Introduction
The most widely occurring soft-tissue sarcoma is
human liposarcoma (LPS) as it constitutes 24-45% of all soft-tissue
sarcomas (1,2). Effective therapeutic methods for
treating this sarcoma are underdeveloped despite of its wide
occurrence and that poses certain issues as metastatic diseases
cannot be treated via surgery, radiotherapy, or chemotherapy
(3). LPS has 3 main subcategories
based on its histopathological manifestations: i)
Well-differentiated (WDLPS) or de-differentiated liposarcomas
(DDLPS), ii) myxoid or round cell LPS (MRC), and iii) pleomorphic
LPS (4). For the majority of LPS
cases (85-90%), a fusion of surgery as well as radiotherapy has
been proven to be successful in hindering its reappearance at the
surgical site (5). However, such
outcomes differ based on the subtype of sarcoma. Radiation therapy
is usually used before, after or even during the surgery to
eliminate the malignant cells and to reduce their reoccurrence at
the same site. The effectiveness of chemotherapy for curing
liposarcoma is yet undefined, and, in the metastatic or
unresectable setting, various liposarcomas are considered
relatively chemotherapy-resistant and there is no consensus to
warrant the use of systemic treatment currently in the adjuvant or
neoadjuvant setting. However, it is used in certain scenarios when
the patients are at a critical stage or when there is a high chance
for reoccurrence of the tumor (6).
Surgical resection also remains the definitive management, and the
vast majority of extremity WDLS can be resected with negative
margins, and their clinical behavior does not warrant the use of
chemotherapy in either the adjuvant or neoadjuvant setting. Thus,
due to therapeutic limitations, new treatment regimens and a better
understanding of LPS are needed to address these drawbacks.
Tumor treating fields (TTFields) are an emerging
field that offers a non-invasive anticancer therapy model. TTFields
(also known as alternating electric field therapy) make use of
transcutaneous delivery of alternating electric fields of
low-intensity (1-3 V/cm) and intermediate-frequency (100-300 kHz),
which apply biophysical forces on charged as well as polarizable
molecules called dipoles (7,8). The
effectiveness of TTFields as anticancer therapy is affected by the
duration of the treatment (for example, the application of TTFields
for more than 18 h per day has been proven to improve the patient's
survival), the intensity of the electrical field (where increased
intensity is directly proportional to reducing tumor
proliferation), and electrical field frequency (the application for
which differs between the different cancers) (9). TTFields has been proven to hinder
tumor growth and induce the elimination of tumor cells in murine
and human cell models (10) via
impeding the proper development of the mitotic spindle apparatus
and the activation of the mitotic spindle checkpoint (7,11).
This causes the blebbing of the plasma membrane and disturbs cell
division, which would ultimately lead to the segregation of
abnormal chromosomes, disrupts cell-division cycle, and the
production of injured cell, subsequently leading to cell death or
apoptosis (12-15).
TTFields have been approved by the Food and Drug Administration
(FDA) in the United States as a modality for monotherapy for newly
diagnosed and recurrent GBM, according to the results of the EF-11
trial (16) and clinical trials of
humans who are being treated for some other tumor types. Moreover,
many preclinical studies (both lab and animal studies) that utilize
TTFields are already in progress for various cancers, such as
breast, cervical, stomach, and liver cancers, etc. (7,17-20).
Some of these studies indicate that TTFields may have better
effectivity with other anti-cancer therapies such as chemotherapy,
immunotherapy, and radiation therapy, leading to a synergistic
effect. For treating LPS, the main modality used as curative
therapy is surgical resection. Moreover, large liposarcomas at an
extreme stage or those occurring in the retroperitoneal area have a
high local reoccurrence rate (15 and 75%) and a generally low
survival rate in patients (21).
In such cases, inculcating neo-adjuvant approaches like
chemotherapy or radiotherapy, might be useful in improving the
local control, although such advancements have been scarce in
improving the survival rate for the disease in the last two decades
(22,23).
Thus, this study investigates the effectivity of
TTFields on treating liposarcoma and their capability in hindering
the proliferation and migration of tumor cells in preclinical
study.
Materials and methods
Experimental setup of the electric
fields
TTFields was generated using a pair of insulated
wires connected to a functional generator and a high-voltage
amplifier, which generated sine-wave signals ranging from 0 to 800
V and resulted in an applied electric field intensity and frequency
of 0.9 V/cm and 150 kHz, respectively (14,24).
We used 0.9 V/cm as the field intensity because of its use in
clinical settings. For TTFields treatment, cells were plated in
100-mm dishes and incubated at 37˚C under humidified conditions and
5% CO2 atmosphere until they reached 70-80%
confluency.
Cell culture
Human liposarcoma SW872 (HTB-92-ATCC) and 94T778
(ATCC CRL-3044) cancer cells were purchased from the ATCC
(Manassas, VA, USA) and cultured in RPMI 1640 medium (GIBCO,
Gaithersburg, MD, USA) supplemented with heat-inactivated 10% fetal
bovine serum (FBS; GIBCO), 0.1 mM non-essential amino acids,
glutamine, 4-(2-hydroxyethyl)1-piperazineethanesulfonic acid
(HEPES), and antibiotics at 37˚C in a 5% CO2-humidified
incubator.
Cell viability assay
To evaluate the effect of cell viability, it was
determined by trypan blue exclusion assay (20). An equal volume of trypan blue
reagent was added to a cell suspension, and the percentage of
viable cells was evaluated using microscopy. Assays were performed
in triplicate.
Water-soluble tetrazolium (WST-1)
assay
For the cytotoxicity assay to evaluate the
proliferation rate, liposarcoma cells were seeded in 96-well
culture plastic plates at a density of 1x103 cells per
well. TTFields was added to the dishes and the cells were incubated
for 48 h followed by application of the water-soluble tetrazolium
(WST)-1 cytotoxicity assay reagent (Roche Diagnostics, Laval,
Quebec, Canada: CAS No.150849-52-8) per the manufacturer's
recommendations. Cell viability was assessed by determining the
A450 nm of the cell culture media after adding WST-1 for 2 h. The
results were reported as a percentage of the optical density of the
untreated control cells, which was designated as 100% cell
viability. Percentage of cytotoxicity was calculated as follows:
(1-Aexp/Acontrol) x100; where Aexp and Acontrol are the absorbance
values of the experimental drug-treated and control untreated
cells, respectively.
Three-dimensional (3D) culture
system
Human SW872 and 94T778 liposarcoma cells were seeded
in 96-well plates at 1x104 cells/well to inhibit the
proliferation by TTFields. In the 3D culture model, 96-well plates
were pre-coated with Matrigel as a basement membrane by adding 40
µl of Matrigel to each well followed by incubation at 37˚C for 30
min. Cells were plated onto the gel in an appropriate medium, and
wells were photographed after a duration of 10 d.
Colony-forming assay
Liposarcoma cancer cells (500-1,000) were seeded
into 6-well plates in triplicate and treated with TTFields (1.0
V/cm; 150 kHz), doxorubicin (Sigma-Aldrich, St. Louis, MO, USA) (5
µM) or both concurrently for 48 h to evaluate the proliferation
after each treatment. After 14-20 d, colonies were fixed with 100%
Methanol and stained with 0.4% crystal violet (Sigma, St Louis, MO,
USA).
Cell death detection assay
To evaluate the cell death after TTFields treatment,
cells were treated, harvested, and stained with Cell Death
Detection ELISA kit (Roche Diagnostics GmbH: 11774425001) in
accordance with the manufacturer's protocols (25). Cell death was then measured using
Multiskan EX (Thermo Fisher Scientific, Germany) at 450 nm.
Caspase-3 activity assay
To evaluate the pNA light emission can be quantified
using a spectrophotometer or the activity of caspase3 after
TTFields treatment, Caspase-3 activity was analyzed in the SW872
and 94T778 cell lines 72 h after concurrent treatment with TTFields
(1.0 V/cm; 150 kHz) and 5 µM doxorubicin using detection kits
(Caspase-Glo 3/7 assay kit: G8091, Promega, Madison, WI, USA). The
assay is based on spectrophotometric detection of the chromophore
p-nitroanilide (pNA) after cleavage from the labeled substrates of
DEVD-pNA (for caspase-3). The microtiter plate reader at 405 nm.
Comparison of the pNA absorbance of apoptotic and control samples
allows the determination of the fold increase in caspase
activity.
ROS assay
Liposarcoma cells were cultured, and harvested at
the indicated times, according to the manufacturer's protocol using
Cellular ROS Assay Kit (ab113851) to confirm the relationship
between ROS production and the enhancement of TTFields-induced
apoptosis, and ROS was then measured using Multiskan EX (Thermo
Fisher Scientific, Germany) at 450 nm (26).
Transwell chamber assay
The migratory ability of liposarcoma cells was
measured using Transwell chambers (Corning Costar, Cambridge, MA,
USA) according to the manufacturer's protocol and reference
(27). Briefly, cells were seeded
onto the membrane of the upper chamber of the Transwell at a
concentration of 4x105 cells/ml in 150 µl of medium and
were left untreated or treated with TTFields for 24 h. The medium
in the upper chamber was serum-free, whereas the medium in the
lower chamber contained 10% (v/v) FBS as a source of
chemo-attractants. Cells that passed through the
Matrigel®/gelatin-coated membrane were stained with Cell
Stain Solution containing crystal violet supplied in the Transwell
chamber assay (Chemicon, Millipore, Billerica, MA, USA) and
photographed after a 24-h incubation period.
Statistical analysis
Statistical significance was determined using
one-way ANOVA and Tukey's post hoc test. Values represent the mean
of three experimental repeats ± SD. Data analysis was performed
using the GraphPad Prism 6 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of TTFields on the
proliferation of liposarcoma cancer cell lines
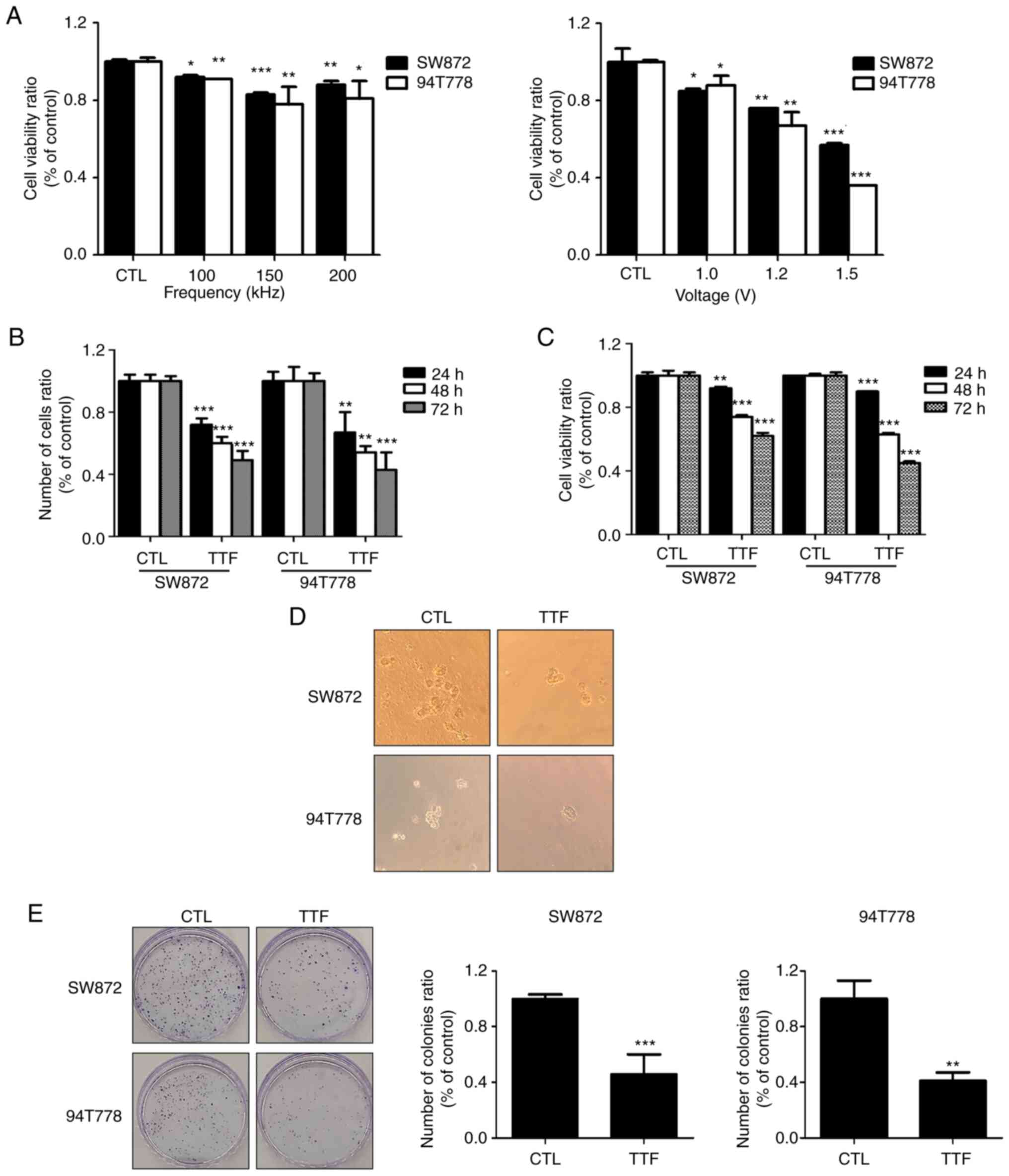
To determine the optimal TTFields voltage and
frequency, SW872 and 94T778 cells were subjected to various
conditions (Voltage, 0, 1.0, 1.2 and 1.5 V/cm; frequency, 0, 100,
150, and 200 kHz) for 48 h (Fig.
1A). The two liposarcoma cancer cell lines exhibited a
voltage-dependent reduction in cell viability (~20% at 1.0 V/cm;
150 kHz). As a result of processing the frequency of various
conditions, the viability of the cell was the most reduced at 150
kHz, the condition used in general various cancer types (28-30).
As shown in Fig. 1B and C showed at first, we indicated that
TTFields restricted the proliferation of cells as well as their
viability in vitro, utilizing a trypan blue exclusion and
WST-1 assays within a time-dependent way in SW872 and 94T778 cells.
Moreover, cell colonies in untreated 3D cultures were larger in
comparison to those formed by TTFields-treated cells (Fig. 1D). Colony forming assays were
incorporated for understanding similar effects in vitro
(Fig. 1E). Collectively, these
findings suggest that TTFields can inhibit the proliferation of
LPS.
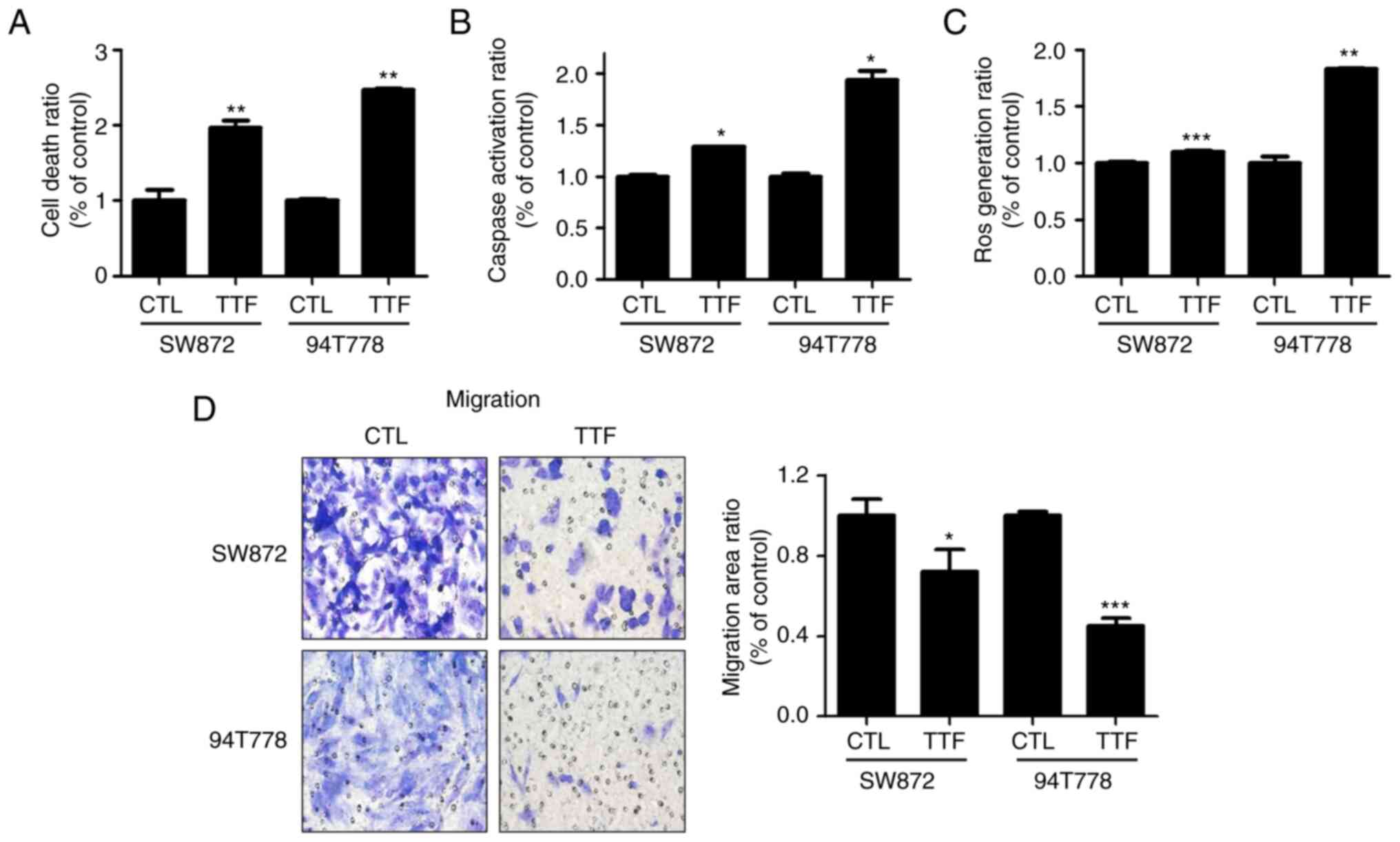
Apoptosis and migration on liposarcoma
is amplified by TTFields
To observe the effect of TTFields inducing apoptosis
on LPS, we analyzed early apoptosis using a cell death detection
kit. In LPS cell lines, it was noticed that a 72-h TTFields
exposure considerably increased the amount of cells undergoing
apoptosis (Fig. 2A). Subsequently,
we studied whether TTFields enhanced cytotoxicity was caused due to
an increased activation of caspase, leading to increased apoptotic
cell death. An increase in the activation of caspase-3 in response
to TTFields treatment was analyzed in comparison to the control
group (Fig. 2B). ROS are small
molecule metabolites of oxygen that tend to participate in redox
reactions because of their high reactivity (31). A link was observed between the
production of ROS and the enhancement of TTFields induced
apoptosis. The production of ROS was synergistically caused by
TTFields for treating liposarcoma cancer cell lines (Fig. 2C) and that ROS created by the TT
Fields treatment increases intracellular caspase signaling and,
consequently, apoptosis. Next, the effects of TTFields on
liposarcoma cells' migratory capacity was evaluated using Matrigel
chamber assays, which demonstrated that treatment using TTFields
majorly impeded the cell migration compared to the control group
(Fig. 2D).
Doxorubicin sensitizes LPS to
TTFields
Doxorubicin was the most common regimen as 1st line
therapy for soft-tissue sarcomas (32). To investigate the effect and
mechanism of enhancing the therapeutic efficacy of Doxorubicin and
TTFields combined treatment for LPS, we first confirmed the cell
viability. To analyze the effect of DOX on LPS cells via WST-1
assay, SW872 and 94T778 cells were treated with different
quantities of DOX for understanding the effect of DOX on LPS
(Fig. 3A). After 48 h, an
inhibition of cell growth was observed with it being statistically
relevant in cells that were treated with ≥5 µg/ml DOX (P<0.05).
Moreover, the data showed that SW872 and 94T778 cells were
sensitive to DOX and were dependent on their concentration. The
treatment using the combination of DOX and TTFields produced
significantly higher antitumor effects on SW872 and 94T778 cells
compared to either treatment being done alone through the use of
trypan blue cell viability and WST-1 assays (Fig. 3B and C). In addition, formation of tumor
colonies in combination-treated cells were smaller than
single-treated 3D cultures (Fig.
3D). In the colony forming assay, survival fraction values were
reduced in the combination containing TTFields and DOX compared
with that of single treatment on liposarcoma (Fig. 3E).
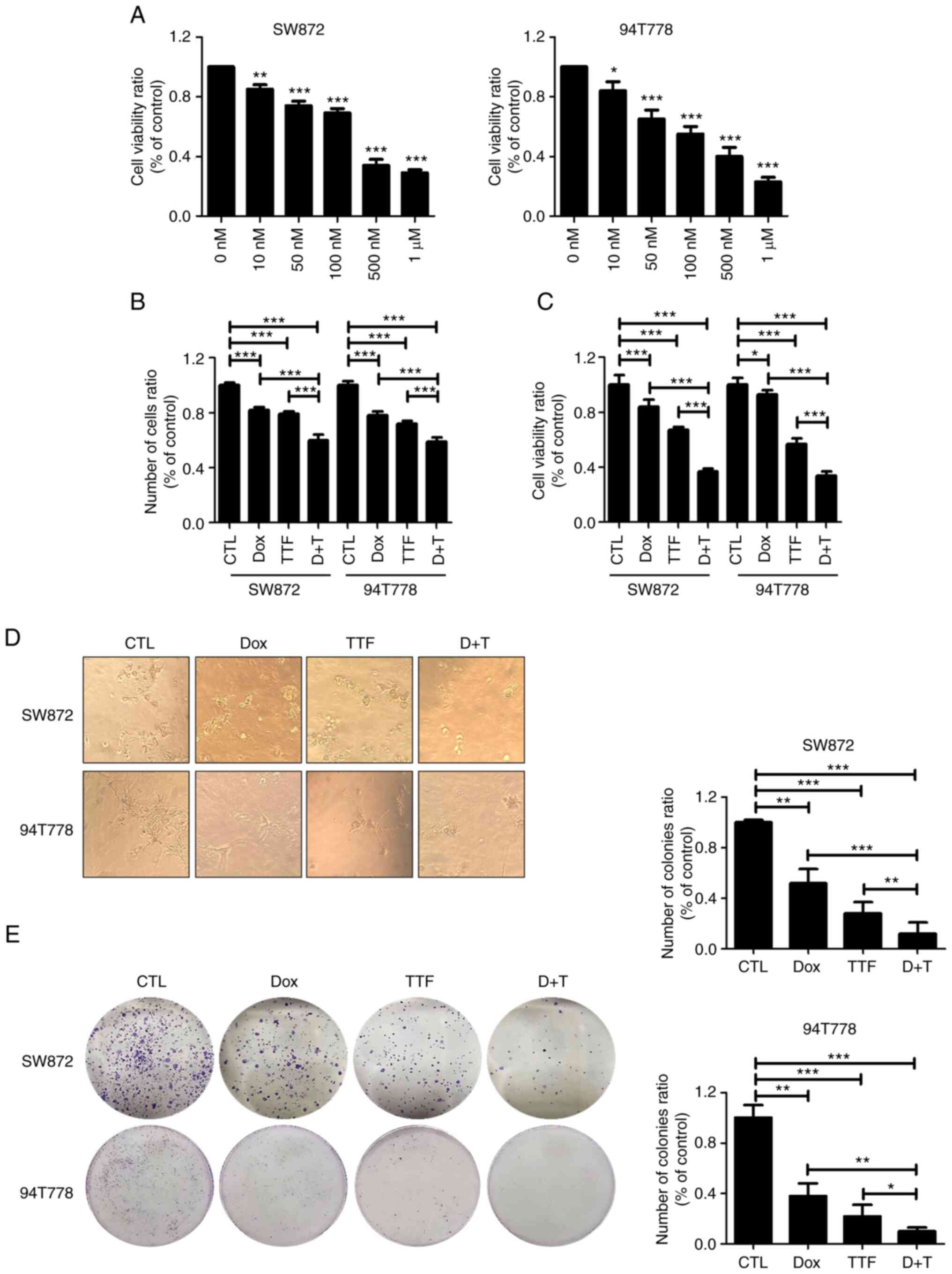 | Figure 3TTFields combined with Doxorubicin
inhibit cell proliferation in liposarcoma. (A) Analysis of WST-1
assay in two liposarcoma cell lines 48 h after each treatment with
TTFields by cell detection kit. *P<0.05,
**P<0.01, ***P<0.001 vs. CTL. (B)
Liposarcoma cells were treated with TTFields, doxorubicin, or
combined treatment for 48 h, and the cell viability was determined
by trypan blue exclusion assay. ***P<0.001. (C) WST-1
assay. *P<0.05, ***P<0.001. Values
represent the means of three experiments. (D) 3D culture assay
(magnification, x400). (E) The sensitivity of liposarcoma cells
treated with TTFields was measured via a colony formation assay.
*P<0.05, **P<0.01,
***P<0.001. CTL, control; TTF, tumor-treating fields;
DOX, doxorubicin; D+T, doxorubicin combined with tumor-treating
fields. |
Combined effect of TTFields and DOX on
apoptosis and migration of liposarcoma cells
To investigate the capacity of Doxorubicin and
TTFields in inducing apoptosis, we analyzed early apoptosis using
cell death detection kit. In the two liposarcoma cell lines, it was
noticed that an exposure of 72 h to Doxorubicin and TTFields
exhibited a remarkable increase in the amount of early apoptotic
cells (Fig. 4A). Such observations
underline an increase in the action of caspase3 in the combined
treatment method compared to Doxorubicin used alone on LPS.
(Fig. 4B). ROS production was
induced more strongly under combined treatment compared to that
under mono treatments (Fig. 4C)
and this can explain the increase in apoptotic rate when
combination treatment is used. Next, we analyzed the effects of
TTFields and DOX on the migratory capacities of LPS cells using
Matrigel chamber assays, which indicated that combined treatment
considerably reduced cell migration in comparison with the single
group on LPS (Fig. 4D).
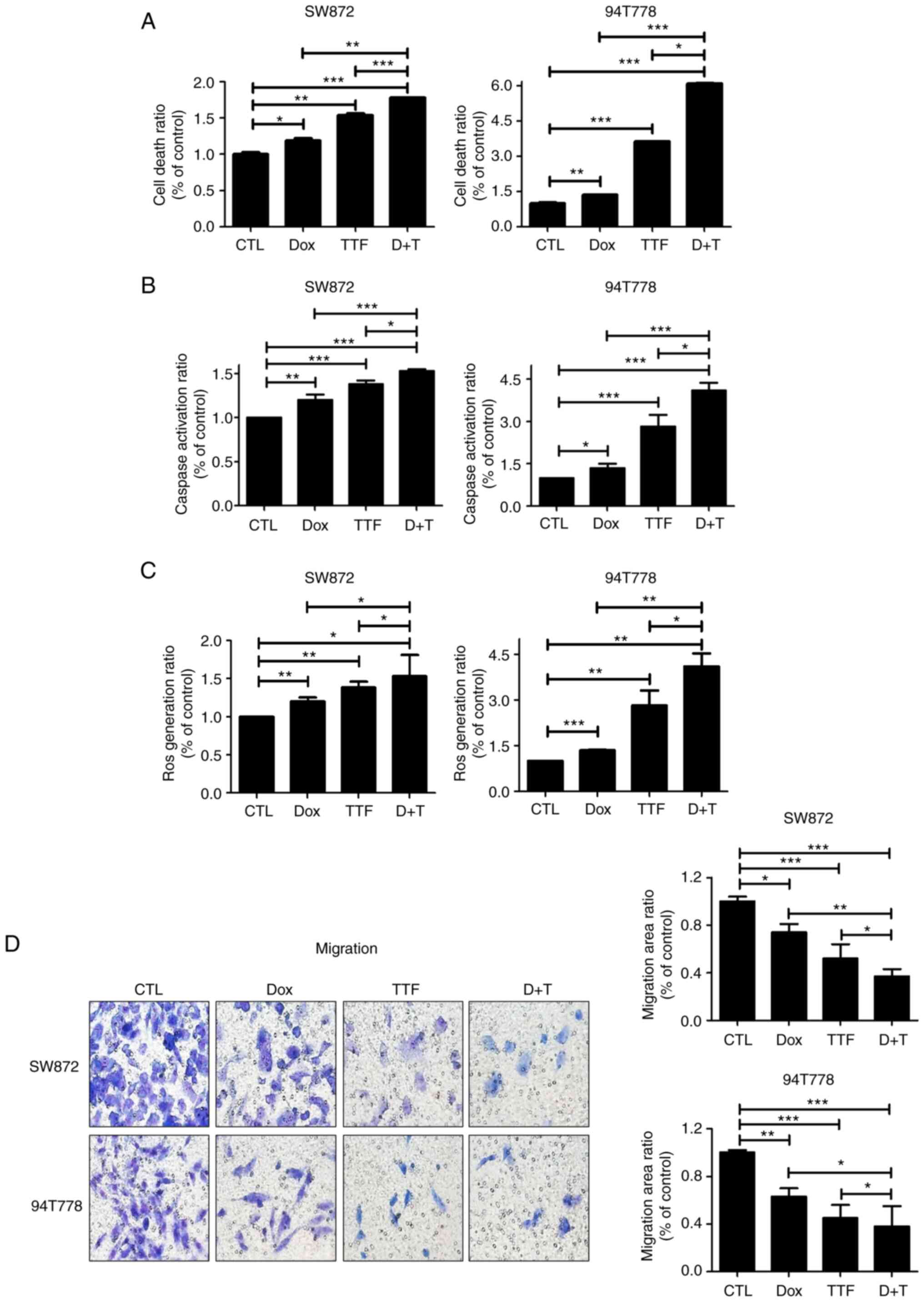 | Figure 4TTFields combined with Doxorubicin
enhance cell death and inhibits the migration on liposarcoma. (A)
Analysis of cell death in two liposarcoma cell lines 72 h after
concurrent treatment with TTFields (1.0 V/cm, 150 kHz) and
doxorubicin (5 µM) using a cell death detection kit.
*P<0.05, **P<0.01,
***P<0.001. (B) Analysis of caspase activity in the
two liposarcoma cell lines 72 h after treatment with TTFields and
doxorubicin by caspase ELISA. Data were obtained using a Multiskan
EX reader at 405 nm. *P<0.05, **P<0.01,
***P<0.001. (C) Analysis of ROS generation in two
liposarcoma cell lines 6 h after treatment with TTFields (1.0 V/cm,
150 kHz) by Cellular ROS Assay Kit. *P<0.05,
**P<0.01, ***P<0.001. (D) Tumor cell
migration after 24-h TTFields, doxorubicin, or combined treatment
examined by Transwell chamber assays. The number of migratory tumor
cells that penetrated through the gelatin was counted using five
high-intensity fields (magnification, x400). *P<0.05,
**P<0.01, ***P<0.001. CTL, control;
TTF, tumor-treating fields; DOX, doxorubicin; D+T, doxorubicin
combined with tumor-treating fields. |
Discussion
As a common soft sarcoma issue, liposarcoma is
observed in approximately 20% of overall sarcomas in adults
(33-35).
Because soft-tissue sarcomas constitute a heterogeneous group of
rare tumors, management by an experienced multidisciplinary team of
specialists is needed the standard of care from the time of
diagnosis. Similar to numerous other sarcoma subtypes, there
remains a paucity of treatment options for locally advanced or
metastatic liposarcoma. Currently, only doxorubicin (36), trabectedin (37) and eribulin (38) have Phase III data to support their
efficacy in advanced soft tissue sarcomas, including liposarcoma.
Several emerging systemic therapeutic agents from a range of
different classes have shown promise in Phase II clinical trials to
date, including tyrosine kinase inhibitors (39-41)
CDK inhibitors (42), mTOR
inhibitors (43),
thiazolidinediones (44), and
Selinexor (41). Several other
agents from the same classes as these agents, as well as
cabazitaxel (45) and the role of
immunotherapy in liposarcoma are currently under investigation in
Phase II clinical trials (46).
Further work in Phase III randomized clinical trials is required to
explore the efficacy of these newer treatments in the management of
liposarcomas, including further biomarker-led studies to
investigate additional targets for treatment.
Against this backdrop, TTFields represents a
noninvasive and novel therapeutic solution to the treatment of
liposarcoma based on our results. Recently, tumor cocktail therapy
has become a popular concept for cancer treatment and according to
preclinical work, because it mainly acts through the combination of
a variety of drugs to inhibit tumor growth at multiple, such as
combining nano- or immunotherapy drugs to target the abnormal tumor
microenvironment (TME) and prevent immune escape or cancer cell
growth to the greatest extent (47). In a broad sense, we described as a
combination of multiple therapeutic regimens, on LPS as like
TTFields and DOX. With advancements in research, TTFields combined
with chemoradiotherapy is being considered as a more effective
approach than radiotherapy and chemotherapy alone, and this has
been confirmed in many clinical trials (48). Currently, many other existing
therapies are becoming more effective when combined with TTFields.
in combination with an immune checkpoint inhibitor, TTFields are
capable of functioning in a synergistic way with some cytotoxic
agents on various cancer types. However, the long-term efficacy of
this therapy that involves TTFields needs additional assessment for
setting on LPS.
The results of this study revealed that TTFields
inhibited cell proliferation and cell viability with approximately
20% viability inhibition in vitro in a time-dependent manner
in liposarcoma cells. Our results also indicate that TTFields has
an inhibitory effect on migratory abilities through TTFields
combined with doxorubicin. Moreover, TTFields treatment
synergistically induced ROS production in liposarcoma cancer cell
lines, thereby suggesting that the TTFields-generated ROS boosts
intracellular caspase signaling and apoptosis on LPS with other
cancer types (49-51).
To broaden the therapeutic application, we
previously published a paper describing how these similar processes
are used to treat glioma, lung cancer, osteosarcoma,
hepatocarcinoma, and colon cancers (49-52).
These cancers were considered for our study because there is a need
to investigate treatment options for cancers that are rare in
addition to cancer types for which radiation therapy is currently
limited. We first performed a TTFields therapy trial on liposarcoma
with this intention. These clinical trials will make it easier to
understand how TTFields fit into treatment plans and determine
whether it is feasible to expand the availability of TTFields to
treat more types of cancer in the future. According to numerous
publications, TTFields cause disruptions in a wide range of
biological activities, including autophagy, DNA repair,
permeability, cell migration, and immune responses, in addition to
their apoptotic effects (1,9,10,13,27,53-58).
According to these reports, TTFields induce autophagy by blocking
the Akt2/miR29b axis in glioblastoma cells (56) and these delay DNA damage repair
following radiation treatment of glioma cells (54). And TTFields increase membrane
permeability in GBM cells (57)
and also induce immunogenic cell death when combined with anti-PD-1
therapy (58). Although there have
been reports of many similarities between the biological mechanisms
of TTFields, there have also been reports that the function of p53
is unclear. Numerous references, including my study, state that
exposure to TTFields causes apoptosis through both p53-independent
and p53-dependent mechanisms (14,59,60).
We need to research into p53's impact using TTFields on
liposarcoma.
Overall, our results show that TTFields is an
effective therapeutic approach for liposarcoma; radiation or
doxorubicin would be the TTFields-sensitizer based on our results
demonstrated the effectiveness of TTFields as a sensitizer of 5-FU
on colon cancers (61). Patient
outcome enhancements have stagnated despite the emergence of
revolutionary regimens that comprise traditional cytotoxic
chemotherapy to treat liposarcoma over the past few decades. There
is a need for optimizing clinical trials of TTFields-based tumor
treatments via preclinical testing using patient samples or in
vivo models and the application of electric fields alone or in
combination with drugs.
In summary, TTFields has been found to curtail cell
migration and proliferation of liposarcoma. These findings provide
a molecular basis for the use of chemotherapeutic drugs as TTFields
sensitizers to treat liposarcoma. The identification of TTFields
seems to be key for the optimization of therapeutic strategies for
liposarcoma and must a be a focus of future studies.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Rare Isotope Science
Project of Institute for Basic Science funded by Ministry of
Science and ICT and NRF of Korea (grant no. 2013M7A1A1075764). This
work was also supported by a National Research Foundation of Korea
(NRF) grant (grant no. 2022R1F1A1073750).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WSL participated in experiments, formal analysis and
used GraphPad Prism 6 software. YJJ was involved in methodology and
investigation. AHC and YHB participated in experiments. YBK and SMY
were involved in formal analysis. EHK designed the project and
wrote the manuscript. WSL and EHK revised the manuscript. EHK and
WSL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Conyers R, Young S and Thomas DM:
Liposarcoma: Molecular genetics and therapeutics. Sarcoma.
2011(483154)2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Crago AM and Singer S: Clinical and
molecular approaches to well differentiated and dedifferentiated
liposarcoma. Curr Opin Oncol. 23:373–378. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Malik AT, Alexander JH, Mayerson JL, Khan
SN and Scharschmidt TJ: Is Surgical resection of the primary site
associated with an improved overall survival for patients with
primary malignant bone tumors who have metastatic disease at
presentation? Clin Orthop Relat Res. 478:2284–2295. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singer S, Socci ND, Ambrosini G, Sambol E,
Decarolis P, Wu Y, O'Connor R, Maki R, Viale A, Sander C, et al:
Gene expression profiling of liposarcoma identifies distinct
biological types/subtypes and potential therapeutic targets in
well-differentiated and dedifferentiated liposarcoma. Cancer Res.
67:6626–6636. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haddox CL and Riedel RF: Recent advances
in the understanding and management of liposarcoma. Fac Rev.
10(1)2021.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Thway K: Well-differentiated liposarcoma
and dedifferentiated liposarcoma: An updated review. Semin Diagn
Pathol. 36:112–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kirson ED, Gurvich Z, Schneiderman R,
Dekel E, Itzhaki A, Wasserman Y, Schatzberger R and Palti Y:
Disruption of cancer cell replication by alternating electric
fields. Cancer Res. 64:3288–3295. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fonkem E and Wong ET: NovoTTF-100A: A new
treatment modality for recurrent glioblastoma. Expert Rev
Neurother. 12:895–899. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rominiyi O, Vanderlinden A, Clenton SJ,
Bridgewater C, Al-Tamimi Y and Collis SJ: Tumour treating fields
therapy for glioblastoma: Current advances and future directions.
Br J Cancer. 124:697–709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Giladi M, Schneiderman RS, Voloshin T,
Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A,
et al: Mitotic spindle disruption by alternating electric fields
leads to improper chromosome segregation and mitotic catastrophe in
cancer cells. Sci Rep. 5(18046)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kirson ED, Dbaly V, Tovarys F, Vymazal J,
Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S,
Gurvich Z, Schneiderman R, et al: Alternating electric fields
arrest cell proliferation in animal tumor models and human brain
tumors. Proc Natl Acad Sci USA. 104:10152–10157. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gera N, Yang A, Holtzman TS, Lee SX, Wong
ET and Swanson KD: Tumor treating fields perturb the localization
of septins and cause aberrant mitotic exit. PLoS One.
10(e0125269)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karanam NK and Story MD: An overview of
potential novel mechanisms of action underlying Tumor Treating
Fields-induced cancer cell death and their clinical implications.
Int J Radiat Biol. 97:1044–1054. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim EH, Kim YH, Song HS, Jeong YK, Lee JY,
Sung J, Yoo SH and Yoon M: Biological effect of an alternating
electric field on cell proliferation and synergistic antimitotic
effect in combination with ionizing radiation. Oncotarget.
7:62267–62279. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee WS, Seo SJ, Chung HK, Park JW, Kim JK
and Kim EH: Tumor-treating fields as a proton beam-sensitizer for
glioblastoma therapy. Am J Cancer Res. 11:4582–4594.
2021.PubMed/NCBI
|
|
16
|
Fabian D, Guillermo Prieto Eibl MDP,
Alnahhas I, Sebastian N, Giglio P, Puduvalli V, Gonzalez J and
Palmer JD: Treatment of Glioblastoma (GBM) with the addition of
tumor-treating fields (TTF): A review. Cancers (Basel).
11(174)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Carrieri FA, Smack C, Siddiqui I,
Kleinberg LR and Tran PT: Tumor treating fields: At the crossroads
between physics and biology for cancer treatment. Front Oncol.
10(575992)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hottinger AF, Pacheco P and Stupp R: Tumor
treating fields: A novel treatment modality and its use in brain
tumors. Neuro Oncol. 18:1338–1349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pless M, Droege C, von Moos R, Salzberg M
and Betticher D: A phase I/II trial of Tumor Treating Fields
(TTFields) therapy in combination with pemetrexed for advanced
non-small cell lung cancer. Lung Cancer. 81:445–450.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jo Y, Hwang SG, Jin YB, Sung J, Jeong YK,
Baek JH, Cho JM, Kim EH and Yoon M: Selective toxicity of tumor
treating fields to melanoma: An in vitro and in vivo study. Cell
Death Discov. 4(46)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mulita F, Verras GI, Liolis E,
Tchabashvili L, Kehagias D, Kaplanis C, Perdikaris I and Kehagias
I: Recurrent retroperitoneal liposarcoma: A case report and
literature review. Clin Case Rep. 9(e04717)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Spalek MJ, Kozak K, Czarnecka AM, Bartnik
E, Borkowska A and Rutkowski P: Neoadjuvant treatment options in
soft tissue sarcomas. Cancers (Basel). 12(2061)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pasquali S and Gronchi A: Neoadjuvant
chemotherapy in soft tissue sarcomas: Latest evidence and clinical
implications. Ther Adv Med Oncol. 9:415–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Romanenko A, Grassellino A, Crawford AC,
Sergatskov DA and Melnychuk O: Ultra-high quality factors in
superconducting niobium cavities in ambient magnetic fields up to
190 mG. Appl Phys Lett. 105(234103)2014.
|
|
25
|
Liu C, Zhu Y, Lou W, Cui Y, Evans CP and
Gao AC: Inhibition of constitutively active Stat3 reverses
enzalutamide resistance in LNCaP derivative prostate cancer cells.
Prostate. 74:201–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ji WO, Lee MH, Kim GH and Kim EH:
Quantitation of the ROS production in plasma and radiation
treatments of biotargets. Sci Rep. 9(19837)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim EH, Song HS, Yoo SH and Yoon M: Tumor
treating fields inhibit glioblastoma cell migration, invasion and
angiogenesis. Oncotarget. 7:65125–65136. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wenger C, Miranda PC, Salvador R,
Thielscher A, Bomzon Z, Giladi M, Mrugala MM and Korshoej AR: A
review on tumor-treating fields (TTFields): Clinical implications
inferred from computational modeling. IEEE Rev Biomed Eng.
11:195–207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bernhardt M, Angerer B, Buss M and
Struppler A: Neural observer based spasticity quantification during
therapeutic muscle stimulation. Conf Proc IEEE Eng Med Biol Soc.
2006:4897–4900. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alesanco A, Garcia J, Serrano P, Ramos L
and Istepanian RH: On the guarantee of reconstruction quality in
ECG wavelet codecs. Conf Proc IEEE Eng Med Biol Soc.
2006:6461–6464. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sies H and Jones DP: Reactive oxygen
species (ROS) as pleiotropic physiological signalling agents. Nat
Rev Mol Cell Biol. 21:363–383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McGovern Y, Zhou CD and Jones RL: Systemic
therapy in metastatic or unresectable
well-differentiated/dedifferentiated liposarcoma. Front Oncol.
7(292)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Codenotti S, Vezzoli M, Monti E and
Fanzani A: Focus on the role of Caveolin and Cavin protein families
in liposarcoma. Differentiation. 94:21–26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jo VY and Fletcher CD: WHO classification
of soft tissue tumours: An update based on the 2013 (4th) edition.
Pathology. 46:95–104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Casali PG, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG,
Brodowicz T, et al: Soft tissue and visceral sarcomas: ESMO-EURACAN
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29 (Suppl 4):iv268–iv269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: Doxorubicin alone versus intensified
doxorubicin plus ifosfamide for first-line treatment of advanced or
metastatic soft-tissue sarcoma: A randomised controlled phase 3
trial. Lancet Oncol. 15:415–423. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Demetri GD, von Mehren M, Jones RL,
Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K,
Tawbi H, et al: Efficacy and safety of trabectedin or dacarbazine
for metastatic liposarcoma or leiomyosarcoma after failure of
conventional chemotherapy: Results of a phase III randomized
multicenter clinical trial. J Clin Oncol. 34:786–793.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schoffski P, Chawla S, Maki RG, Italiano
A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha SY, et
al: Eribulin versus dacarbazine in previously treated patients with
advanced liposarcoma or leiomyosarcoma: A randomised, open-label,
multicentre, phase 3 trial. Lancet. 387:1629–1637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Samuels BL, Chawla SP, Somaiah N, Staddon
AP, Skubitz KM, Milhem MM, Kaiser PE, Portnoy DC, Priebat DA,
Walker MS and Stepanski EJ: Results of a prospective phase 2 study
of pazopanib in patients with advanced intermediate-grade or
high-grade liposarcoma. Cancer. 123:4640–4647. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oki E, Makiyama A, Miyamoto Y, Kotaka M,
Kawanaka H, Miwa K, Kabashima A, Noguchi T, Yuge K, Kashiwada T, et
al: Trifluridine/tipiracil plus bevacizumab as a first-line
treatment for elderly patients with metastatic colorectal cancer
(KSCC1602): A multicenter phase II trial. Cancer Med. 10:454–461.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tchekmedyian V, Sherman EJ, Dunn L, Tran
C, Baxi S, Katabi N, Antonescu CR, Ostrovnaya I, Haque SS, Pfister
DG and Ho AL: Phase II study of lenvatinib in patients with
progressive, recurrent or metastatic adenoid cystic carcinoma. J
Clin Oncol. 37:1529–1537. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dickson MA, Schwartz GK, Keohan ML,
D'Angelo SP, Gounder MM, Chi P, Antonescu CR, Landa J, Qin LX,
Crago AM, et al: Progression-Free survival among patients with
well-differentiated or dedifferentiated liposarcoma treated with
CDK4 inhibitor palbociclib: A phase 2 clinical trial. JAMA Oncol.
2:937–940. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chawla SP, Staddon AP, Baker LH, Schuetze
SM, Tolcher AW, D'Amato GZ, Blay JY, Mita MM, Sankhala KK, Berk L,
et al: Phase II study of the mammalian target of rapamycin
inhibitor ridaforolimus in patients with advanced bone and soft
tissue sarcomas. J Clin Oncol. 30:78–84. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Demetri GD, Fletcher CD, Mueller E, Sarraf
P, Naujoks R, Campbell N, Spiegelman BM and Singer S: Induction of
solid tumor differentiation by the peroxisome
proliferator-activated receptor-gamma ligand troglitazone in
patients with liposarcoma. Proc Natl Acad Sci USA. 96:3951–3956.
1999.PubMed/NCBI View Article : Google Scholar
|
|
45
|
U.S. National Library of Medicine: Ph II
Cabazitaxel DD Liposarcoma. ClinicalTrials.gov, 2013. Accessed August 1,
2013.
|
|
46
|
Keung EZ, Lazar AJ, Torres KE, Wang WL,
Cormier JN, Ashleigh Guadagnolo B, Bishop AJ, Lin H, Hunt KK, Bird
J, et al: Phase II study of neoadjuvant checkpoint blockade in
patients with surgically resectable undifferentiated pleomorphic
sarcoma and dedifferentiated liposarcoma. BMC Cancer.
18(913)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dizon DS, Krilov L, Cohen E, Gangadhar T,
Ganz PA, Hensing TA, Hunger S, Krishnamurthi SS, Lassman AB,
Markham MJ, et al: Clinical cancer advances 2016: Annual report on
progress against cancer from the American society of clinical
oncology. J Clin Oncol. 34:987–1011. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs. maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee WS and Kim EH: Combination therapy of
Doxorubicin with TTFields and radiation: Newer approaches to combat
lung cancer. Am J Cancer Res. 12:2673–2685. 2022.PubMed/NCBI
|
|
50
|
Kim EH, Lee WS and Oh HK: Tumor-treating
fields in combination with sorafenib curtails the growth of
colorectal carcinoma by inactivating AKT/STAT3 signaling. Transl
Cancer Res. 11:2553–2561. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Oh JY, Lee YJ and Kim EH: Tumor-Treating
fields inhibit the metastatic potential of osteosarcoma cells.
Technol Cancer Res Treat. 19(1533033820947481)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jang Y, Lee WS, Sai S, Kim JY, Kim JK and
Kim EH: Tumor-treating fields in combination with sorafenib
restrain the proliferation of liver cancer in vitro. Oncol Lett.
24(338)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Park JI, Song KH, Jung SY, Ahn J, Hwang
SG, Kim J, Kim EH and Song JY: Tumor-Treating fields induce
RAW264.7 macrophage activation via NK-κB/MAPK signaling pathways.
Technol Cancer Res Treat. 18(1533033819868225)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Giladi M, Munster M, Schneiderman RS,
Voloshin T, Porat Y, Blat R, Zielinska-Chomej K, Hååg P, Bomzon Z,
Kirson ED, et al: Tumor treating fields (TTFields) delay DNA damage
repair following radiation treatment of glioma cells. Radiat Oncol.
12(206)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tanzhu G, Chen L, Xiao G, Shi W, Peng H,
Chen D and Zhou R: The schemes, mechanisms and molecular pathway
changes of Tumor Treating Fields (TTFields) alone or in combination
with radiotherapy and chemotherapy. Cell Death Discov.
8(416)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kim EH, Jo Y, Sai S, Park MJ, Kim JY, Kim
JS, Lee YJ, Cho JM, Kwak SY, Baek JH, et al: Tumor-treating fields
induce autophagy by blocking the Akt2/miR29b axis in glioblastoma
cells. Oncogene. 38:6630–6646. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chang E, Patel CB, Pohling C, Young C,
Song J, Flores TA, Zeng Y, Joubert LM, Arami H, Natarajan A, et al:
Tumor treating fields increases membrane permeability in
glioblastoma cells. Cell Death Discov. 4(113)2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Voloshin T, Kaynan N, Davidi S, Porat Y,
Shteingauz A, Schneiderman RS, Zeevi E, Munster M, Blat R, Tempel
Brami C, et al: Tumor-treating fields (TTFields) induce immunogenic
cell death resulting in enhanced antitumor efficacy when combined
with anti-PD-1 therapy. Cancer Immunol Immunother. 69:1191–1204.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Schneiderman RS, Voloshin T, Giladi M,
Porat Y, Munster M, Blat R, Sherbo S, Kirson ED, Weinberg U and
Palti Y: ATPS-25p53 Status dependence of tumor treating fields
(TTFIELDS) efficacy against glioma cancer cells. Neuro Oncol. 17
(Suppl 5)(v23)2015.
|
|
60
|
Lee YJ, Seo HW, Baek JH, Lim SH, Hwang SG
and Kim EH: Gene expression profiling of glioblastoma cell lines
depending on TP53 status after tumor-treating fields (TTFields)
treatment. Sci Rep. 10(12272)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Lee YJ, Cho JM, Sai S, Oh JY, Park JA, Oh
SJ, Park M, Kwon J, Shin US, Beak JH, et al: 5-Fluorouracil as a
Tumor-treating field-sensitizer in colon cancer therapy. Cancers
(Basel). 11(1999)2019.PubMed/NCBI View Article : Google Scholar
|