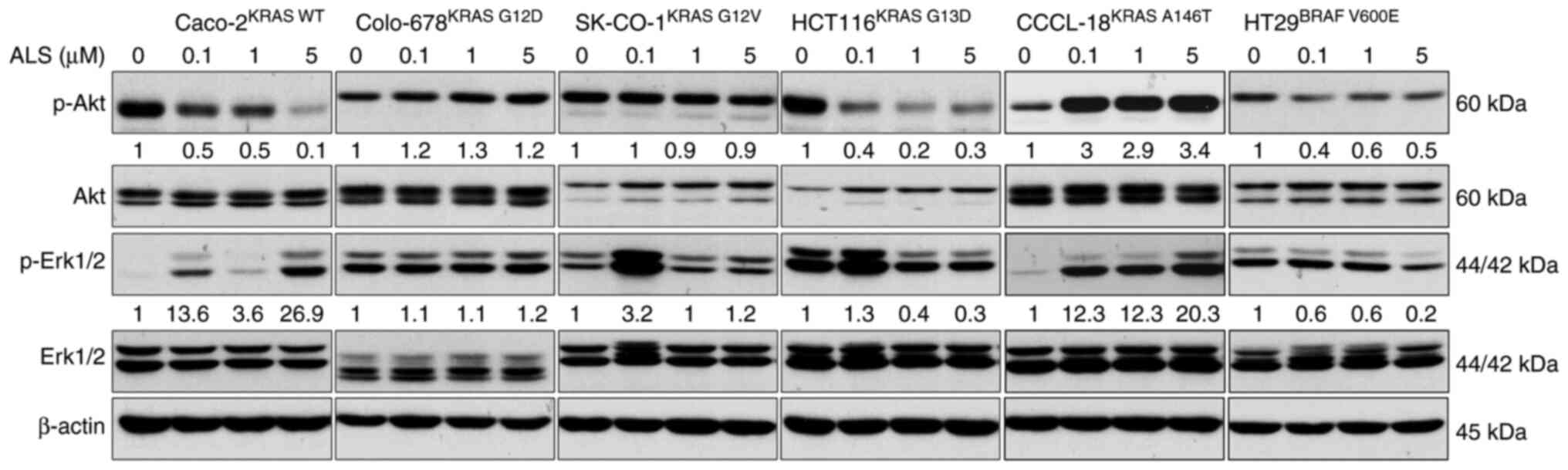
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Colorectal cancer burden in EU-27. Source:
ECIS-European Cancer Information System. https://ecis.jrc.ec.europa.eu, accessed
15/01/2021.
|
|
3
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cancer Stat Facts: Colorectal Cancer,
2022.
|
|
5
|
Global Cancer Observatory, World Health
Organization. https://gco.iarc.fr/.
|
|
6
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen H, Lu B and Dai M: Colorectal cancer
screening in China: Status, challenges, and prospects-China, 2022.
China CDC Wkly. 4:322–328. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chu E: An update on the current and
emerging targeted agents in metastatic colorectal cancer. Clin
Colorectal Cancer. 11:1–13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y,
Chen H and Dai M: Incidence, mortality, survival, risk factor and
screening of colorectal cancer: A comparison among China, Europe,
and northern America. Cancer Lett. 522:255–268. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lombardi L, Morelli F, Cinieri S, Santini
D, Silvestris N, Fazio N, Orlando L, Tonini G, Colucci G and
Maiello E: Adjuvant colon cancer chemotherapy: Where we are and
where we'll go. Cancer Treat Rev. 36 (Suppl 3):S34–S41.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu G, Pei L, Xia H, Tang Q and Bi F: Role
of oncogenic KRAS in the prognosis, diagnosis and treatment of
colorectal cancer. Mol Cancer. 20(143)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jackson EL, Willis N, Mercer K, Bronson
RT, Crowley D, Montoya R, Jacks T and Tuveson DA: Analysis of lung
tumor initiation and progression using conditional expression of
oncogenic K-ras. Genes Dev. 15:3243–3248. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pylayeva-Gupta Y, Grabocka E and Bar-Sagi
D: RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer.
11:761–774. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Graziano SL, Gamble GP, Newman NB, Abbott
LZ, Rooney M, Mookherjee S, Lamb ML, Kohman LJ and Poiesz BJ:
Prognostic significance of K-ras codon 12 mutations in patients
with resected stage I and II non-small-cell lung cancer. J Clin
Oncol. 17:668–675. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu
N, Sakurada A, Whitehead M, Zhu CQ, Livingston R, Johnson DH, et
al: Prognostic and predictive importance of p53 and RAS for
adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol.
25:5240–5247. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Andreyev HJ, Norman AR, Cunningham D,
Oates JR and Clarke PA: Kirsten ras mutations in patients with
colorectal cancer: The multicenter ‘RASCAL’ study. J Natl Cancer
Inst. 90:675–684. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simanshu DK, Nissley DV and McCormick F:
RAS proteins and their regulators in human disease. Cell.
170:17–33. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schubbert S, Shannon K and Bollag G:
Hyperactive Ras in developmental disorders and cancer. Nat Rev
Cancer. 7:295–308. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stephen AG, Esposito D, Bagni RK and
McCormick F: Dragging ras back in the ring. Cancer Cell.
25:272–281. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bolanos-Garcia VM: Aurora kinases. Int J
Biochem Cell Biol. 37:1572–1577. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gautschi O, Heighway J, Mack PC, Purnell
PR, Lara PN Jr and Gandara DR: Aurora kinases as anticancer drug
targets. Clin Cancer Res. 14:1639–1648. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dotan E, Meropol NJ, Zhu F, Zambito F,
Bove B, Cai KQ, Godwin AK, Golemis EA, Astsaturov I and Cohen SJ:
Relationship of increased aurora kinase A gene copy number,
prognosis and response to chemotherapy in patients with metastatic
colorectal cancer. Br J Cancer. 106:748–755. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Baba Y, Nosho K, Shima K, Irahara N, Kure
S, Toyoda S, Kirkner GJ, Goel A, Fuchs CS and Ogino S: Aurora-A
expression is independently associated with chromosomal instability
in colorectal cancer. Neoplasia. 11:418–425. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Du R, Huang C, Liu K, Li X and Dong Z:
Targeting AURKA in cancer: Molecular mechanisms and opportunities
for cancer therapy. Mol Cancer. 20(15)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Umstead M, Xiong J, Qi Q, Du Y and Fu H:
Aurora kinase A interacts with H-Ras and potentiates Ras-MAPK
signaling. Oncotarget. 8:28359–28372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dauch D, Rudalska R, Cossa G, Nault JC,
Kang TW, Wuestefeld T, Hohmeyer A, Imbeaud S, Yevsa T, Hoenicke L,
et al: A MYC-aurora kinase A protein complex represents an
actionable drug target in p53-altered liver cancer. Nat Med.
22:744–753. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xie Y, Zhu S, Zhong M, Yang M, Sun X, Liu
J, Kroemer G, Lotze M, Zeh HJ III, Kang R and Tang D: Inhibition of
Aurora kinase A induces necroptosis in pancreatic carcinoma.
Gastroenterology. 153:1429–1443.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang-Bishop L, Chen Z, Gomaa A, Lockhart
AC, Salaria S, Wang J, Lewis KB, Ecsedy J, Washington K, Beauchamp
RD and El-Rifai W: Inhibition of AURKA reduces proliferation and
survival of gastrointestinal cancer cells with activated KRAS by
preventing activation of RPS6KB1. Gastroenterology. 156:662–675.e7.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Davis SL, Robertson KM, Pitts TM, Tentler
JJ, Bradshaw-Pierce EL, Klauck PJ, Bagby SM, Hyatt SL, Selby HM,
Spreafico A, et al: Combined inhibition of MEK and Aurora A kinase
in KRAS/PIK3CA double-mutant colorectal cancer models. Front
Pharmacol. 6(120)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dos Santos EO, Carneiro-Lobo TC, Aoki MN,
Levantini E and Bassères DS: Aurora kinase targeting in lung cancer
reduces KRAS-induced transformation. Mol Cancer.
15(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wan XB, Long ZJ, Yan M, Xu J, Xia LP, Liu
L, Zhao Y, Huang XF, Wang XR, Zhu XF, et al: Inhibition of Aurora-A
suppresses epithelial-mesenchymal transition and invasion by
downregulating MAPK in nasopharyngeal carcinoma cells.
Carcinogenesis. 29:1930–1937. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ren BJ, Zhou ZW, Zhu DJ, Ju YL, Wu JH,
Ouyang MZ, Chen XW and Zhou SF: Alisertib induces cell cycle
arrest, apoptosis, autophagy and suppresses EMT in HT29 and Caco-2
cells. Int J Mol Sci. 17(41)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Poulin EJ, Bera AK, Lu J, Lin YJ, Strasser
SD, Paulo JA, Huang TQ, Morales C, Yan W, Cook J, et al:
Tissue-Specific Oncogenic Activity of KRASA146T. Cancer
Discov. 9:738–755. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Haigis KM: KRAS alleles: The devil is in
the detail. Trends Cancer. 3:686–697. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lito P, Solomon M, Li LS, Hansen R and
Rosen N: Allele-specific inhibitors inactivate mutant KRAS G12C by
a trapping mechanism. Science. 351:604–608. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
McFall T, Diedrich JK, Mengistu M,
Littlechild SL, Paskvan KV, Sisk-Hackworth L, Moresco JJ, Shaw AS
and Stites EC: A systems mechanism for KRAS mutant allele-specific
responses to targeted therapy. Sci Signal.
12(eaaw8288)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hong DS, Fakih MG, Strickler JH, Desai J,
Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS,
et al: KRASG12C inhibition with sotorasib in advanced
solid tumors. N Engl J Med. 383:1207–1217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao Y, Murciano-Goroff YR, Xue JY, Ang A,
Lucas J, Mai TT, Da Cruz Paula AF, Saiki AY, Mohn D, Achanta P, et
al: Diverse alterations associated with resistance to KRAS(G12C)
inhibition. Nature. 599:679–683. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J,
Kiedrowski LA, Michel AG, Syed MU, Fella KA, Sakhi M, et al:
Clinical acquired resistance to KRASG12C inhibition
through a novel KRAS switch-II pocket mutation and polyclonal
alterations converging on RAS-MAPK reactivation. Cancer Discov.
11:1913–1922. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Awad MM, Liu S, Rybkin II, Arbour KC,
Dilly J, Zhu VW, Johnson ML, Heist RS, Patil T, Riely GJ, et al:
Acquired resistance to KRASG12C Inhibition in cancer. N
Engl J Med. 384:2382–2393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hong X, O'Donnell JP, Salazar CR, Van
Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown
SL, Mikkelsen T and Lehman NL: The selective Aurora-A kinase
inhibitor MLN8237 (alisertib) potently inhibits proliferation of
glioblastoma neurosphere tumor stem-like cells and potentiates the
effects of temozolomide and ionizing radiation. Cancer Chemother
Pharmacol. 73:983–990. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhou N, Singh K, Mir MC, Parker Y, Lindner
D, Dreicer R, Ecsedy JA, Zhang Z, The BT, Almasan A and Hansel DE:
The investigational Aurora kinase A inhibitor MLN8237 induces
defects in cell viability and cell-cycle progression in malignant
bladder cancer cells in vitro and in vivo. Clin Cancer Res.
19:1717–1728. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
D'Assoro AB, Liu T, Quatraro C, Amato A,
Opyrchal M, Leontovich A, Ikeda Y, Ohmine S, Lingle W, Suman V, et
al: The mitotic kinase Aurora-a promotes distant metastases by
inducing epithelial-to-mesenchymal transition in ERα(+) breast
cancer cells. Oncogene. 33:599–610. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Boss DS, Beijnen JH and Schellens JH:
Clinical experience with aurora kinase inhibitors: A review.
Oncologist. 14:780–793. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X,
Wang D, Yang T, Wang NJ, Zhao RJ and Zhou SF: Inhibition of mitotic
Aurora kinase A by alisertib induces apoptosis and autophagy of
human gastric cancer AGS and NCI-N78 cells. Drug Des Devel Ther.
9:487–508. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST,
He ZX, Edelman JL, Wang D, Yang YX, Zhang X, et al: Alisertib, an
Aurora kinase A inhibitor, induces apoptosis and autophagy but
inhibits epithelial to mesenchymal transition in human epithelial
ovarian cancer cells. Drug Des Devel Ther. 9:425–464.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang F, Li H, Yan XG, Zhou ZW, Yi ZG, He
ZX, Pan ST, Yang YX, Wang ZZ, Zhang X, et al: Alisertib induces
cell cycle arrest and autophagy and suppresses
epithelial-to-mesenchymal transition involving PI3K/Akt/mTOR and
sirtuin 1-mediated signaling pathways in human pancreatic cancer
cells. Drug Des Devel Ther. 9:575–601. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH,
He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, et al: Pro-apoptotic and
pro-autophagic effects of the Aurora kinase A inhibitor alisertib
(MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the
activation of mitochondria-mediated pathway and inhibition of p38
MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther.
9:1555–1584. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Prior IA, Lewis PD and Mattos C: A
comprehensive survey of Ras mutations in cancer. Cancer Res.
72:2457–2467. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Imamura Y, Lochhead P, Yamauchi M, Kuchiba
A, Qian ZR, Liao X, Nishihara R, Jung S, Wu K, Nosho K, et al:
Analyses of clinicopathological, molecular, and prognostic
associations of KRAS codon 61 and codon 146 mutations in colorectal
cancer: Cohort study and literature review. Mol Cancer.
13(135)2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ihle NT, Byers LA, Kim ES, Saintigny P,
Lee JJ, Blumenschein GR, Tsao A, Liu S, Larsen JE, Wang J, et al:
Effect of KRAS oncogene substitutions on protein behavior:
Implications for signaling and clinical outcome. J Natl Cancer
Inst. 104:228–239. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hammond DE, Mageean CJ, Rusilowicz EV,
Wickenden JA, Clague MJ and Prior IA: Differential reprogramming of
isogenic colorectal cancer cells by distinct activating KRAS
mutations. J Proteome Res. 14:1535–1546. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhou ZW, Ambrogio C, Bera AK, Li Q, Li XX,
Li L, Son J, Gondi S, Li J, Campbell E, et al: KRASQ61H
preferentially signals through MAPK in a RAF dimer-dependent manner
in non-small cell lung cancer. Cancer Res. 80:3719–3731.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ecker V, Stumpf M, Brandmeier L, Neumayer
T, Pfeuffer L, Engleitner T, Ringshausen I, Nelson N, Jücker M,
Wanninger S, et al: Targeted PI3K/AKT-hyperactivation induces cell
death in chronic lymphocytic leukemia. Nat Commun.
12(3526)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Burgess MR, Hwang E, Mroue R, Bielski CM,
Wandler AM, Huang BJ, Firestone AJ, Young A, Lacap JA, Crocker L,
et al: KRAS allelic imbalance enhances fitness and modulates MAP
kinase dependence in cancer. Cell. 168:817–829.e15. 2017.PubMed/NCBI View Article : Google Scholar
|