Introduction
NENs are a heterogeneous group of tumors arising
from enterochromaffin cells, being multipotent stem cells that
migrate from the neural crest to the gut ectoderm (1). The most common primary sites are the
gastro-intestinal and respiratory tract. Due to its heterogeneity,
these tumors exhibit diverse clinical and biological
characteristics (2). Grade,
largely based on Ki67 proliferation index, has proven to be a
powerful prognostic indicator. Apart from grade, stage, with
referral to size, depth of invasion and metastatic status, has a
prognostic value as well (2).
Small intestinal neuroendocrine tumors (siNENs)
represent the fastest growing cohort of gastroenteropancreatic NENs
(3). Well-differentiated siNENs in
general behave more indolently but nevertheless tend to
metastasize, with preference to the liver (4). The peritoneum is reportedly the third
most common site of metastasis after the liver and lymph nodes
(5). Peritoneal metastasis, but
not hepatic metastasis alone, is associated with shorter
disease-specific survival (6). The
combination of aggressive behavior and a confirmed very low Ki67
index in siNEN is speculative.
Case report
A 68-year-old Caucasian female, with no remarkable
medical history, presented herself at AZ Sint-Jan (Bruges, Belgium)
in April 2017. She was referred to us because of altered defecation
in association with lower abdominal cramps, and significant weight
loss despite normal appetite. A CT scan of chest and abdomen
revealed multifocal liver metastasis and a characteristic cartwheel
at the level of the mesentery, suggestive of neuroendocrine origin
(Fig. 1). Patient's basic
laboratory values were normal. Serum chromogranin was raised at
39,300 mcg/l. Further imaging with 68Ga-DOTATATE PET-CT identified
the mesenteric cartwheel lesion lacking SSTR expression, a focus
with increased somatostatin receptor expression in the middle
section of the ileum, diffuse liver metastasis and lymph nodes in
the right inguinal region with high SSTR expression. No other
lesions suspected for a primary tumor site outside the ileum were
detected. On endoscopic ultrasound, no primary pancreatic lesion
was identified. A core biopsy of one of the liver lesions confirmed
the neuroendocrine origin with a Ki67 index of less than 3%.
Because of sub-obstructive symptoms, the patient was sent for
surgery. On histomorphological and immunohistochemical analysis of
the oncologically resected ileal segment a G1 NEN with Ki67 index
1%, without clear mitosis, and with pronounced perineural and
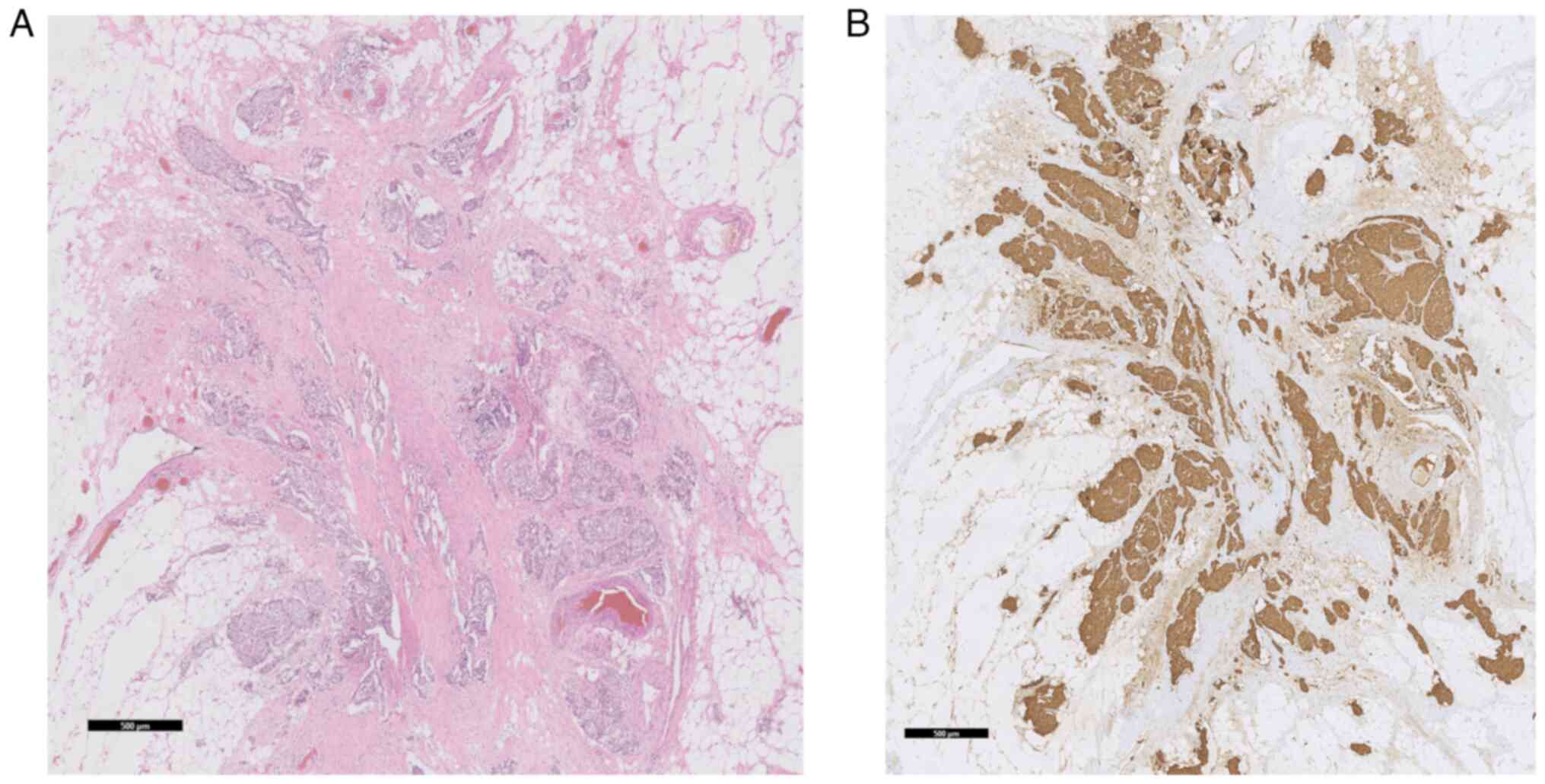
vascular invasion was diagnosed. The investigated lesions were
found within the ileal mesenteric fat (Fig. 2) and not in the wall of the
provided transverse ileal sections. Two out of 5 lymph nodes were
positive for metastatic involvement, with capsular invasion in one
of them. The multidisciplinary oncology board confirmed the
diagnosis of siNEN and a treatment with lanreotide was initiated at
90 mg on a monthly basis. During the following year, the patient
started losing weight and periodically had mild symptoms of
intestinal obstruction with a spontaneous resolution after a mean
of 3 days. Chromogranin remained globally unchanged. One year
later, the patient presented to the emergency department with bowel
obstruction. A CT scan revealed disease progression with de novo
peritoneal implants, ascites, an increased number of liver lesions,
with central necrosis in some of them. Serum chromogranin raised
from 7,320 mcg/l (value after segmental ileal resection) to 22,600
mcg/l. Symptoms resolved on supportive therapy. A 68Ga DOTATATE
PET-CT was repeated, revealing clinically evident small bowel
sub-obstruction as well as omental carcinomatosis, an implant in
the right iliac fossa as a new finding, and progression of the
liver metastases. A 18FDG PET-CT was planned and an exploratory
laparoscopy was performed. The histomorphological examination of
one of the peritoneal tumor nodules revealed lympho-vascular
invasion and a Ki67 index of 2% (Fig.
3). Consequently, the dose of the octreotide analogue was
augmented to 240 mg monthly. Peptide receptor radionuclide therapy
(PRRT) and everolimus were not considered a valid therapeutic
option after multidisciplinary consultation. 18FDG PET-CT did show
a hazy infiltration of the mesentery, but no avidity in the liver
lesions, and no pulmonary or osseous lesions. Three months later
the patient presented once again to the emergency department with
malaise, nausea, vomiting, and abdominal tenderness. Blood analysis
showed an acute renal insufficiency and significantly raised
inflammatory parameters (CRP 441 mg/l). On urgent CT scan an
intestinal perforation was obvious. The patient underwent an
emergency laparotomy which uncovered an inoperable state of bowel
obstruction with necrotic small bowel segments and diffuse tumor
invasion. Shortly after the intervention she developed sepsis. In
agreement with the family as well as the medical team, life support
was withdrawn, and the patient passed some hours later.
Discussion
What stands out in the presented case is the rapid
disease progression and the development of peritoneal metastases
despite what appeared to be a histologically grade 1 siNEN, and
which to our knowledge has been reported only twice (1,7).
In accordance with the latest 2019 WHO
classification of tumors of the digestive system, neuroendocrine
tumors are divided into NEN and neuroendocrine carcinoma, based on
their molecular differences. Mutations in MEN1, DAXX,
and ATRX are entity-defining for well-differentiated NENs,
whereas NECs usually have TP53 or RB1 mutations
(8). Whole-exome sequencing on
siNENs has shown quite low mutation rates, and it is felt that
epigenetic processes might be more important in tumor propagation
and metastasis, accounting for the more indolent behavior (9).
The diagnosis of siNEN remains a difficult task due
to the lack of overt symptoms (10). As a result the vast majority of
patients have metastatic disease at the time of diagnosis. Site of
metastasis seems to play an important role in survival of
metastatic NEN patients, independent of commonly described
prognostic factors (9). Peritoneal
metastasis, but not hepatic metastasis alone, is associated with
shorter disease-specific survival (6). The presence of peritoneal metastasis
has always been thought to be a rare finding in digestive NENs.
More recently, this rate has been estimated close to 14% (11).
In case of peritoneal metastasis, malignant cells
originating from primary abdominal organs usually spread through a
transcoelomic mechanism, responsible for the preferred areas for
metastases such as the omentum, paracolic gutters and the right
diaphragm (12). In case of NEN,
dissemination usually occurs through lymphatic spread, revolving
around the ligaments and mesentery (12). The complex, multistage process
involves multilevel reactions among molecular and cellular
components of the primary tumor site as well as the peritoneum,
depending on the combination of specific intrinsic characteristics
of the tumor cells and a specific receptive environment of the
peritoneum, the so-called pre-metastatic niche (13).
The peritoneum, being of mesodermal origin, exhibits
both mesenchymal and epithelial characteristics, and is composed of
distinctive layers: the glycocalyx, mesothelial cells, the basal
lamina, the submesothelial stroma, and the elastic lamina (13). In order to metastasize tumor cells
need to acquire a mobile and invasive phenotype, and therefore
undergo epithelial-to-mesenchymal transition. One of the changes
involved in this process is the cadherin switch, promoting the
detachment of cells from the primary tumor, as well as the
subsequent invasion and angiogenesis (13). Another, most critical step,
is the attachment to the submesothelial stroma, being a rich source
of all the necessary factors required for proliferation, and
protected by the mesothelial barrier (13). In case of neuro-endocrine tumors,
the stroma may be reached by invasion of physiological
intercellular spaces between mesothelial cells, the lymphatic
stomata. Stomata are small gaps between mesothelial cells with a
direct connection with the lymphatic system (13). The subsequent interactive intertalk
between metastatic cells, stromal cells, like cancer-associated
fibroblasts, and the specific microenvironment, is until nowadays
disappointingly poorly understood.
The fact that the metastatic lesions in our patient
did not show a convincing grade shift, in contrast to the evolution
to high aggressiveness, is an argument in the direction of
epigenetic changes and transcriptome dysregulation, not affecting
the Ki67 index. Besides, although Ki67 expression is tightly
correlated with proliferation, the possibility has been raised that
the contribution may be cell type specific and correlated with
distinct stages of the cell cycle (14). Re-expression of stem-cell markers
as part of the ‘homing’ of the recipient stroma may be a decisive
contributor as well (15).
Furthermore, the presented case was initially
diagnosed with mesenteric tumor deposits (MTDs), of which
multifocality and not the mere presence, is carrying a stronger
negative prognostic impact than true lymph node metastasis
(11). It seems probable that
venous invasion is the initial step for development of MTDs. The
access of tumor cells in MTDs to the enterohepatic venous system
further explains why they are a strong predictor for liver
metastases in patients with midgut NEN (11). To our knowledge no information on a
grade shift if any in MDTs, based on Ki67 labeling, is available in
the literature.
Thus, a twofold metastatic mechanism, interrelated
or not, and both raising several unanswered questions, may have
played part in the fast fatal course of the disease in the
presented patient.
The therapeutic approach of peritoneal
carcinomatosis is challenging, mainly due to lack of broad
knowledge of biological mechanisms and predictive factors, taking
the neoplastic environment as a whole. Translated to the presented
case, no benefit had to be expected from the traditional approach,
whatsoever (16).
The ultimate disposition of epigenetic drugs, and of
an availability-expanded arsenal of tumor-homing peptides and
optimized nanocarriers most probably will allow multipronged and
personally adjusted approaches to peritoneal carcinomatosis drug
delivery, and thus result in a better prognosis (17).
As the importance of an accurate tumor
classification lies in its prognostic implications, the present
case also obviously illustrates the need of implementing indices
different from Ki67, and correlated to the subcellular and
molecular level.
In conclusion, the development of peritoneal
carcinomatosis in NENs had initially been thought of as a rare
finding. The literature, however, alludes to the fact that it is
not quite as rare as previously believed, except in
well-differentiated G1 NENs. A more powerful predictive system is
needed to identify those patients at increased risk of developing
rapidly progressive metastatic disease. A complete understanding of
the interactions between the peritoneum and metastatic
neuro-endocrine cells at subcellular and molecular level should
lead to new treatment strategies for peritoneal carcinomatosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the article. VDW and KW
designed the study. JVH was responsible for data collection and
analysis with a focus on histopathology. CDW was responsible for
data collection and analysis, with a focus on radiologic imaging.
VDW and KW were responsible for data collection, analyzed the
literature, and drafted, edited and reviewed the manuscript. VDW
and KW confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of the data
and accompanying images was given by the patient's husband.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoniadou F, Korkolis D, Koufopoulos N,
Manatakis D and Sakellariou S: A well differentiated neuroendocrine
tumor of the jejunum with peritoneal carcinomatosis: A case report.
Mol Clin Oncol. 9:651–655. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim JY, Hong SM and Ro JY: Recent updates
on grading and classification of neuroendocrine tumors. Ann Diagn
Pathol. 29:11–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamaguchi T, Fujimori T, Tomita S,
Ichikawa K, Mitomi H, Ohno K, Shida Y and Kato H: Clinical
validation of the gastrointestinal NET grading system: Ki67 index
criteria of the WHO 2010 classification is appropriate to predict
metastasis or recurrence. Diagn Pathol. 8(65)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Norlen O, Edfeldt K, Akerstrom G, Westin
G, Hellman P, Bjorklund P and Stalberg P: Peritoneal carcinomatosis
from small intestinal neuroendocrine tumors: Clinical course and
genetic profiling. Surgery. 156:1512–1521. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wright MF, Cates J, Gonzales RS, Das S,
Berlin JD and Shi C: Impact of peritoneal metastasis on survival of
patients with small intestinal neuroendocrine tumor. Am J Surg
Pathol. 43:559–563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Celotti A, Pulcini G, Schieppati M,
Ministrini S, Berruti A and Ronconi M: An unusual case of a
well-differentiated neuroendocrine tumour of the ileum with
peritoneal carcinomatosis: A case report. World J Surg Oncol.
13(169)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA:
WHO Classification of Tumours Editorial Board. The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Trikalinos NA, Tan BR, Amin M, Liu J,
Govindan R and Morgensztern D: Effect of metastatic site on
survival in patients with neuroendocrine neoplasm (NENs). An
analysis of SEER data from 2010 to 2014. BMC Endocr Disord.
20(44)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Strosberg JR, Weber JM, Feldman M, Coppola
D, Meredith K and Kvols LK: . Prognostic validity of the American
joint committee on cancer staging classification for midgut
neuroendocrine tumors. J Clin Oncol. 31:420–425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Das S, Shi C, Koyama T, Huang Y, Gonzalez
R, Idrees K, Bailey CE and Berlin J: Peritoneal carcinomatosis in
well-differentiated small-intestinal neuroendocrine tumors with
mesenteric tumor deposits. J Med Surg Pathol. 4:1–10.
2019.PubMed/NCBI
|
|
12
|
Desai JP and Moustarah F: Peritoneal
metastasis. In: StatPearls (Internet). StatPearls Publishing,
Treasure Island, FL, 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541114/.
|
|
13
|
van Baal JOAM, van Noorden CJF, Nieuwland
R, Van de Vijver KK, Sturk A, van Driel WJ, Kenter GG and Lok CAR:
Development of peritoneal carcinomatosis in epithelial ovarian
cancer: A review. J Histochem Cytochem. 66:67–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Di Domenico A, Wiedmer T, Marinoni I and
Perren A: Genetic and epigenetic drives of neuroendocrine tumours
(NET). Endocr Relat Cancer. 24:R315–R334. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Berardi R, Morgese F, Torniai M, Savini A,
Partelli S, Rinaldi S, Caramanti M, Ferrini C, Falconi M and
Cascinu S: Medical treatment for gastro-entero-pancreatic
neuroendocrine tumours. World J Gastrointest Oncol. 8:389–401.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Simon-Gracia L, Hunt H and Teesalu T:
Peritoneal carcinomatosis targeting with tumor homing peptides.
Molecules. 23(1190)2018.PubMed/NCBI View Article : Google Scholar
|