neurochem. 82:1137–1147.
2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Su B, Wang X, Lee HG, Tabaton M, Perry G,
Smith MA and Zhu X: Chronic oxidative stress causes increased tau
phosphorylation in M17 neuroblastoma cells. Neurosci Lett.
468:267–271. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kumar V and Gill KD: Oxidative stress and
mitochondrial dysfunction in aluminium neurotoxicity and its
amelioration: A review. Neurotoxicology. 41:154–166.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lim J and Yue Z: Neuronal aggregates:
Formation, clearance, and spreading. Dev Cell. 32:491–501.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sabayan B, Namazi MR, Mowla A and Moniri
SA: Are patients with Darier and Haily-Haily diseases susceptible
to Alzheimer's disease? A theory based on abnormal intraneuronal
Ca2+ homeostasis. J Alzheimers Dis. 16:521–523. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Singh N, Haldar S, Tripathi AK, McElwee
MK, Horback K and Beserra A: Iron in neurodegenerative disorders of
protein misfolding: A case of prion disorders and Parkinson's
disease. Antioxid Redox Signal. 21:471–484. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hare DJ and Double KL: Iron and dopamine:
A toxic couple. Brain. 139:1026–1035. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Batarseh YS, Bharate SS, Kumar V, Kumar A,
Vishwakarma RA, Bharate SB and Kaddoumi A: Crocus sativus
extract tightens the blood-brain barrier, reduces Amyloid β load
and related toxicity in 5XFAD mice. ACS Chem Neurosci. 8:1756–1766.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Papandreou MA, Kanakis CD, Polissiou MG,
Efthimiopoulos S, Cordopatis P, Margarity M and Lamari FN:
Inhibitory activity on amyloid-beta aggregation and antioxidant
properties of Crocus sativus stigmas extract and its crocin
constituents. J Agric Food Chem. 54:8762–8768. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shati AA, Elsaid FG and Hafez EE:
Biochemical and molecular aspects of aluminium chloride-induced
neurotoxicity in mice and the protective role of Crocus
sativus L. extraction and honey syrup. Neuroscience. 175:66–74.
2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Reeta KH, Singh D and Gupta YK: Chronic
treatment with taurine after intracerebroventricular streptozotocin
injection improves cognitive dysfunction in rats by modulating
oxidative stress, cholinergic functions and neuroinflammation.
Neurochem Int. 108:146–156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bharate SS, Kumar V, Singh G, Singh A,
Gupta M, Singh D, Kumar A, Vishwakarma RA and Bharate SB:
Preclinical development of Crocus sativus-based botanical
lead IIIM-141 for Alzheimer's disease: Chemical standardization,
efficacy, formulation development, pharmacokinetics, and safety
pharmacology. ACS Omega. 3:9572–9585. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kapucu A: Crocin ameliorates oxidative
stress and suppresses renal damage in streptozotocin induced
diabetic male rats. Biotech Histochem. 96:153–160. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Naghizadeh B, Mansouri MT, Ghorbanzadeh B,
Farbood Y and Sarkaki A: Protective effects of oral crocin against
intracerebroventricular streptozotocin-induced spatial memory
deficit and oxidative stress in rats. Phytomedicine. 20:537–542.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Asadi F, Jamshidi AH, Khodagholi F, Yans
A, Azimi L, Faizi M, Vali L, Abdollahi M and Ghahremani MH:
Reversal effects of crocin on amyloid β-induced memory deficit:
Modification of autophagy or apoptosis markers. Pharmacol Biochem
Behav. 139:47–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang C, Cai X, Hu W, Li Z, Kong F, Chen X
and Wang D: Investigation of the neuroprotective effects of crocin
via antioxidant activities in HT22 cells and in mice with
Alzheimer's disease. Int J Mol Med. 43:956–966. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Osaki LH and Gama P: MAPKs and signal
transduction in the control of gastrointestinal epithelial cell
proliferation and differentiation. Int J Mol Sci. 14:10143–10161.
2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shehzad A and Lee YS: Molecular mechanisms
of curcumin action: Signal transduction. Biofactors. 39:27–36.
2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Vriz S, Reiter S and Galliot B: Cell
death: A program to regenerate. Curr Top Dev Biol. 108:121–151.
2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rafieipour F, Hadipour E, Emami SA, Asili
J and Tayarani-Najaran Z: Safranal protects against beta-amyloid
peptide-induced cell toxicity in PC12 cells via MAPK and PI3K
pathways. Metab Brain Dis. 34:165–172. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Baluchnejadmojarad T, Mohamadi-Zarch SM
and Roghani M: Safranal, an active ingredient of saffron,
attenuates cognitive deficits in amyloid β-induced rat model of
Alzheimer's disease: Underlying mechanisms. Metab Brain Dis.
34:1747–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Alonso AC, Zaidi T, Grundke-Iqbal I and
Iqbal K: Role of abnormally phosphorylated tau in the breakdown of
microtubules in Alzheimer disease. Proc Natl Acad Sci USA.
91:5562–5566. 1994.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chalatsa I, Arvanitis DA, Koulakiotis NS,
Giagini A, Skaltsounis AL, Papadopoulou-Daifoti Z, Tsarbopoulos A
and Sanoudou D: The Crocus sativus compounds trans-crocin 4
and trans-crocetin modulate the amyloidogenic pathway and tau
misprocessing in Alzheimer disease neuronal cell culture models.
Front Neurosci. 13(249)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Rashedinia M, Lari P, Abnous K and
Hosseinzadeh H: Protective effect of crocin on acrolein-induced tau
phosphorylation in the rat brain. Acta Neurobiol Exp (Wars).
75:208–219. 2015.PubMed/NCBI
|
|
66
|
Koulakiotis NS, Purhonen P, Gikas E,
Hebert H and Tsarbopoulos A: Crocus-derived compounds alter the
aggregation pathway of Alzheimer's disease: Associated beta amyloid
protein. Sci Rep. 10(18150)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Haeri P, Mohammadipour A, Heidari Z and
Ebrahimzadeh-Bideskan A: Neuroprotective effect of crocin on
substantia nigra in MPTP-induced Parkinson's disease model of mice.
Anat Sci Int. 94:119–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rao SV, Muralidhara Yenisetti SC and
Rajini PS: Evidence of neuroprotective effects of saffron and
crocin in a Drosophila model of parkinsonism. Neurotoxicology.
52:230–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Finley JW and Gao S: A Perspective on
Crocus sativus L. (saffron) constituent crocin: A potent
water-soluble antioxidant and potential therapy for Alzheimer's
disease. J Agric Food Chem. 65:1005–1020. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chen WW, Zhang X and Huang WJ: Role of
neuroinflammation in neurodegenerative diseases (Review). Mol Med
Rep. 13:3391–3396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Spencer JP, Vafeiadou K, Williams RJ and
Vauzour D: Neuroinflammation: Modulation by flavonoids and
mechanisms of action. Mol Aspects Med. 33:83–97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
McManus RM and Heneka MT: Role of
neuroinflammation in neurodegeneration: New insights. Alzheimers
Res Ther. 9(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Simpson DSA and Oliver PL: ROS Generation
in microglia: Understanding oxidative stress and inflammation in
neurodegenerative disease. Antioxidants (Basel).
9(743)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Spagnuolo C, Moccia S and Russo GL:
Anti-inflammatory effects of flavonoids in neurodegenerative
disorders. Eur J Med Chem. 153:105–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Korani S, Korani M, Sathyapalan T and
Sahebkar A: Therapeutic effects of crocin in autoimmune diseases: A
review. Biofactors. 45:835–843. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang J, Wang Y, Dong X and Liu J:
Crocetin attenuates inflammation and amyloid-β accumulation in
APPsw transgenic mice. Immun Ageing. 15(24)2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Jurcau A and Simion A: Neuroinflammation
in cerebral ischemia and ischemia/reperfusion injuries: From
pathophysiology to therapeutic strategies. Int J Mol Sci.
23(14)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Das J and G K R: Post stroke depression:
The sequelae of cerebral stroke. Neurosci Biobehav Rev. 90:104–114.
2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wang SH, Zhang ZJ, Guo YJ, Sui YX and Sun
Y: Involvement of serotonin neurotransmission in hippocampal
neurogenesis and behavioral responses in a rat model of post-stroke
depression. Pharmacol Biochem Behav. 95:129–137. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Xu QQ, Qian XD, Sun F, Liu H, Dou ZJ, Gong
J and Zhang XR: Effects of crocin on inflammatory response and
TLR4/MyD88/NF-κB signaling pathway in post-stroke depression rats.
Chin J Immunol. 37:179–185. 2021.
|
|
81
|
Johannsen DL and Ravussin E: The role of
mitochondria in health and disease. Curr Opin Pharmacol. 9:780–786.
2009.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Hernández-Reséndiz S, Buelna-Chontal M,
Correa F and Zazueta C: Targeting mitochondria for cardiac
protection. Curr Drug Targets. 14:586–600. 2013.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Shults CW: Mitochondrial dysfunction and
possible treatments in Parkinson's disease-a review. Mitochondrion.
4:641–648. 2004.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zhang GF, Zhang Y and Zhao G: Crocin
protects PC12 cells against MPP(+)-induced injury through
inhibition of mitochondrial dysfunction and ER stress. Neurochem
Int. 89:101–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Vongsfak J, Pratchayasakul W, Apaijai N,
Vaniyapong T, Chattipakorn N and Chattipakorn SC: The alterations
in mitochondrial dynamics following cerebral ischemia/reperfusion
injury. Antioxidants (Basel). 10(1384)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wen B: Study on the protective effect of
crocin pretreatment on hippocampal mitochondria in the rats with
ischemic brain injury. Mod J Integr Tradit Chin West Med.
29(6)2020.
|
|
88
|
Zhang YH, Cong WH and Liu JX: Effect of
crocin on mitochondrial dynamics in SH-SY5Y cells against injury
induced by oxygen-glucose deprivation. Chin Pharmacol Bull.
32:986–990. 2016.
|
|
89
|
Zhang YH, Yao MJ, Cong WH and Liu JX:
Effect of extraction of saffron crocus on mitochondrial dynamics in
ischemia/reperfusion rats. Chin Pharmacol Bull. 34:770–775.
2018.
|
|
90
|
Reddy PH: Abnormal tau, mitochondrial
dysfunction, impaired axonal transport of mitochondria, and
synaptic deprivation in Alzheimer's disease. Brain Res.
1415:136–148. 2011.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lin L, Liu G and Yang L: Crocin improves
cognitive behavior in rats with Alzheimer's disease by regulating
endoplasmic reticulum stress and apoptosis. Biomed Res Int.
2019(9454913)2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Hadipour M, Kaka G, Bahrami F, Meftahi GH,
Pirzad Jahromi G, Mohammadi A and Sahraei H: Crocin improved
amyloid beta induced long-term potentiation and memory deficits in
the hippocampal CA1 neurons in freely moving rats. Synapse.
72(e22026)2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Naghibi SM, Hosseini M, Khani F, Rahimi M,
Vafaee F, Rakhshandeh H and Aghaie A: Effect of aqueous extract of
Crocus sativus L. on morphine-induced memory impairment. Adv
Pharmacol Sci. 2012(494367)2012.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Mazumder AG, Sharma P, Patial V and Singh
D: Crocin attenuates kindling development and associated cognitive
impairments in mice via inhibiting reactive oxygen species-mediated
NF-κB activation. Basic Clin Pharmacol Toxicol. 120:426–433.
2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Canal CE, Chang Q and Gold PE: Amnesia
produced by altered release of neurotransmitters after
intraamygdala injections of a protein synthesis inhibitor. Proc
Natl Acad Sci USA. 104:12500–12505. 2007.PubMed/NCBI View Article : Google Scholar
|
|
96
|
D'Amato RJ, Zweig RM, Whitehouse PJ, Wenk
GL, Singer HS, Mayeux R, Price DL and Snyder SH: Aminergic systems
in Alzheimer's disease and Parkinson's disease. Ann Neurol.
22:229–236. 1987.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Müller ML and Bohnen NI: Cholinergic
dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep.
13(377)2013.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hall H, Reyes S, Landeck N, Bye C, Leanza
G, Double K, Thompson L, Halliday G and Kirik D: Hippocampal Lewy
pathology and cholinergic dysfunction are associated with dementia
in Parkinson's disease. Brain. 137:2493–2508. 2014.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Geromichalos GD, Lamari FN, Papandreou MA,
Trafalis DT, Margarity M, Papageorgiou A and Sinakos Z: Saffron as
a source of novel acetylcholinesterase inhibitors: Molecular
docking and in vitro enzymatic studies. J Agric Food Chem.
60:6131–6138. 2012.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Pitsikas N and Sakellaridis N: Crocus
sativus L. extracts antagonize memory impairments in different
behavioural tasks in the rat. Behav Brain Res. 173:112–115.
2006.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Pitsikas N, Zisopoulou S, Tarantilis PA,
Kanakis CD, Polissiou MG and Sakellaridis N: Effects of the active
constituents of Crocus sativus L., crocins on recognition
and spatial rats' memory. Behav Brain Res. 183:141–146.
2007.PubMed/NCBI View Article : Google Scholar
|
|
102
|
White LD, Cory-Slechta DA, Gilbert ME,
Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A,
Lasley SM, Qian YC and Basha MR: New and evolving concepts in the
neurotoxicology of lead. Toxicol Appl Pharmacol. 225:1–27.
2007.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Bakulski KM, Seo YA, Hickman RC, Brandt D,
Vadari HS, Hu H and Park SK: Heavy metals exposure and Alzheimer's
disease and related dementias. J Alzheimers Dis. 76:1215–1242.
2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Butnariu M, Quispe C, Herrera-Bravo J,
Sharifi-Rad J, Singh L, Aborehab NM, Bouyahya A, Venditti A, Sen S,
Acharya K, et al: The pharmacological activities of Crocus
sativus L.: A review based on the mechanisms and therapeutic
opportunities of its phytoconstituents. Oxid Med Cell Longev.
2022(8214821)2022.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Tamegart L, Abbaoui A, Makbal R, Zroudi M,
Bouizgarne B, Bouyatas MM and Gamrani H: Crocus sativus
restores dopaminergic and noradrenergic damages induced by lead in
Meriones shawi: A possible link with Parkinson's disease. Acta
Histochem. 121:171–181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Sartori E and Edan G: Assessment of
cognitive dysfunction in multiple sclerosis. J Neurol Sci.
245:169–175. 2006.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Glanz BI, Healy BC, Hviid LE, Chitnis T
and Weiner HL: Cognitive deterioration in patients with early
multiple sclerosis: A 5-year study. J Neurol Neurosurg Psychiatry.
83:38–43. 2012.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Ziehn MO, Avedisian AA, Tiwari-Woodruff S
and Voskuhl RR: Hippocampal CA1 atrophy and synaptic loss during
experimental autoimmune encephalomyelitis, EAE. Lab Invest.
90:774–786. 2010.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Ghaffari S, Hatami H and Dehghan G:
Saffron ethanolic extract attenuates oxidative stress, spatial
learning, and memory impairments induced by local injection of
ethidium bromide. Res Pharm Sci. 10:222–232. 2015.PubMed/NCBI
|
|
110
|
Bonuccelli U, Meco G, Fabbrini G,
Tessitore A, Pierantozzi M, Stocchi F, Ceravolo R, Caltagirone C,
Silvestrini M, Morgante F, et al: A non-comparative assessment of
tolerability and efficacy of duloxetine in the treatment of
depressed patients with Parkinson's disease. Expert Opin
Pharmacother. 13:2269–2280. 2012.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Dobkin RD, Menza M, Bienfait KL, Gara M,
Marin H, Mark MH, Dicke A and Friedman J: Depression in Parkinson's
disease: Symptom improvement and residual symptoms after acute
pharmacologic management. Am J Geriatr Psychiatry. 19:222–229.
2011.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Lozupone M, La Montagna M, D'Urso F,
Piccininni C, Sardone R, Dibello V, Giannelli G, Solfrizzi V, Greco
A, Daniele A, et al: Pharmacotherapy for the treatment of
depression in patients with alzheimer's disease: A
treatment-resistant depressive disorder. Expert Opin Pharmacother.
19:823–842. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Novais F and Starkstein S: Phenomenology
of depression in Alzheimer's disease. J Alzheimers Dis. 47:845–855.
2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Pålhagen S, Qi H, Mårtensson B, Wålinder
J, Granérus AK and Svenningsson P: Monoamines, BDNF, IL-6 and
corticosterone in CSF in patients with Parkinson's disease and
major depression. J Neurol. 257:524–532. 2010.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Schrag A and Taddei RN: Depression and
anxiety in Parkinson's disease. Int Rev Neurobiol. 133:623–655.
2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Xiao Q, Xiong Z, Yu C, Zhou J, Shen Q,
Wang L, Xie X and Fu Z: Antidepressant activity of crocin-I is
associated with amelioration of neuroinflammation and attenuates
oxidative damage induced by corticosterone in mice. Physiol Behav.
212(112699)2019.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Ghadrdoost B, Vafaei AA, Rashidy-Pour A,
Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR and
Pahlvan S: Protective effects of saffron extract and its active
constituent crocin against oxidative stress and spatial learning
and memory deficits induced by chronic stress in rats. Eur J
Pharmacol. 667:222–229. 2011.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Halataei BAS, Khosravi M, Arbabian S,
Sahraei H, Golmanesh L, Zardooz H, Jalili C and Ghoshooni H:
Saffron (Crocus sativus) aqueous extract and its constituent
crocin reduces stress-induced anorexia in mice. Phytother Res.
25:1833–1838. 2011.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Ghasemi T, Abnous K, Vahdati F, Mehri S,
Razavi BM and Hosseinzadeh H: Antidepressant effect of Crocus
sativus aqueous extract and its effect on CREB, BDNF, and VGF
transcript and protein levels in rat hippocampus. Drug Res
(Stuttg). 65:337–343. 2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Vahdati Hassani F, Naseri V, Razavi BM,
Mehri S, Abnous K and Hosseinzadeh H: Antidepressant effects of
crocin and its effects on transcript and protein levels of CREB,
BDNF, and VGF in rat hippocampus. Daru. 22(16)2014.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Cano A, Fonseca E, Ettcheto M,
Sánchez-López E, de Rojas I, Alonso-Lana S, Morató X, Souto EB,
Toledo M, Boada M, et al: Epilepsy in neurodegenerative diseases:
Related drugs and molecular pathways. Pharmaceuticals (Basel).
14(1057)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Vezzani A, French J, Bartfai T and Baram
TZ: The role of inflammation in epilepsy. Nat Rev Neurol. 7:31–40.
2011.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Filippini A, D'Amore A and D'Alessio A:
Calcium mobilization in endothelial cell functions. Int J Mol Sci.
20(4525)2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Maklad A, Sharma A and Azimi I: Calcium
signaling in brain cancers: Roles and therapeutic targeting.
Cancers (Basel). 11(145)2019.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Schrank S, Barrington N and Stutzmann GE:
Calcium-handling defects and neurodegenerative disease. Cold Spring
Harb Perspect Biol. 12(a035212)2020.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Xing B, Li S, Yang J, Lin D, Feng Y, Lu J
and Shao Q: Phytochemistry, pharmacology, and potential clinical
applications of saffron: A review. J Ethnopharmacol.
281(114555)2021.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Zhao Z, Zheng B, Li J, Wei Z, Chu S, Han
X, Chu L, Wang H and Chu X: Influence of crocetin, a natural
carotenoid dicarboxylic acid in saffron, on L-type Ca2+
current, intracellular Ca2+ handling and contraction of
isolated rat cardiomyocytes. Biol Pharm Bull. 43:1367–1374.
2020.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Moshiri M, Vahabzadeh M and Hosseinzadeh
H: Clinical Applications of saffron (Crocus sativus) and its
constituents: A review. Drug Res (Stuttg). 65:287–295.
2015.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Omidkhoda SF and Hosseinzadeh H: Saffron
and its active ingredients against human disorders: A literature
review on existing clinical evidence. Iran J Basic Med Sci.
25:913–933. 2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Foster ER: Themes from the special issue
on neurodegenerative diseases: What have we learned, and where can
we go from here? Am J Occup Ther. 68:6–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Akhondzadeh S, Shafiee Sabet M, Harirchian
MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH,
Alimardani R, Jamshidi A, et al: A 22-week, multicenter,
randomized, double-blind controlled trial of Crocus sativus
in the treatment of mild-to-moderate Alzheimer's disease.
Psychopharmacology (Berl). 207:637–643. 2010.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Asadollahi M, Nikdokht P, Hatef B, Sadr
SS, Sahraei H, Assarzadegan F and Pirzad Jahromi G: Protective
properties of the aqueous extract of saffron (Crocus sativus
L.) in ischemic stroke, randomized clinical trial. J
Ethnopharmacol. 238(111833)2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Zandi N, Pazoki B, Momeni Roudsari N,
Lashgari NA, Jamshidi V, Momtaz S, Abdolghaffari AH and Akhondzadeh
S: Prospects of saffron and its derivatives in Alzheimer's disease.
Arch Iran Med. 24:233–252. 2021.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Marzabadi LR, Fazljou SMB, Araj-Khodaei M,
Sadigh-Eteghad S, Naseri A and Talebi M: Saffron reduces some
inflammation and oxidative stress markers in donepezil-treated
mild-to-moderate Alzheimer's disease patients: A randomized
double-blind placebo-control trial. J Herb Med. 34(100574)2022.
|