Introduction
Alzheimer's disease (AD) is a neurodegenerative
disease that causes the most common form of dementia worldwide
(1). Clinical manifestations of AD
include progressive memory loss, language and intellectual
disorders, and inability to take care of oneself (2). Amyloid β (Aβ) deposition is one of
the main pathological mechanisms of AD. Aβ aggregation can have a
direct toxic effect on nerve cells, and oligomers formed by Aβ
aggregation can stimulate the graded activation of inflammation and
oxidative stress, which in turn promote further Aβ aggregation in a
positive feedback loop (3,4). Therefore, the mechanism of Aβ
deposition in AD has been increasingly investigated in recent years
(5-8).
The drugs currently used to treat AD are symptomatic and have very
limited effects on improving the course of the disease (9). According to estimations in the World
Alzheimer's Disease Report 2021, there will be >78 million AD
patients worldwide by 2030 (https://www.alzint.org/resource/world-alzheimer-report-2021/).
Therefore, the development of drugs to prevent and treat AD is
urgently needed.
Traditional Chinese medicine and other natural
plant-based therapies have been used to prevent and treat AD. For
example, Liuwei Dihuang, as a representative prescription for
nourishing yin and tonifying kidneys, has a long history of use.
Liuwei Dihuang increases antioxidant activity in nematodes by
increasing the expression of heat shock proteins (HSPs), and
decreasing reactive oxygen species (ROS) levels to alleviate Aβ
protein toxicity (10).
Polysaccharides from Coptis chinensis Franch can regulate
the expression of HSPs, thereby delaying aging in nematodes, and
can inhibit Aβ deposition and thus reduce its toxicity (11). This demonstrates that traditional
Chinese medicine has the potential to play an increasingly
important role in the treatment of diseases.
The dried tuber of the orchid, Gastrodia
elata Bl., is one of the most valuable Chinese medicines.
Pharmacological research shows that Gastrodia elata can
improve cognitive function, protect nerves and delay aging, and has
the potential to treat AD (12-15).
The active ingredients of ethyl acetate Gastrodia elata
extract (EEGE) include phosphorylated (p)-hydroxybenzyl alcohol and
p-hydroxybenzaldehyde, which have anti-aging and anti-oxidative
stress effects and regulate Aβ protein, which may be key in the
treatment of AD (16). However,
the mechanism by which EEGE regulates Aβ is not clear.
Transgenic Caenorhabditis elegans (C.
elegans) can express human Aβ in muscle cells and neurons. This
is a powerful model for elucidating the mechanisms of AD and for
studying AD-related drugs (17,18).
Therefore, the present study used a transgenic C. elegans
model of AD to study how EEGE affects Aβ toxicity.
Materials and methods
Chemicals and reagents
4-Hydroxybenzyl alcohol, 4-hydroxybenzaldehyde and
4,4-dihydroxydiphenylmethane were purchased from Chengdu Alfa
Biotechnology Co., Ltd.; trolox and 2,4,6-tripyridine-s-triazine
(TPTZ) were purchased from Macklin Biochemical Co., Ltd.;
2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was
purchased from Shanghai Yi En Chemical Technology Co., Ltd.; and
2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) was purchased from
Shanghai Yuanye Bio-Technology Co., Ltd.
Strains and maintenance
The C. elegans strains employed in the
present study were: Wild-type N2 (Bristol), the transgenic strains
CL2006 {dvls2 [pCL12(unc-54/human Aβ1-42) + rol-6 (su1006)]},
CL4176 {dvIs27 [myo-3p::Aβ (1-42)::let-851
3'UTR] + rol-6(su1006) X}. Escherichia coli OP50 was
obtained from the Caenorhabditis Genetics Center (University of
Minnesota). CL4176 is a temperature-sensitive transgenic model in
which the Serine/threonine-protein kinase 1 system is inactivated
when the temperature increases from 16 to 25˚C, which leads to
overexpression of Aβ in muscles and a paralysis phenotype. All
C. elegans were cultured on solid nematode growth medium
(NGM; consisting of 300 ml deionized water including 0.82 g
peptone, Oxoid Limited; Thermo Fisher Scientific; 1.2 g NaCl,
Tianjin Fengchuan Chemical Reagent Co., Ltd.; 5.15 g agar, Beijing
Solarbio Science & Technology Co., Ltd.; autoclaved and
supplemented 1 mM MgSO4, 1 mM CaCl2, Tianjin
Fengchuan Chemical Reagent Co., Ltd.; 12.9 mM cholesterol solution,
Macklin, Inc.) containing E. coli OP50. CL4176 was
maintained at 16˚C, while N2 and CL2006 were maintained at
20˚C.
Preparation of EEGE and analysis of
its main components by high-performance liquid chromatography
(HPLC)
Gastrodia elata Bl. was purchased from Yunnan
Huide Pharmaceutical Co., Ltd. The samples were collected from
artificially cultivated Gastrodia elata in Xiaocaoba
(Yiliang County, Zhaotong, Yunnan, China), and analyzed by
Associate Professor Zili Yin, Yunnan University of Chinese Medicine
(Kunming, China). Drug extraction was performed as previously
described (19). The specified
amount of Gastrodia elata Bl. was extracted three times with
95% ethanol under heating reflux at 85˚C. The organic solvent was
evaporated under reduced pressure and the remaining extract was
dissolved in deionized water and extracted with ethyl acetate three
times. Samples were concentrated in a rotary evaporator at 60˚C
under reduced pressure, and dried. The obtained EEGE was analyzed
by HPLC (Agilent 1290 InfinityIIHPLC system; Agilent Technologies,
Inc.). The gradient elution process was performed as follows on a
ZORBAX SB-C18 column (4.6x250 mm; 5 µm; Agilent Technologies,
Inc.). Mobile phase A was acetonitrile, and mobile phase B was
water (0-25 min, from 13% A to 58% A; 25-26 min, from 58% A to 100%
A). The detection wavelength was 221 nm. EEGE and standards were
dissolved in methanol, the flow rate was 1.0 ml/min, and the column
was at room temperature, with a sample quantity of 10 µl.
Determination of total flavonoid and
total phenol amount and total antioxidant capacity
Total phenolic content of EEGE was measured by the
Folin-Ciocalteu method (20).
Briefly, gallic acid of known concentration was used as a standard
and Folin-Ciocalteu reagent was allowed to react with different
concentrations of standard and sample for 5 min,
Na2CO3 was then added, mixed thoroughly, and
incubated at room temperature in the dark for 2 h. Absorbance at
760 nm was then measured. The total flavonoid content was
determined with reference to Navarro-Hortal et al (21). Rutin was used as a standard. The
samples were reacted with NaNO2 for 6 min and then
incubated with AlCl3 for 5 min (at room temperature).
Finally, 4 ml NaOH was added, thoroughly mixed and reacted at room
temperature for 5 min. Absorbance was determined at 510 nm. The
results for total phenolic and flavonoid content are expressed as
mg gallic acid equivalent/g dry extract and mg rutin equivalent/g
dry extract, respectively. The total antioxidant capacity of EEGE
was evaluated by three methods including ferric reducing
antioxidant power (FRAP), DPPH and ABTS. In the FRAP method
(22), when iron and TPTZ are
complexed in sodium acetate solution, the color changes and the
absorbance is measured at 593 nm, which can be used to evaluate the
ability of samples to reduce Fe3+ to Fe2+.
DPPH detection was performed according to Qadir et al
(23). At a wavelength of 517 nm,
the stronger the reducing power of the compound in the sample, the
faster the color elimination of DPPH. Finally, oxidation of ABTS by
K2S2O8 results in green
ABTS+ free radicals (24). At a wavelength of 734 nm, the
stronger the anti-oxidation ability of the sample, the faster the
color elimination of ABTS+ radicals. Absorbance was
measured using a Varioskan Flash instrument (Thermo Fisher
Scientific, Inc.). Each experiment was repeated three times. The
results are expressed as mM trolox equivalent/g dry extract.
Paralysis assays
CL4176 worms synchronized to the L1 stage were
transferred to 35 mm culture plates with or without EEGE (0.125,
0.25, 0.5, 1 and 2 mg/ml) and cultured at 16˚C for 36 h. The
temperature was then raised to 25˚C for transgene induction. After
culture at 25˚C for 24 h the paralyzed C. elegans were
observed every 2 h. Worms were considered paralyzed when they did
not move and did not respond to platinum wire stimulation.
Lifespan assay
L4 stage CL4176 nematodes were transferred to NGM
plates with or without EEGE. Three replica plates and no less than
70 nematodes for each group were prepared. The nematodes were
incubated at 16˚C. To prevent the influence of egg and larval
development on the nematode counts, oviposition was inhibited by
adding 12 mM fluorouracil to the NGM medium. The number of
nematodes surviving on each culture plate was counted every 2 days
until all nematodes died. C. elegans death was determined by
the absence of movement and swallowing, and no reaction after being
touched by a platinum wire. Nematodes that burrowed into the agar
or climbed the wall of the plate and died of desiccation were
excluded from the statistics. The experiment was repeated
independently three times.
Heat stress resistance assays
L4 stage N2 nematodes were transferred to NGM plates
with or without EEGE. Three replica plates and no less than 70
nematodes for each group were prepared. The nematodes were
incubated at 20˚C for 48 h. The temperature was then changed to
35˚C, and the number of nematodes surviving in each culture plate
was counted every hour until all nematodes died. C. elegans
death was determined by the absence of movement and swallowing, and
no reaction after being touched by a platinum wire. The experiment
was repeated independently three times.
Juglone induction of stress in
wild-type N2
The effect of EEGE on juglone-induced oxidative
stress was evaluated using wild-type N2 worms (10). N2 nematodes were cultured to the L1
stage after synchronization, and transferred to NGM plates with or
without EEGE. A total of three replica plates for each group were
prepared. The nematodes developed to the L4 stage at 20˚C and were
then transferred to NGM plates supplemented with juglone (300 µM)
(Shanghai Yuanye Biotechnology Co., Ltd.). The survival of the
nematodes was observed every hour until all had died. Worms that
were rigid and unresponsive to light and slight vibrations were
recorded as dead.
Locomotion assay and reproduction
assay
The reproductive and locomotor abilities of C.
elegans are physiological markers related to senescence
(25). After synchronization, N2
nematodes were cultured at 20˚C to the L4 stage and transferred to
NGM medium with or without EEGE (0.5 or 1 mg/ml), with three
parallel plates in each group and at least 15 nematodes in each
plate. After culture for 48 h, nematodes were transferred to blank
NGM medium to observe the number of sinusoidal movements within 20
sec. A reproduction assay was performed according to Meng et
al (26); two N2 nematodes at
the L4 stage were selected from each group and fed separately at
20˚C (three replica plates were prepared for each group). This was
recorded as the first day of the reproduction assay. They were
transferred to new plates every 24 h until the reproductive
capacity of the nematodes was lost. The egg-laying boards were
incubated at 20˚C for 48 h and the number of offspring was counted
(in this experiment, the number of nematode offspring indirectly
reflected the number of eggs laid).
Cytosolic ROS measurement
Cytoplasmic ROS were detected as reported (27) after nematodes were incubated for 4
h with a fluorescent probe, 2,7-dichlorofluorescein diacetate at 25
µM. The probe becomes fluorescent after combining with reactive
oxygen species in the cytoplasm. N2 nematodes were synchronized and
cultured to the L1 stage, then transferred to an NGM culture plate
with or without EEGE, and developed to the L4 stage at 20˚C. Then,
5 mM paraquat (Aladdin) was added to the NGM plate and the
nematodes incubated for 4 h. The nematodes were then rinsed with M9
buffer solution, placed on a slide and covered with a cap. The
nematodes were observed using a positive fluorescence microscope
(Axio Scope A1; Carl Zeiss AG). The fluorescence intensity was
quantified using ImageJ v1.8.0. software (National Institutes of
Health).
Fluorescent staining of Aβ
deposits
Transgenic C. elegans strain CL2006
synchronized to the L4 stage (early adult stage) was inoculated on
NGM plates with or without EEGE. N2 worms were used as a negative
control for Aβ deposition (28).
After incubation at 20˚C for 48 h, worms were collected with M9
buffer and fixed in 4% paraformaldehyde/PBS (pH 7.4) (cat. no.
BL539A; Biosharp Life Sciences) at 4˚C for 24 h. The worms were
then incubated in 5% β-mercaptoethanol (cat. no. M828395; Macklin,
Inc.), 1% Triton X-100 (cat. no. MB2486; meilunbio) and 125 mM Tris
(pH 7.4) (cat. no. T8060; Beijing Solarbio Science & Technology
Co., Ltd.) at 37˚C for 24 h. The worms were then stained with
0.125% thioflavin S (cat. no. S19293; Shanghai Yuanye Biotechnology
Co., Ltd.) in 50% ethanol at room temperature for 2 min, and then
rinsed in 50% ethanol 2-3 times. The worms were then placed on a
glass slide for observation under a laser scanning confocal
microscope (LSM900; Carl Zeiss AG). The amount of thioflavin S
deposition in the prepharyngeal region of each nematode was scored
to quantify amyloid deposits.
Measurement of ROS, malondialdehyde
(MDA), superoxide dismutase (SOD) and catalase (CAT) in
nematodes
With reference to Song et al (6), CL4176 nematodes were synchronized at
16˚C for 48 h, cultured at 25˚C for 40 h then rinsed twice with M9
buffer to remove E. coli. The nematodes were then
homogenized and protein abundance was measured by the bicinchoninic
acid assay (Beyotime Institute of Biotechnology). The levels of
MDA, SOD and CAT were determined using Total Superoxide Dismutase
Assay (Beyotime Institute of Biotechnology), Catalase Assay
(Beyotime Institute of Biotechnology) and Malondialdehyde Detection
(Beijing Solarbio Science & Technology Co., Ltd.) according to
the manufacturer's instructions. ROS accumulation was determined
with reference to Wang et al (29). Briefly, 50 µl of nematode
supernatant was added to each well of a 96-well plate and 50 µl of
100 µM DCFH-DA solution (a fluorescent probe) (Beyotime Institute
of Biotechnology) was added to give a final DCFH-DA concentration
of 50 µM, The solutions were thoroughly mixed by shaking for 30
sec. Fluorescence detection was performed using a microplate reader
at an excitation wavelength of 485 nm and an emission wavelength of
538 nm. Detection was conducted once every 10 min, and ROS changes
within 100 min were counted.
RNA-sequencing (RNA-seq) analysis
Gene expression in transgenic CL4176 nematodes
treated with EEGE (1 mg/ml) and controls was analyzed by RNA-seq.
Extraction and purification of total RNA, library construction and
sequencing were performed at Beijing Fruit Shell Biotechnology Co.,
Ltd. using the Illumina Novaseq 6000 system (Illumina, Inc.). The
quality of the data sets was evaluated using an Agilent bioanalyzer
2100 (Agilent Technologies, Inc.). Transcript levels were estimated
using fragments per kilobase of transcript per million mapped reads
values to allow different genes or samples to be compared.
Settings: Two-fold change in expression levels and a false
discovery rate with a P-value <0.05 were used to screen the
RNA-seq data for differentially expressed genes (DEGs). All
analyses were performed at Beijing Fruit Shell Biotechnology Co.,
Ltd. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) analysis of DEGs was carried out using the DAVID (https://david.ncifcrf.gov/) online platform. The R
package ggplot2 (version 3.3.2) (30) was used to generate volcano, GO and
KEGG bubble maps to visualize the distribution of DEGs.
Validation of the RNA-seq results via
reverse transcription-quantitative PCR (RT-qPCR)
To verify the RNA-seq results, synchronized L1 stage
CL4176 nematodes were transferred onto NGM plates with or without
EEGE (1 mg/ml) (~500 nematodes per plate) and cultured at 15˚C for
36 h. The temperature was then raised to 25˚C, and the culture
continued for ~40 h. The nematodes were then collected with M9
buffer into an EP tube, and washed three times. Total RNA was
extracted from nematodes using an RNA extraction kit (Tiangen
Biotech Co., Ltd.) according to the manufacturer's instructions.
RNA concentration and purity were measured using an ultra-micro
spectrophotometer (SMA6000; Merinton Instrument, Ltd.). cDNA
(Promega Beijing Biotech Co., Ltd.) was generated by RT-PCR in a
PCR instrument (Veriti™ 96-Well Fast Thermal Cycler; Applied
Biosystems; Thermo Fisher Scientific, Inc.), and then the target
mRNA was quantified (GoTaq® qPCR and RT-qPCR Systems,
Promega Beijing Biotech Co., Ltd.) using a real-time PCR instrument
(C1000 Touch PCR; Bio-Rad Laboratories, Inc.). RT-qPCR was
performed with the following cycling conditions: 95˚C for 10 min,
followed by 40 cycles of 95˚C for 15 sec, 55˚C for 30 sec, 72˚C for
30 sec and then maintained at 4˚C. Relative gene expression was
calculated by the 2-∆∆Cq method, using β-actin as a
housekeeping gene (31). The
analysis was performed in triplicate for each group. Primer 3 Plus
(https://www.primer3plus.com/) was used
to design the primers and they were synthesized by TsingKe
Biological Technology. The primers are listed in Table SI.
Statistical analysis
Using GraphPad prism 8.0.0 (GraphPad Software, Inc.)
statistical software for analysis and processing, if the data
conformd to the normal distribution and the variance was uniform
(P>0.05), ANOVA followed by Bonferroni's multiple comparison
test was used. For lifespan and paralysis assays, Kaplan Meier
survival was utilized and P-values were calculated using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference, and all values were expressed as means ±
standard deviation. All experiments were repeated three times.
Results
Quality control of EEGE
As revealed by HPLC analysis, three main compounds
were detected in EEGE, namely: 4-Hydroxybenzyl alcohol,
4-hydroxybenzaldehyde, 4,4-Dihydroxydiphenylmethane (Fig. 1A and B). All three compounds have benzene rings
in their structures (Fig. 1C). The
total phenols, total flavonoids, total antioxidant capacity (DPPH,
FRAP and ABTS) and relative contents of main compounds of EEGE are
presented in Table I. These
results indicate that EEGE has a strong antioxidant capacity in
vitro.
 | Table IQuality analysis of EEGE. |
Table I
Quality analysis of EEGE.
| Parameter | Mean ± SEM |
|---|
| Total flavonoids
content (mg rutin equivalent/g EEGE) | 95.41±1.77 |
| Total phenolic
content (mg gallic acid equivalent/g EEGE) | 3.47±0.06 |
| FRAP (µM TE/g
EEGE) | 68.89±5.18 |
| DPPH (mM TE/g
EEGE) | 7.51±0.01 |
| ABTS (mM TE/g
EEGE) | 0.53±0.11 |
| 4-Hydroxybenzyl
alcohol (mg/g EEGE) | 32.40±0.07 |
|
4-hydroxybenzaldehyde (mg/g EEGE) | 36.25±0.05 |
|
4,4-Dihydroxydiphenylmethane (mg/g
EEGE) | 11.18±0.05 |
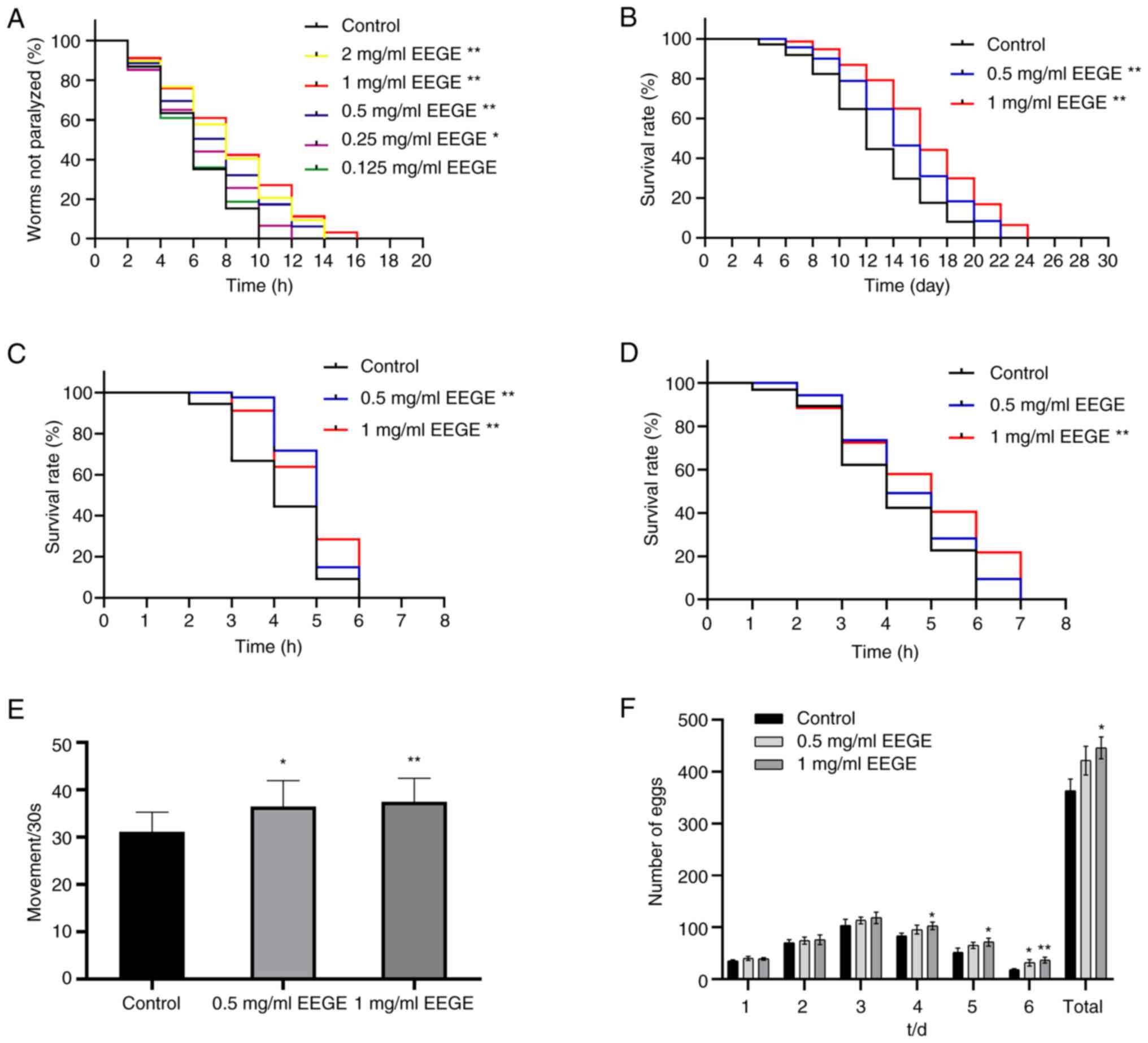
Effects of EEGE on paralysis
The present study first studied the effect of
different concentrations of EEGE (0.125, 0.25, 0.5, 1 and 2 mg/ml)
on the Aβ-induced toxicity of the transgenic C. elegans
strain CL4176. None of the concentrations were lethally toxic to
CL4176 nematodes (Fig. 2A).
Compared with the control group, 0.125 mg/ml EEGE had no
significant effect on CL4176 nematodes (P>0.05). EEGE (0.25,
0.5, 1 and 2 mg/ml) could prolong the paralysis time of nematodes.
The drug effect was dose-dependent within the concentration range
of 1 mg/ml, and the efficacy was weakened when the drug
concentration reached 2 mg/ml. Therefore, 1 mg/ml was the optimal
concentration for EEGE. These results suggested that the potential
of EEGE to protect CL4176 from Aβ-induced toxicity.
Effect of EEGE on lifespan
Senescence plays an important role in the
development of AD. Survival analysis showed that EEGE treatment
significantly shifted the survival curve of CL4176 to the right
(Fig. 2B). Compared with the
control group, 0.5 and 1 mg/ml EEGE increased the maximum lifespan
of CL4176 nematodes by 10.0% (P<0.01), 20.0% (P<0.01),
respectively. These data indicated that EEGE could delay the
senescence of CL4176 nematodes.
Effect of EEGE on heat stress
Compared with the control group, 0.5 and 1 mg/ml
EEGE could delay the survival rate of N2 nematodes under heat
stress induced by high temperature (P<0.01; Fig. 2C), although the mortality time of
C. elegans could not be prolonged, the overall survival rate
of C. elegans was increased. The results showed that EEGE
could improve the heat stress resistance of C. elegans.
EEGE reduces oxidative stress in C.
elegans
The present study further revealed that EEGE could
also protect wild-type N2 nematodes from oxidative stress produced
by juglone, and after 6 h of juglone stress, all nematodes in the
control group died, which was not significantly different compared
with the control group with 0.5 mg/ml EEGE (P>0.05), and the
maximum survival rate of the worms in the 1 mg/ml EEGE group was
significantly improved by 16.7% (P<0.01; Fig. 2D). The results showed that the
worms subjected to EEGE intervention exhibited significant
protection against oxidative stress induced by juglone (300
µM).
Effect of EEGE on reproduction and
locomotion
As the locomotor behavior of C. elegans
decreased with age, the present study investigated whether EEGE
affected the locomotor ability of C. elegans. Compared with
the control group, EEGE intervention increased the activity of N2
wild-type nematodes (Fig. 2E), and
the effect of 1 mg/ml EEGE was the most significant (P<0.01).
Nematodes begin to lay eggs when they enter the adult stage. As the
nematodes gradually aged, their egg-laying rate gradually decreased
and reached the peak of growth on the 3rd day of adult life.
Compared with the blank group, the egg laying rate of the EEGE
group was not significantly different on Days 1 to 3 (P>0.05;
Fig. 2F), and the egg laying rate
was significantly increased on Days 4 to 6. 1 mg/ml EEGE could
significantly increase the total oviposition of nematodes
(P<0.05), while 0.5 mg/ml EEGE had no statistical significance
(P>0.05). The results showed that EEGE could increase the number
of nematode progeny and improve the reproductive ability of
nematode.
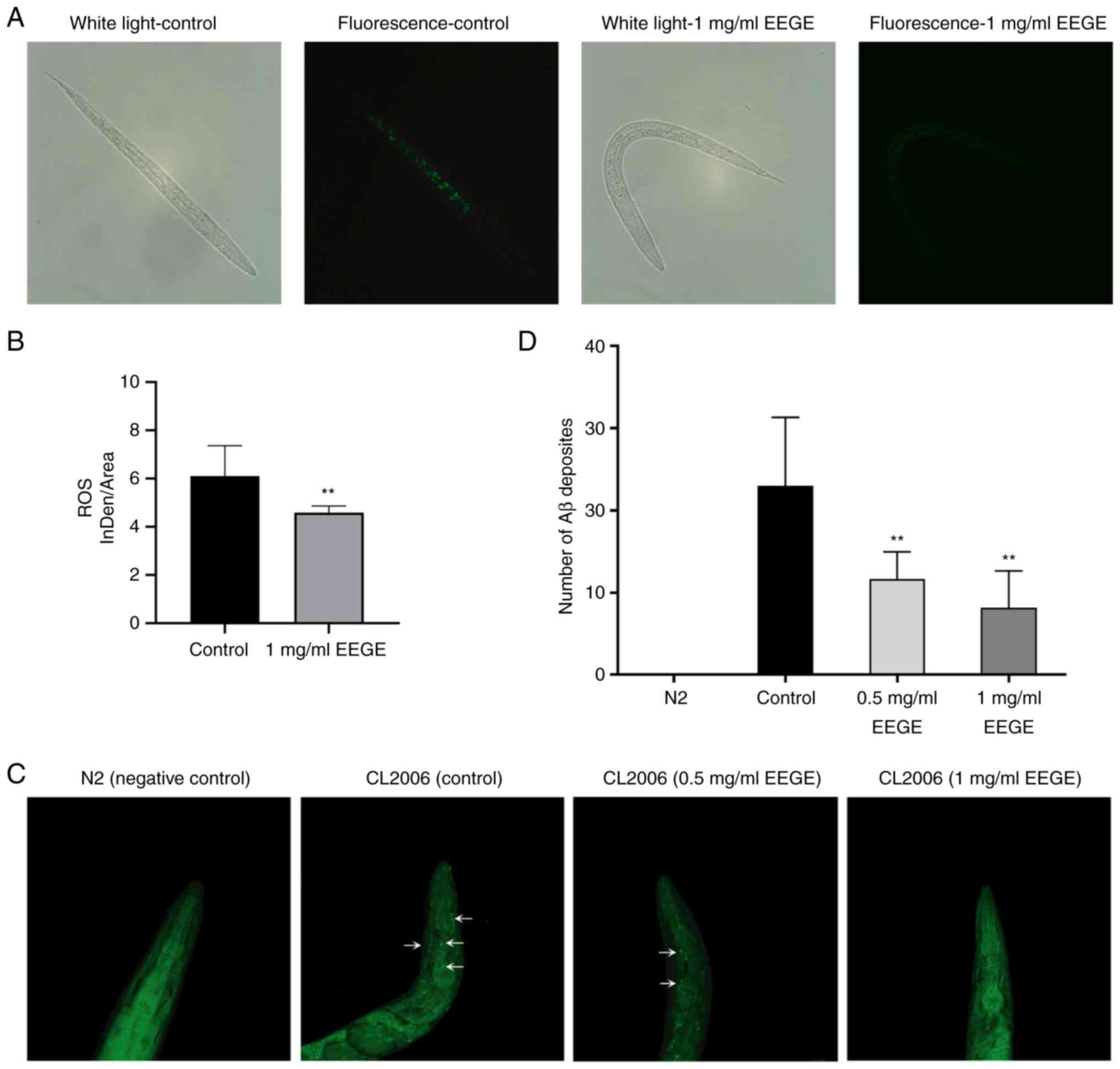
Effects of EEGE on ROS production
Paraquat can induce the increase of free radicals in
C. elegans, and EEGE has strong antioxidant capacity in
vitro (27). The present study
further determined the effect of EEGE on ROS in C. elegans.
The higher the amount of green fluorescence in nematodes, the more
ROS accumulation. Compared with the control group, the green
fluorescence in EEGE group was decreased (Fig. 3A), and the fluorescence intensity
of ROS per unit area was significantly decreased (P<0.01;
Fig. 3B). The results showed that
EEGE could inhibit the generation of free radicals and improve the
antioxidant capacity of C. elegans.
Effects of EEGE on Aβ aggregation
The present study observed the formation of amyloid
fibrils using the thioflavin-S fluorescence method to study the
effect of EEGE on the aggregation of Aβ. The transgenic C.
elegans strain CL2006 used in the present study expressed Aβ
protein fragments that are associated with the development of AD.
C. elegans demonstrated that the expression and aggregation
of Aβ in muscle led to progressive paralysis. CL2006 were stained
with triterpenes for Aβ at the end of EEGE treatment (showing a
green fluorescent spot). Fluorescence imaged of the heads of CL2006
nematodes demonstrated that, compared with untreated worms
(negative control), Aβ deposition in C. elegans treated with
EEGE was significantly decreased. Wild N2 strain has no Aβ
deposition in the whole animal (Fig.
3C). EEGE (0.5 and 1 mg/ml) significantly reduced the number of
Aβ oligomers (P<0.01; Fig. 3D),
and these results indicated that EEGE directly inhibited the
aggregation and deposition of Aβ in transgenic nematode muscle
cells, thereby delaying nematode paralysis.
Effects of EEGE on SOD and CAT
activities, and MDA and ROS levels
Oxidative stress has been shown to play an important
role in Aβ-induced toxicity (32).
The present study investigated the effects of EEGE on Aβ-induced
SOD, CAT, MDA and ROS. As shown in Fig. 4, compared with the control group,
EEGE was able to increase the CAT and SOD activities in nematodes
(P<0.01; Fig. 4A and B), reduce the MDA level in nematodes
(P<0.01; Fig. 4C), and inhibit
the rising trend of ROS in vivo (P<0.01; Fig. 4D), indicating that after
intervention with EEGE, the expression of antioxidant enzymes such
as CAT and SOD in nematodes was increased, the accumulation of
lipid peroxides was reduced, and the generation of free radicals
was reduced to improve the antioxidant capacity of the body,
thereby reversing the symptoms of AD to exert neuroprotective
function.
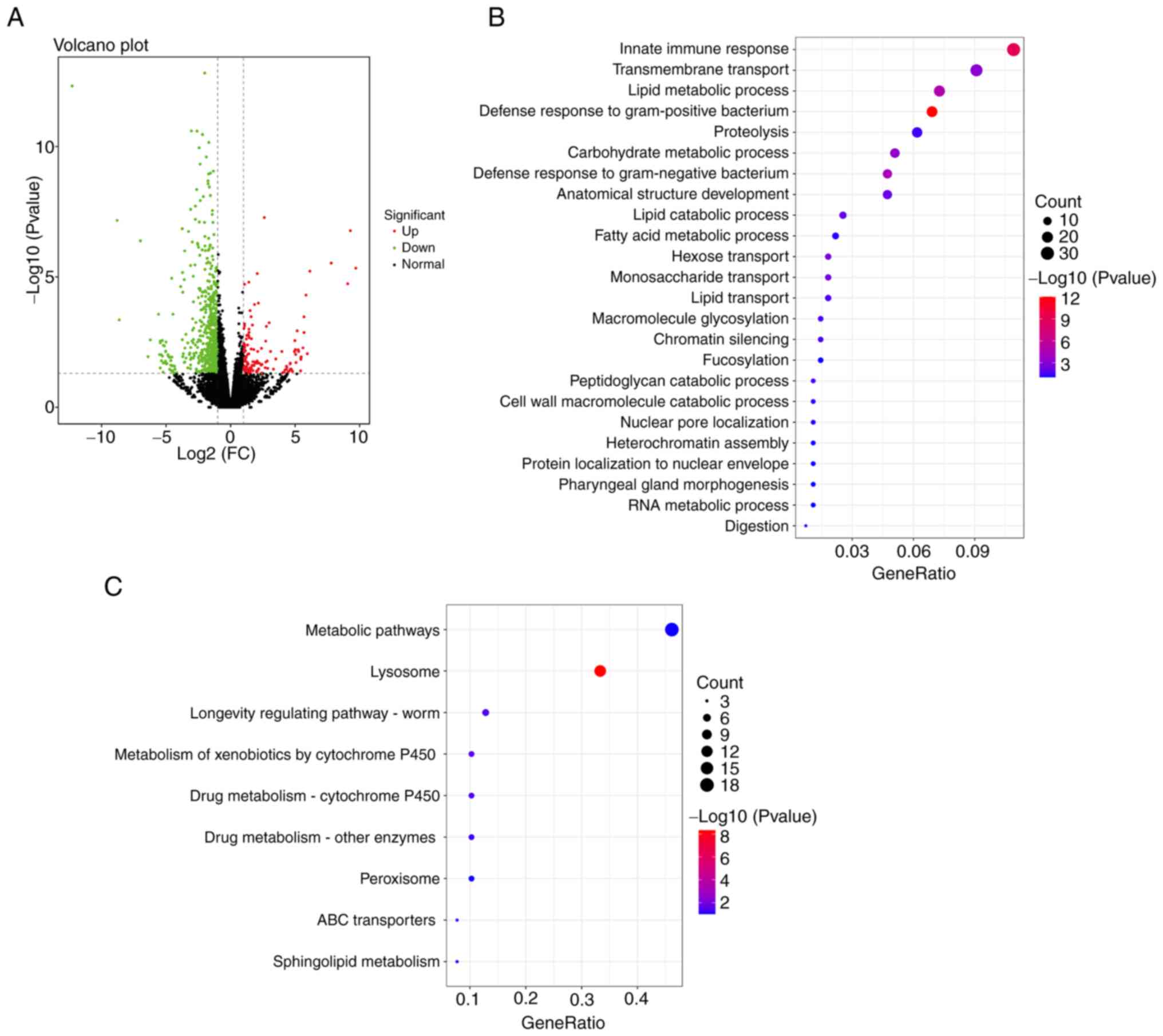
Genome-wide transcriptional profiling
of transgenic C. elegans treated with EEGE
A total of 763 DEGs were identified by RNA-Seq
analysis, including 145 upregulated genes and 618 downregulated
genes (Fig. 5A). The present study
demonstrated the biological process in GO analysis. GO analysis
showed that the regulatory mechanisms of EEGE involved 25
biological processes such as ‘innate immune response’,
‘transmembrane transport’ and ‘lipid metabolic process’ (Fig. 5B). In addition, nine pathways were
identified by KEGG enrichment analysis, which were related to, for
example, ‘metabolic pathways’, ‘lysosome’ and ‘longevity regulating
pathway-worm’ (Fig. 5C). The
results demonstrated that the treatment of diseases with complex
pathogenesis by a single target is limited, highlighting the
advantages of multi-component and multi-target treatment of
diseases by traditional Chinese medicine.
Validation of DEGs using qPCR
To verify the results of RNA-Seq, qPCR analyses were
performed. A total of 11 genes involved in nematode longevity,
oxidative stress, immunity, aging and regulation of Aβ protein were
selected for verification. These 11 genes and their functions were
considered to be closely related to AD pathogenesis (Table II). Compared with the control
group, the expression levels of gst-4, gst-25, hsp-12.3, hsp-12.6,
ugt-37 and ugt-63 genes were upregulated (Fig. 6A) and the expression levels of
fat-7, ins-7, ins-23, rgba-1 and dod-22 genes were downregulated
(P<0.05) (Fig. 6B). These 11
genes each showed the same tendency as observed in the RNA-Seq
experiments, and the present study inferred that the EEGE
regulation mechanism was likely related to the insulin pathway
based on the function and characteristics of the genes.
 | Table IIBackground information of 11
genes. |
Table II
Background information of 11
genes.
| Gene symbol | Gene name | Gene function |
|---|
| Gst-4 | Glutathione
S-transferase-4 | Enhances the
activity of glutathione transferase and participates in |
| Gst-25 | Glutathione
S-transferase-25 | the glutathione
metabolism. It is an important detoxification enzyme that regulates
the oxidation level and lifespan of C. elegans |
| Hsp-12.3 | Heat shock protein
12.3 | Improving the
stress resistance of C. elegans |
| Hsp-12.6 | Heat shock protein
12.6 | |
| Dod-22 | Downstream of
DAF-16 protein DOD-22 | Involved in the
defense response to gram-negative bacteria and innate immune
response |
| Fat-7 | δ(9)-fatty-acid
desaturase fat-7 | Regulating the
dysregulation of lipid metabolism associated with Aβ toxicity |
| Ins-7 | Insulin-like
peptide 7 | It is expected to
enhance the hormone activity of C. elegans and |
| Ins-23 | Insulin-like
peptide 23 | participate in the
regulation of olfaction and learning of C. elegans |
| Rgba-1 | Regulatory gene for
behavioral aging-1 | Regulating life
span and reproduction of C. elegans |
| Ugt-37 |
UDP-glucuronosyltransferase-37 | It can enhance the
activity of glucuronosyltransferase and the expression of UGTs can
be used to increase antioxidant stress and aging |
Discussion
At the forefront of AD research is the mechanism of
Aβ deposition (33). Gastrodia
elata Bl. can improve the memory of rats given bilateral
hippocampal injections of Aβ25-35 by reducing Aβ deposition in the
hippocampus, and can have a protective effect in this AD rat model
(34). However, its anti-Aβ effect
has not been systematically studied. Therefore, the present study
investigated the effect of EEGE on Aβ using transgenic C.
elegans expressing the human Aβ gene (35,36).
This revealed that that EEGE intervention delayed the paralysis of
C. elegans. However, the current study observed a weaker
potency of 2 mg/ml EEGE compared with 1 mg/ml EEGE, which may be
related to the drug metabolism pattern in vivo. Some drugs
need transporters to metabolize in the body, and when the dose is
too high, there will be overload (25). CL4176 is a strain obtained from
wild-type N2 by transgenic technology. The present study did not
find a higher mortality rate in the EEGE group compared with in the
control group with CL4176. Therefore, the present study considered
the concentrations in the experiment to be safe for C.
elegans. In addition, EEGE not only promoted the movement and
reproduction of C. elegans, but also extended the life span
of C. elegans.
The present study then explored the underlying
mechanism by which EEGE functions. The activities of SOD and CAT
increased, and the levels of ROS and lipid peroxide MDA decreased,
indicating that the antioxidant level of C. elegans
increased. Accumulation of Aβ in the wireworm head was
significantly reduced by EEGE (37), which might be key for EEGE
reversing the paralytic phenotype of nematodes. These data indicate
that EEGE has a protective effect against Aβ-induced neurotoxicity,
and that this effect delays senescence in C. elegans. AD can
be a pathological manifestation of the aging process (38) and the present results indicated
that EEGE had an anti-aging effect on the AD model C.
elegans. Therefore, anti-aging may play an important role in
the prevention of AD. The anti-AD effect of EEGE is closely related
to its antioxidant properties (39).
The present study determined the main components of
EEGE by HPLC. The main constituents of EEGE are p-hydroxybenzyl
alcohol, p-hydroxybenzaldehyde and 4,4'-dihydroxydiphenylmethane,
all of which are phenolic components of Gastrodia elata and
have strong antioxidant capacity (19). The present EEGE extraction method
yielded higher total phenol and total flavonoids contents compared
with that used by Song et al (40). P-Hydroxybenzyl alcohol, the active
ingredient of Gastrodia elata, can reduce ROS accumulation
and inhibit Aβ mRNA by regulating the transcription factor
FOXO/DAF-16, thereby delaying nematode paralysis and playing a
neuroprotective role (41).
However, studies separately analyzing p-hydroxybenzaldehyde and
4,4'-dihydroxydiphenylmethane in AD have not been reported yet. It
is well known that traditional Chinese medicine functions through
synergistic effects of multiple components on multiple targets, and
that the active substances may be a group of components with
similar structures. Therefore, the more purified the active
substance, the more its biological activity is lost (42,43).
The large number of compounds contained in EEGE means that there
may be other types of chemical besides phenolic compounds that have
anti-AD effects. The present study hypothesizes that
p-hydroxybenzaldehyde and 4,4'-dihydroxydiphenyl methane have
potential anti-AD activity; therefore, in future studies, the
authors will investigate the biological activity of these two
components against AD.
The current study used RNA-seq technology to analyze
AD-related gene expression changes and to explore the molecular
mechanism of EEGE against AD. After querying gene function, 11
genes were identified that might be related to the inhibition of Aβ
toxicity by EEGE. They were gst-4, gst-25,
hsp-12.3, hsp-12.6, dod-22, fat-7,
ins-7, ins-23, rgba-1, ugt-37 and
ugt-63. These genes are involved in the regulation of
nematode longevity and Aβ protein expression. qPCR showed that the
relative expression of all 11 genes to have the same trend as that
observed by RNA-seq. Among these genes, hsp-12.3 and
hsp-12.6 were further studied. Hsp-12.3 and
hsp-12.6 belong to the HSP family and are regulated by the
insulin/insulin-like growth factor-1 signaling (IIS) pathway
(44). HSPs are stress-reactive
proteins that are expressed in the majority of organisms under heat
and oxidative stress. The production of HSPs contributes to
longevity extension and stress resistance of nematodes (11). In addition, the IIS pathway also
regulates antioxidant genes such as gst-4 and gst-25
(45), which helps to increase
oxidation in C. elegans, thereby prolonging lifespan.
Rgba-1 regulates behavior and aging and can be activated by
the mitochondrial unfolded protein reaction regulated by
SIR-2.1(46). Fat-7 is
related to lipogenesis, and can regulate the lipid metabolism
pathway of C. elegans to inhibit Aβ deposition (47,48).
Ins-7 can reduce oxidative stress to reduce ROS production,
improve neuronal damage and prolong the lifespan of nematodes
(49). Dod-22 is regulated
by daf-2 and daf-16, and participates in the
regulation of nematode life-span (50).
There are three transcription factors in the IIS
pathway: SKN-1, DAF-16 and HSF-1. Insulin signaling plays a central
role in regulating metabolism and senescence in nematodes and a
number of other species (51-53).
KEGG analysis showed DEGS to be significantly related to the
regulation of nematode metabolism and longevity. The present study
therefore deduced that the regulation of Aβ toxicity by EEGE was
likely to occur through the IIS pathway, but this needs
verification.
In the IUCN red list, Gastrodia elata is
listed as a vulnerable species, but, as it is a completely
heterotrophic plant, it has been artificially cultivated in China
(54). In addition, Gastrodia
elata has gastrodin, p-hydroxybenzyl alcohol and other
components that reduce inflammatory factors, reduce Aβ deposition
and other pharmacological activities, which have unique advantages
for neuroprotection (12,55). It can be seen that Gastrodia
elata has great development value in the treatment of
neurodegenerative diseases and is a hope for the treatment of
dementia (15). However, there are
some limitations to the present study. Although C. elegans
is transferred into human Aβ gene to form Aβ deposition, the
specific mechanism of Aβ formation cannot be completely simulated.
In addition, C. elegans is only a low organism, and the
pathogenesis of AD is complex. In the future, we will study the
mechanism of action of EEGE in animal models such as rats and mice,
in order to obtain data that may be clinically used.
In conclusion, the present study investigated the
effect of EEGE on alleviating Aβ toxicity in a nematode AD model.
The protective effect of EEGE on transgenic C. elegans was
to reduce the aggregation of Aβ protein, improve antioxidant
levels, effectively remove free radicals and regulate the
expression of genes related to the IIS pathway, thereby reducing
the toxicity induced by Aβ and delaying the paralysis of C.
elegans. This study reveals the potential for EEGE to have a
positive effect in preventing AD, and also provides a theoretical
basis for the prevention and treatment of aging-related diseases by
EEGE. It is necessary to further clarify the active compounds in
EEGE and to verify their pharmacodynamics using AD models in more
complex animals, such as rats.
Supplementary Material
Primer sequences.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81960733), the Open Project
of Yunnan Key Laboratory of Dai and Yi Medicines (grant no.
202210ZD2206), the Xingdian Talent Support Program - Special for
Young Talent (grant no. XDYC-QNRC-2022-0284), the National
Administration of Traditional Chinese Medicine High-level Key
Discipline Construction Project ‘Dai Medicine’ and ‘Dai
Pharmacy’.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The sequence data from this study have been submitted to
the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra), with accession
number SRP440005.
Authors' contributions
XS, XY, LY and XD made considerable contributions to
the experimental design, statistical data analysis and English
language editing. XD and LY are responsible for drafting the
manuscript and revising it for important intellectual content. XS
and XY confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lane CA, Hardy J and Schott JM:
Alzheimer's disease. Eur J Neurol. 25:59–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
De Strooper B and Karran E: The cellular
phase of Alzheimer's disease. Cell. 164:603–615. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mohsenzadegan M and Mirshafiey A: The
immunopathogenic role of reactive oxygen species in Alzheimer
disease. Iran J Allergy Asthma Immunol. 11:203–216. 2012.PubMed/NCBI
|
|
4
|
Alafuzoff I, Pikkarainen M, Arzberger T,
Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E,
Ferrer I, et al: Inter-laboratory comparison of neuropathological
assessments of beta-amyloid protein: A study of the BrainNet Europe
consortium. Acta Neuropathol. 115:533–546. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang X, Kang X, Du L, Zhang L, Huang Y,
Wang J, Wang S, Chang Y, Liu Y and Zhao Y: Tanshinone IIA loaded
chitosan nanoparticles decrease toxicity of β-amyloid peptide in a
Caenorhabditis elegans model of Alzheimer's disease. Free Radical
BiolMed. 193:81–94. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song X, Sun Y, Wang Z, Su Y, Wang Y and
Wang X: Exendin-4 alleviates β-Amyloid peptide toxicity via DAF-16
in a Caenorhabditis elegans model of Alzheimer's disease. Front
Aging Neurosci. 14(955113)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Du F, Zhao H, Yao M, Yang Y, Jiao J and Li
C: Deer antler extracts reduce amyloid-beta toxicity in a
Caenorhabditis elegans model of Alzheimer's disease. J
Ethnopharmacol. 285(114850)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhi D, Yang W, Yue J, Xu S, Ma W, Zhao C,
Wang X and Wang D: HSF-1 mediated combined ginsenosides
ameliorating Alzheimer's disease like symptoms in Caernorhabditis
elegans. Nutr Neurosci. 25:2136–2148. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Anand R, Gill KD and Mahdi AA:
Therapeutics of Alzheimer's disease: Past, present and future.
Neuropharmacology. 76 Pt A:27–50. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sangha JS, Sun X, Wally OS, Zhang K, Ji X,
Wang Z, Wang Y, Zidichouski J, Prithiviraj B and Zhang J: Liuwei
Dihuang (LWDH), a traditional Chinese medicinal formula, protects
against β-amyloid toxicity in transgenic Caenorhabditis elegans.
PLoS One. 7(e43990)2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Guan S, Liu C, Chen X, Zhu Y, Xie Y,
Wang J, Ji X, Li L, Li Z, et al: Neuroprotective effects of Coptis
Chinensis Franch polysaccharide on amyloid-beta (Aβ)-induced
toxicity in a transgenic Caenorhabditis elegans model of
Alzheimer's disease (AD). Int J Biol Macromol. 113:991–995.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ding Y, Bao X, Lao L, Ling Y, Wang Q and
Xu S: p-Hydroxybenzyl alcohol prevents memory deficits by
increasing neurotrophic factors and decreasing inflammatory factors
in a mice model of Alzheimer's disease. J Alzheimers Dis.
67:1007–1019. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He F, Duan X, Dai R, Wang W, Yang C and
Lin Q: Protective effects of ethyl acetate extraction from
Gastrodia elata blume on blood-brain barrier in focal cerebral
ischemia reperfusion. Afr J Tradit Complement Altern Med.
13:199–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang Q, Zhang C, Qu S, Dong S, Ma Q, Hao
Y, Liu Z, Wang S, Zhao H and Shi Y: Chinese herbal extracts exert
neuroprotective effect in alzheimer's disease mouse through the
dopaminergic synapse/apoptosis signaling pathway. Front Pharmacol.
13(817213)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Heese K: Gastrodia elata Blume (Tianma):
Hope for brain aging and dementia. Evid Based Complement Alternat
Med. 2020(8870148)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mishra M, Huang J, Lee YY, Chua DSK, Lin
X, Hu JM and Heese K: Gastrodia elata modulates amyloid precursor
protein cleavage and cognitive functions in mice. Biosci Trends.
5:129–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen XY, Liao DC, Sun ML, Cui XH and Wang
HB: Essential oil of acorus tatarinowii schott ameliorates
Aβ-induced toxicity in caenorhabditis elegans through an autophagy
pathway. Oxid Med Cell Longev. 2020(3515609)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lublin AL and Link CD: Alzheimer's disease
drug discovery: In vivo screening using Caenorhabditis elegans as a
model for β-amyloid peptide-induced toxicity. Drug Discov Today
Technol. 10:e115–e119. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dai R, Wang T, Si X, Jia Y, Wang L, Yuan
Y, Lin Q and Yang C: Vasodilatory effects and underlying mechanisms
of the ethyl acetate extracts from Gastrodia elata. Can J Physiol
Pharmacol. 95:564–571. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Romero-Márquez JM, Navarro-Hortal MD,
Jiménez-Trigo V, Vera-Ramírez L, Forbes-Hernández TJ, Esteban-Muñoz
A, Giampieri F, Bullón P, Battino M, Sánchez-González C and Quiles
JL: An oleuropein rich-olive (Olea europaea L.) leaf extract
reduces β-amyloid and tau proteotoxicity through regulation of
oxidative- and heat shock-stress responses in Caenorhabditis
elegans. Food Chem Toxicol. 162(112914)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Navarro-Hortal MD, Romero-Márquez JM,
Esteban-Muñoz A, Sánchez-González C, Rivas-García L, Llopis J,
Cianciosi D, Giampieri F, Sumalla-Cano S, Battino M and Quiles JL:
Strawberry (Fragaria × ananassa cv. Romina) methanolic extract
attenuates Alzheimer's beta amyloid production and oxidative stress
by SKN-1/NRF and DAF-16/FOXO mediated mechanisms in C. elegans.
Food Chem. 372(131272)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Romero-Márquez JM, Navarro-Hortal MD,
Jiménez-Trigo V, Muñoz-Ollero P, Forbes-Hernández TY, Esteban-Muñoz
A, Giampieri F, Noya ID, Bullón P, Vera-Ramírez L, et al: An
olive-derived extract 20% Rich in hydroxytyrosol prevents β-amyloid
aggregation and oxidative stress, two features of Alzheimer
disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans.
Antioxidants (Basel). 11(629)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qadir A, Aqil M, Ali A, Ahmad FJ, Ahmad S,
Arif M and Khan N: GC-MS analysis of the methanolic extracts of
Smilax China and Salix alba and their antioxidant activity. Turk J
Chem. 44:352–363. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
DanQing L, YuJie G, ChengPeng Z, HongZhi
D, Yi H, BiSheng H and Yan C: N-butanol extract of Hedyotis diffusa
protects transgenic Caenorhabditis elegans from Aβ-induced
toxicity. Phytother Res. 35:1048–1061. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Meng F, Li J, Rao Y, Wang W and Fu Y:
Gengnianchun extends the lifespan of caenorhabditis elegans via the
Insulin/IGF-1 signalling pathway. Oxid Med Cell Longev.
2018(4740739)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dilberger B, Baumanns S, Schmitt F,
Schmiedl T, Hardt M, Wenzel U and Eckert GP: Mitochondrial
oxidative stress impairs energy metabolism and reduces stress
resistance and longevity of C. elegans. Oxid Med Cell Longev.
2019(6840540)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhi D, Wang D, Yang W, Duan Z, Zhu S, Dong
J, Wang N, Wang N, Fei D, Zhang Z, et al: Dianxianning improved
amyloid β-induced pathological characteristics partially through
DAF-2/DAF-16 insulin like pathway in transgenic C. elegans. Sci
Rep. 7(11408)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang X, Yi K and Zhao Y: Fucoidan inhibits
amyloid-β-induced toxicity in transgenic Caenorhabditis elegans by
reducing the accumulation of amyloid-β and decreasing the
production of reactive oxygen species. Food Funct. 9:552–560.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ito K and Murphy D: Application of ggplot2
to pharmacometric graphics. CPT Pharmacometrics Syst Pharmacol.
2(e79)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Markesbery WR: Oxidative stress hypothesis
in Alzheimer's disease. Free Radic Biol Med. 23:134–147.
1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chiang PK, Lam MA and Luo Y: The many
faces of amyloid beta in Alzheimer's disease. Curr Mol Med.
8:580–584. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang GB, Zhao T, Muna SS, Jin HM, Park
JL, Jo KS, Lee BH, Chae SW, Kim SY, Park SH, et al: Therapeutic
potential of Gastrodia elata Blume for the treatment of Alzheimer's
disease. Neural Regen Res. 8:1061–1070. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Link CD: Expression of human beta-amyloid
peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci
USA. 92:9368–9372. 1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fay DS, Fluet A, Johnson CJ and Link CD:
In vivo aggregation of beta-amyloid peptide variants. J Neurochem.
71:1616–1625. 1998.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu S, Li H, Dong J, Yang W, Liu T, Wang
Y, Wang X, Wang M and Zhi D: Rose essential oil delayed Alzheimer's
disease-like symptoms by SKN-1 pathway in C. elegans. J Agric Food
Chem. 65:8855–8865. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pagano G, Rengo G, Pasqualetti G,
Femminella GD, Monzani F, Ferrara N and Tagliati M: Cholinesterase
inhibitors for Parkinson's disease: A systematic review and
meta-analysis. J Neurol Neurosurg Psychiatry. 86:767–773.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang Y, Li H, Jin S, Lu Y, Peng Y, Zhao L
and Wang X: Cannabidiol protects against Alzheimer's disease in C.
elegans via ROS scavenging activity of its phenolic hydroxyl
groups. Eur J Pharmacol. 919(174829)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Song E, Chung H, Shim E, Jeong JK, Han BK,
Choi HJ and Hwang J: Gastrodia elata blume extract modulates
antioxidant activity and ultraviolet a-irradiated skin aging in
human dermal fibroblast cells. J Med Food. 19:1057–1064.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu Y, Lu YY, Huang L, Shi L, Zheng ZY,
Chen JN, Qu Y, Xiao HT, Luo HR and Wu GS: Para-Hydroxybenzyl
alcohol delays the progression of neurodegenerative diseases in
models of caenorhabditis elegans through activating multiple
cellular protective pathways. Oxid Med Cell Longev.
2022(8986287)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xin L, Yamujala R, Wang Y, Wang H, Wu WH,
Lawton MA, Long C and Di R: Acetylcholineestarase-inhibiting
alkaloids from Lycoris radiata delay paralysis of amyloid
beta-expressing transgenic C. elegans CL4176. PLoS One.
8(e63874)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Takahashi A, Watanabe T, Fujita T,
Hasegawa T, Saito M and Suganuma M: Green tea aroma fraction
reduces β-amyloid peptide-induced toxicity in Caenorhabditis
elegans transfected with human β-amyloid minigene. Biosci
Biotechnol Biochem. 78:1206–1211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang J, Huang XB, Wan QL, Ding AJ, Yang
ZL, Qiu MH, Sun HY, Qi SH and Luo HR: Otophylloside B protects
against Aβ toxicity in caenorhabditis elegans models of Alzheimer's
disease. Nat Prod Bioprospect. 7:207–214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pandey S, Phulara SC, Mishra SK, Bajpai R,
Kumar A, Niranjan A, Lehri A, Upreti DK and Chauhan PS: Betula
utilis extract prolongs life expectancy, protects against amyloid-β
toxicity and reduces Alpha Synuclien in Caenorhabditis elegans via
DAF-16 and SKN-1. Comp Biochem Physiol Toxicol Pharmacol.
228(108647)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yin JA, Gao G, Liu XJ, Hao ZQ, Li K, Kang
XL, Li H, Shan YH, Hu WL, Li HP and Cai SQ: Genetic variation in
glia-neuron signalling modulates ageing rate. Nature. 551:198–203.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yue Y, Shen P, Chang AL, Qi W, Kim KH, Kim
D and Park Y: trans-Trismethoxy resveratrol decreased fat
accumulation dependent on fat-6 and fat-7 in Caenorhabditis
elegans. Food Funct. 10:4966–4974. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jia W, Su Q, Cheng Q, Peng Q, Qiao A, Luo
X, Zhang J and Wang Y: Neuroprotective effects of palmatine via the
enhancement of antioxidant defense and small heat shock protein
expression in Aβ-transgenic caenorhabditis elegans. Oxid Med Cell
Longe. 2021(9966223)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mendler M, Riedinger C, Schlotterer A,
Volk N, Fleming T, Herzig S, Nawroth PP and Morcos M: Reduction in
ins-7 gene expression in non-neuronal cells of high glucose exposed
Caenorhabditis elegans protects from reactive metabolites,
preserves neuronal structure and head motility, and prolongs
lifespan. J Diabetes Complications. 31:304–310. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Murphy CT, McCarroll SA, Bargmann CI,
Fraser A, Kamath RS, Ahringer J, Li H and Kenyon C: Genes that act
downstream of DAF-16 to influence the lifespan of Caenorhabditis
elegans. Nature. 424:277–283. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lin C, Zhang X, Zhuang C, Lin Y, Cao Y and
Chen Y: Healthspan improvements in caenorhabditis elegans with
traditional chinese herbal tea. Oxid Med Cell Longev.
2020(4057841)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yang ZZ, Yu YT, Lin HR, Liao DC, Cui XH
and Wang HB: Lonicera japonica extends lifespan and healthspan in
Caenorhabditis elegans. Free Radic Biol Med. 129:310–322.
2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Brunet A: Aging and the control of the
insulin-FOXO signaling pathway. Med Sci (Paris). 28:316–320.
2012.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
54
|
Liu T, Hua Z, Han P, Zhao Y, Zhou J, Jin
Y, Li X, Huang L and Yuan Y: Mycorrhizosphere bacteria, rahnella
sp. HPDA25, promotes the growth of armillaria gallica and its
parasitic host gastrodia elata. Front Microbiol.
13(842893)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hu Y, Li C and Shen W: Gastrodin
alleviates memory deficits and reduces neuropathology in a mouse
model of Alzheimer's disease. Neuropathology. 34:370–377.
2014.PubMed/NCBI View Article : Google Scholar
|