Introduction
In normal physiological conditions, chondrocytes
respond to degradative inflammatory cascades by upregulating the
biosynthesis of extracellular matrix (ECM) and secreting
anti-inflammatory cytokines, such as IL-4, IL-10 and IL-13(1). However, during the pathological
development of osteoarthritis, the excessive generation and
accumulation of inflammatory cytokines and MMPs interrupt the
balance, which lead to cartilage degradation and chondrocyte cell
death (1). Recent studies have
indicated that pyroptosis, a lytic and inflammatory form of
programmed cell death, is also involved in the development of
osteoarthritis (2-4).
The activation of caspase-1 induces the generation of
proinflammatory cytokines, such as IL-1β and IL-18, resulting in
pyroptosis (5).
Ginseng is a well-known herbal medicine in Asia.
Panax ginseng is the most commonly used type of ginseng and
has been applied to ameliorate rheumatoid arthritis and
osteoarthritis in Asian countries (6). Ginsenosides are the major active
compounds that can be found in ginseng (6). A number of ginsenosides have been
reported to exert potent anti-inflammatory effects and are
potential therapeutic agents for bone remodeling (7). In human osteoarthritis chondrocytes
and a rat model of anterior cruciate ligament transection,
Ginsenoside Rg1 reduced the expression of IL-1β-induced MMP13,
cyclooxygenase-2 (COX-2) and prostaglandin E2, whilst also
reversing the degradation of collagen II and aggrecan (8). Furthermore, ginsenoside Rb1 was
previously demonstrated to alleviate monoiodoacetate-induced
osteoarthritis by reducing cartilage degradation (9,10).
Mechanistically, ginsenoside Rb1 was reported to suppress MMP13
expression by downregulating the Notch signaling pathway (11). Additionally, ginsenoside Rb1 can
prevent chondrocyte apoptosis by reducing the production of
reactive oxygen species and activating the NF-κB signaling pathway
(12).
Ginsenoside compound K (GCK) is a secondary
ginsenoside that is bio-transformed from major ginsenosides, such
as ginsenoside Rb1, Rb2 and Rc (13). Compared with their parental forms,
GCK has higher bioavailability and increased water solubility
(14,15). A previous study confirmed the
potent antiarthritic and bone-protective effect of GCK (16). To facilitate the clinical
utilization of GCK, it is necessary to elucidate its molecular
mechanisms. The anti-inflammatory effects of GCK (17) and its parent forms (18) have been associated with their
anti-pyroptotic properties. Ginsenoside Rb1 can reduce the
pyroptosis of cardiomyocytes triggered by aconitine (19), whilst ginsenoside Rb2 can inhibit
adipocyte pyroptosis and improve insulin resistance (20).
Therefore, the present study aimed to explore the
potential effects of GCK on osteoarthritis and its regulatory
effects on the pyroptosis of chondrocytes.
Materials and methods
Ethics statement
All animal-related experiments were approved by the
Institutional Animal Care and Use Committee of Chinese People's
Liberation Army General Hospital [approval no. SCXK(JING)2019-0010;
Beijing, China]. In addition, it is confirmed that animals were
anesthetized and sacrificed using acceptable methods and
techniques.
Primary mouse chondrocytes (PMCs)
culture
PMCs were prepared and cultured following the
protocols described previously (21). Briefly, three 5-day-old neonatal
C57BL/6 mice that had been euthanized with CO2
inhalation (30% volume/min for at least 50 min) were purchased from
SPEF (Beijing) Biotechnology Co., Ltd. Subsequently, the knee
cartilage was isolated and digested using collagenase D solution at
0.5 mg/ml overnight at 37˚C (MilliporeSigma) to obtain PMCs. PMCs
were cultured in DMEM/F12 (HyClone; Cytiva) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 0.1 mg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) in a culture dish at a density of
8x103 cells/cm2 under standard conditions
(37˚C, 5% CO2). After achieving confluence by days 6-7
of culture, PMCs were harvested using 0.25% Trypsin-EDTA (Gibco;
Thermo Fisher Scientific, Inc.) and passaged. Passage two cells
were used for all experiments.
Cell viability assay and ELISA
PMCs were seeded into 96-well plates (5,000
cells/well) and then subjected to the selective treatments in
sequential order: i) GCK (Chengdu Zhibiao Pure Biotechnology Co.,
Ltd.) pre-treatment (10 or 50 µM) for 24 h; ii) TNF-α (20 ng/ml;
Beyotime Institute of Biotechnology) treatment for 12 h; and iii)
GCK post-treatment (10 or 50 µM) for 12 h. Cells that received DMSO
served as a negative control. Three sets of experiment groups were
designed: Treatment i alone; treatment ii alone; and treatment i,
ii and iii in combination. To check the influence of GCK on the
viability of PMCs, cells in 96-well plates (5,000 cells/well) were
cultured in the presence of different concentrations of GCK (0, 10,
20, 50, 100 and 150 µM) for 48 h. The cell viability of PMCs was
then measured using a Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.) following the manufacturer's
instructions. In brief, 10 µl of CCK-8 solution was added to each
well and incubated for 2 h. The absorbance at 450 nm was measured
using an ELISA reader (Hangzhou Lianke Biotechnology Co.,
Ltd.).
Commercial ELISA kits were used to determine the
concentration of IL-6 (cat. no. PI326; Beyotime Institute of
Biotechnology), MMP13 (cat. no. YB-MMP13-Mu; Shanghai Yubo
Biotechnology Co., Ltd.), A Disintegrin and Metalloproteinase with
Thrombospondin Motifs 5 (ADAMTS5) (cat. no. LS-F32114; LifeSpan
BioSciences, Inc.), MMP3 (cat. no. YB-MMP3-Mu; Shanghai Yubo
Biotechnology Co., Ltd.), aggrecan (cat. no. YB-AGC-Mu; Shanghai
Yubo Biotechnology Co., Ltd.) and collagen II (cat. no.
YB-PIINP-Mu; Shanghai Yubo Biotechnology Co., Ltd.) in the culture
supernatants after the aforementioned treatments, following the
manufacturer's protocols.
Immunofluorescence staining
PMCs (1x105) were cultured on glass
coverslips in 24-well plates and then, in sequential order, were
treated as follows: i) Pre-treated with GCK (50 µM) for 24 h; ii)
treated with TNF-α (20 ng/ml) for 12 h; and iii) post-treated with
GCK (50 µM) for another 12 h. Cells that received DMSO treatment
served as a negative control. Three sets of experiment groups were
designed: Treatment i alone; treatment ii alone; and treatment i,
ii and iii in combination. Following treatment, the cells were
fixed in 4% paraformaldehyde for 15 min at room temperature,
treated with 0.1% Triton X-100 and blocked using 3% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The coverslips were then incubated with anti-MMP13
(1:200; cat. no. 18165-1-AP; Wuhan Sanying Biotechnology) or
anti-collagen II (1:200; cat. no. ab34712; Abcam) at 4˚C overnight.
After washing three times with PBS (5 min/time) at room
temperature, the coverslips were incubated with a secondary
antibody (1:1,000; CoraLite488-conjugated anti-rabbit; cat. no.
SA00013-2; Wuhan Sanying Biotechnology) at room temperature for 2
h. The nucleus was stained using DAPI (300 nM; Thermo Fisher
Scientific, Inc.) for 5 min at room temperature in the dark.
For TUNEL staining, the fixation and Triton X-100
treatments were the same as aforementioned. A One Step TUNEL
Apoptosis Assay Kit (cat. no. C1086, Beyotime Institute of
Biotechnology) was used. TUNEL detection solution (50 µl) was added
to the sample and incubated at 37˚C in the dark for 60 min. After
washing two times with PBS (5 min/time) at room temperature, the
fluorescence was detected. TUNEL-positive cells in five random
views were quantified by manual counting. Fluorescent images were
captured using a confocal microscope under x20 or x40 magnification
(Leica Microsystems GmbH).
Animal studies
Pathogen-free WT C57BL/6J male mice (n=24;
2-month-old; body weight, 25-30 g) were obtained from SPEF
(Beijing) Biotechnology Co., Ltd. The mice were housed in a
temperature-controlled environment (temperature, 25±2˚C; relative
humidity, 45-60%) with a 12-h light/dark cycle and received food
and water ad libitum.
Before the experimental surgery, mice were
anesthetized with 250 mg/kg intraperitoneal tribromoethanol
(Avertin; Sigma-Aldrich; Merck KGaA). To prepare the 100% Avertin
stock solution, 10 g tribromoethanol was added into a centrifuge
tube with 10 ml tertiary amyl alcohol. The tube was then shaken in
hand until the tribromoethanol was completely dissolved. The
solution was then filtered using a 0.22-µm filter membrane. A
working solution of 2.5% Avertin was prepared by diluting the 100%
Avertin stock solution to 2.5% (1:40) with 0.9% NaCl. The working
solution was stored at 4˚C in the dark and used within 2 weeks.
Experimental osteoarthritis was induced in the mice
through transection of the medial meniscotibial ligament and medial
collateral ligament in the right knee [destabilization of the
medial meniscus (DMM) mice]. The left knees not subjected to
surgery in the DMM alone group and was used as the control (sham)
for both DMM and DMM + GCK groups. According to previous studies,
40 mg/kg GCK was sufficient to exert anti-inflammatory effects in
both rat and mouse models of induced arthritis (22,23).
The day after the surgery, the mice were fed for 8 weeks either
with a control diet (normal diet) or with diets supplemented with
GCK (40 mg/kg) (biological replicates, n=12 per group). Mice were
euthanized with CO2 inhalation (30% volume/min for at
least 10 min) after 8 weeks of surgery. Euthanasia using
CO2 was conducted following the AVMA Guidelines for the
Euthanasia of Animals (2020 edition). The mice were left in the
CO2 environment until the cessation of breathing and
heartbeat, when fully dilated pupils were observed. Knee joints
were then collected for histological analysis.
Immunohistochemistry (IHC), Safranin
O-Fast Green and H&E staining
IHC, Safranin O-Fast Green and H&E staining were
conducted in paraffin-embedded tissues using the BOND-III Automated
IHC Stainer (Leica Microsystems GmbH). Knee joints were fixed in
neutral buffered formalin at a concentration of 10%, at room
temperature for 16 h. Dehydration was performed in graded alcohols
(70, 95 and 100% ethanol). Next, the tissues were cleared in a
clearing agent (xylene substitute) to remove alcohol and prepare
them for infiltration with paraffin wax. The tissues were then
infiltrated with liquid paraffin wax at 56-60˚C for 6 h until they
became fully embedded. After that, the tissues were transferred
into fresh liquid paraffin wax and allowed to cool and solidify.
Sections (5 µm) were set in the Stainer. Antigens were retrieved by
heating the tissue sections in a BOND Epitope Retrieval ER2
Solution contained in the BOND IHC Polymer Detection Kit (cat. no.
DS9800) for 20 min at 100˚C. Tissues sections were then subjected
to peroxide blocking using the hydrogen peroxide included in the
BOND IHC Polymer Detection Kit (cat. no. DS9800) for 5 min. The
following primary antibodies were used: Anti-collagen II (1:600;
cat. no. 28459-1-AP; Wuhan Sanying Biotechnology), anti-MMP13
(1:250; cat. no. 18165-1-AP; Wuhan Sanying Biotechnology), anti-NLR
family pyrin domain-containing 3 (anti-NLRP3; 1:200; cat. no.
19771-1-AP; Wuhan Sanying Biotechnology) and anti-Gasdermin D-N
terminal (anti-GSDMD-NT; 1:800; cat. no. 36425; Cell Signaling
Technology, Inc.). The tissue sections were incubated with the
diluted primary antibodies for 30 min at room temperature and then
washed three times with BOND Wash Solution (2 min/time). Next, the
sections were incubated with an HRP-conjugated secondary antibody
(1:2,000) contained in the BOND IHC Polymer Detection Kit (cat. no.
DS9800) for 10 min at room temperature. Chromogenic detection was
conducted by incubating tissue sections with DAB for 10 min.
Counterstaining was conducted by incubating the tissue sections
with hematoxylin for 5 min at room temperature. DAB and hematoxylin
were contained in the BOND IHC Polymer Detection Kit (cat. no.
DS9800). The IHC staining was quantified using a scoring system
described previously (24). The
percentage of positive cells was scored as follows: i) Score of 1,
≤24%; ii) score of 2, 25-50%; iii) score of 3, 51-75%; and iv)
score of 4, ≥76%. The intensity of IHC staining was scored as
follows: i) Score of 0, negative; ii) score of 1, weak; iii) score
of 2, moderate; and (iv) score of 3, strong. The total score was
calculated as follows: Total score = positive percentage score x
intensity score.
For Safranin O-Fast Green and H&E staining, the
Safranin O-Fast Green staining kit (cat. no. PH1852; Phygene) and
the H&E staining kit (cat. no. PH0516; Phygene) were loaded
into BOND-III Automated IHC Stainer. For Safranin O-Fast Green
staining, after deparaffinization and rehydration as
aforementioned, the tissue sections were incubated with Safranin O
solution for 10 min at the room temperature. Next, the sections
were washed with 70% ethanol for 30 sec. The sections were then
incubated with Fast Green solution for 5 min. After the staining,
the sections were washed with graded alcohols (80, 95 and 100%) for
30 sec each. Finally, the sections were washed with xylene (2X) for
1 min. For H&E saining, after deparaffinization and
rehydration, the tissue sections were incubated hematoxylin
solution for 10 min at the room temperature and then washed with
bluing reagent for 5 min. Next, the sections were incubated with
eosin solution for 5 min. Washing with graded alcohols and with
xylene was as aforementioned. The staining images were captured
using a DM4000 B LED microscope (Leica Microsystems, Inc.) at x20
or x40 magnification.
Western blotting
Conventional western blotting was performed as
previously described (25).
Briefly, total proteins were extracted from the PMCs using RIPA
lysis buffer (Beyotime Institute of Biotechnology) and protein
concentration was determined using a BCA kit (cat. no. P0012;
Beyotime Institute of Biotechnology). The proteins (25 µg/lane)
were separated on 10% gels using SDS-PAGE, transferred onto
nitrocellulose membranes, blocked using 5% BSA (Gibco; Thermo
Fisher Scientific, Inc.) in TBST (0.1% Tween-20) at 37˚C for 30 min
and then incubated with primary antibodies at 4˚C overnight. The
following antibodies were used: Anti-NLRP3 (1:1,000; cat. no.
19771-1-AP; Wuhan Sanying Biotechnology), anti-GSDMD-NT (1:1,000;
cat. no. 36425; Cell Signaling Technology, Inc.), anti-cleaved
caspase-1 (1:1,000; cat. no. 89332; Cell Signaling Technology,
Inc.), anti-mature IL-1β (1:1,000; cat. no. 83186; Cell Signaling
Technology, Inc.) and anti-β-actin (1:2,000; cat. no. 20536-1-AP;
Wuhan Sanying Biotechnology). The membranes were washed with 1X
TBST three times (5 min/time) and incubated with HRP-conjugated
secondary antibodies (1:5,000; cat. no. SA00001-2; Wuhan Sanying
Biotechnology) at room temperature for 1 h. The protein bands were
visualized using an enhanced chemiluminescence kit (BeyoECL Star;
Beyotime Institute of Biotechnology) and ChemiScope 6200T imager
(Clinx Science Instruments Co., Ltd.). The intensities of protein
bands were quantitated using ImageJ (v1.5.4; National Institutes of
Health) based on three biological repeats.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was conducted using GraphPad Prism 8.10 (GraphPad
Software; Dotmatics). The Wilcoxon's signed-rank test was performed
for Sham vs. DMM comparisons due to the paired nature of these two
groups, whilst the Wilcoxon's rank-sum test was performed for DMM
vs. DMM + GCK comparisons due to the unpaired nature of these two
groups. Bonferroni's correction was conducted on all P-values
yielded by these two aforementioned tests. Since two tests were
performed within each group, P<0.025 was considered to indicate
a statistically significant difference in these two cases. Either
Kruskal-Wallis test followed by Dunn's test (staining scores) or
one-way ANOVA followed by Tukey's post hoc test (numerical data)
was used for the rest of the multiple comparisons in Figs. 1 and 3. P<0.05 was considered to indicate a
statistically significant difference.
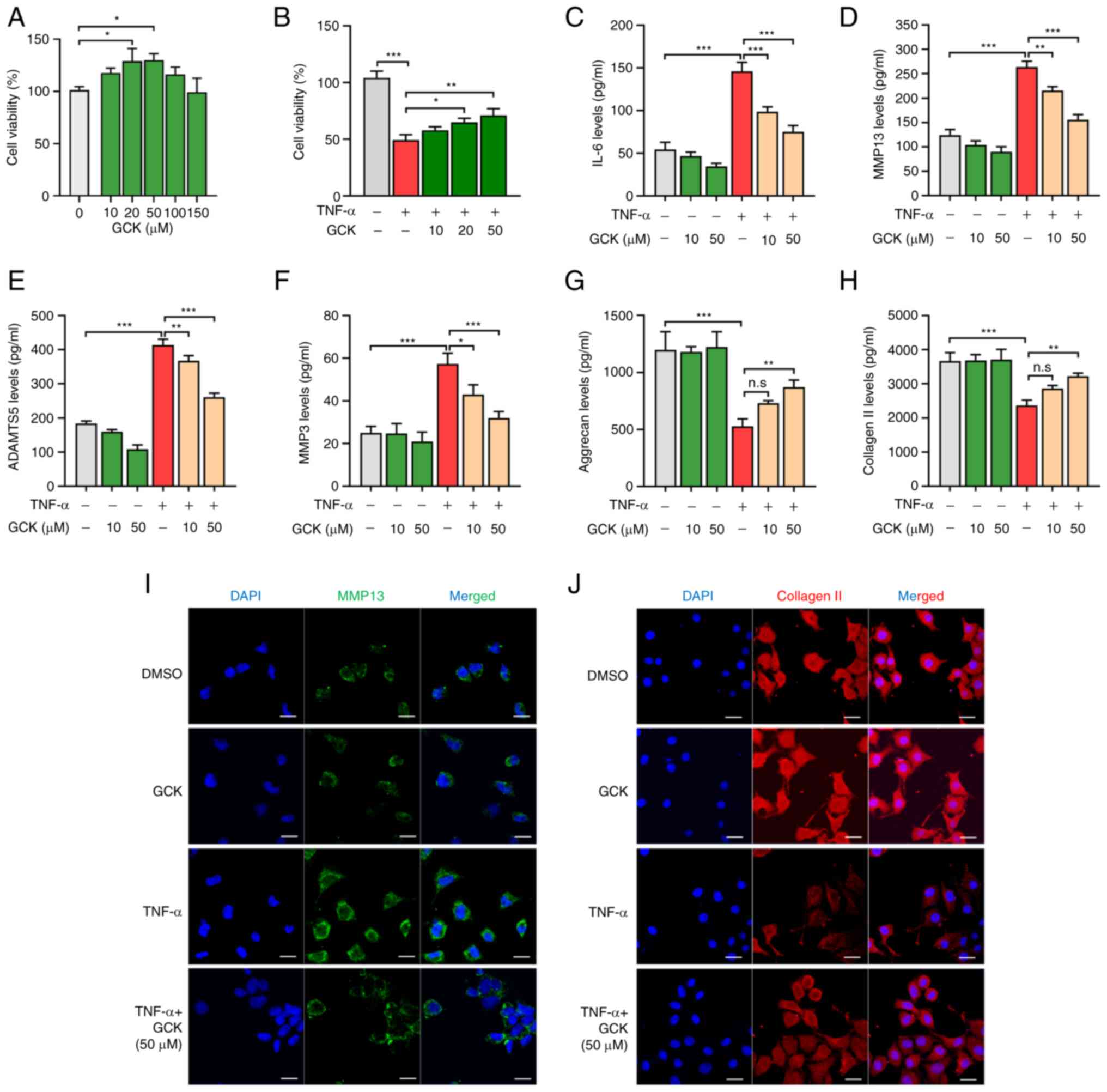 | Figure 1GCK reduces TNF-α-induced imbalance
of extracellular matrix homeostasis in chondrocytes in
vitro. (A and B) Cell viability of normal primary mouse
chondrocytes after treatment with different concentrations of GCK
(A) alone or (B) in combination with TNF-α treatment (20 ng/ml) for
48 h. Effects of TNF-α and GCK on (C) IL-6, (D) MMP13, (E) ADAMTS5,
(F) MMP3, (G) aggrecan and (H) Collagen II production in
chondrocytes after the indicated treatments. The protein
concentrations were determined using ELISA (n=3).
Immunofluorescence staining of (I) MMP13 (green) and (J) collagen
II and the nucleus (DAPI; blue) in chondrocytes following the
indicated treatments. The data are presented as the mean ± SD
(n=3). Scale bar, 20 µm. *P<0.05,
**P<0.01 and ***P<0.001. GCK,
ginsenoside compound K; ADAMTS5, A Disintegrin and
Metalloproteinase with Thrombospondin Motifs 5; Col II, collagen
II; n.s, not significant. |
Results
GCK reduces the TNF-α-induced
imbalance of ECM homeostasis in chondrocytes in vitro
To check the influence of GCK on the viability of
PMCs, cells were cultured in the presence of different
concentrations of GCK (0, 10, 20, 50, 100 and 150 µM) for 48 h.
CCK-8 assay results revealed that GCK conferred no cytotoxicity
towards PMCs, even at the concentration of 150 µM (Fig. 1A). Furthermore, concentrations of
20 and 50 µM GCK significantly increased cell viability compared
with that in the control cells (Fig.
1A). A total of 10 and 50 GCK µM were used in the following
studies to assess whether GCK had dose-dependent effects on
PMCs.
In addition, the viability of PMCs was found to be
significantly reduced by TNF-α treatment. However, co-treatment
with GCK (20 and 50 µM) significantly restored their cell viability
(Fig. 1B). To assess the influence
of GCK on TNF-α-induced inflammation and ECM dysregulation, the
secretion of ECM regulatory factors were measured using ELISA. The
results indicated that TNF-α induced a significant increase in
IL-6, MMP13, ADAMTS5 and MMP3 levels (Fig. 1C-F), whilst significantly
decreasing aggrecan and collagen II levels (Fig. 1G and H). GCK appeared to have reversed these
alterations in a dose-dependent manner (Fig. 1C-H). Immunofluorescence staining
also revealed that GCK partially suppressed the MMP13 expression
that was induced by TNF-α whilst rescuing the collagen II
expression that was reduced by TNF-α in PMCs (Fig. 1I and J).
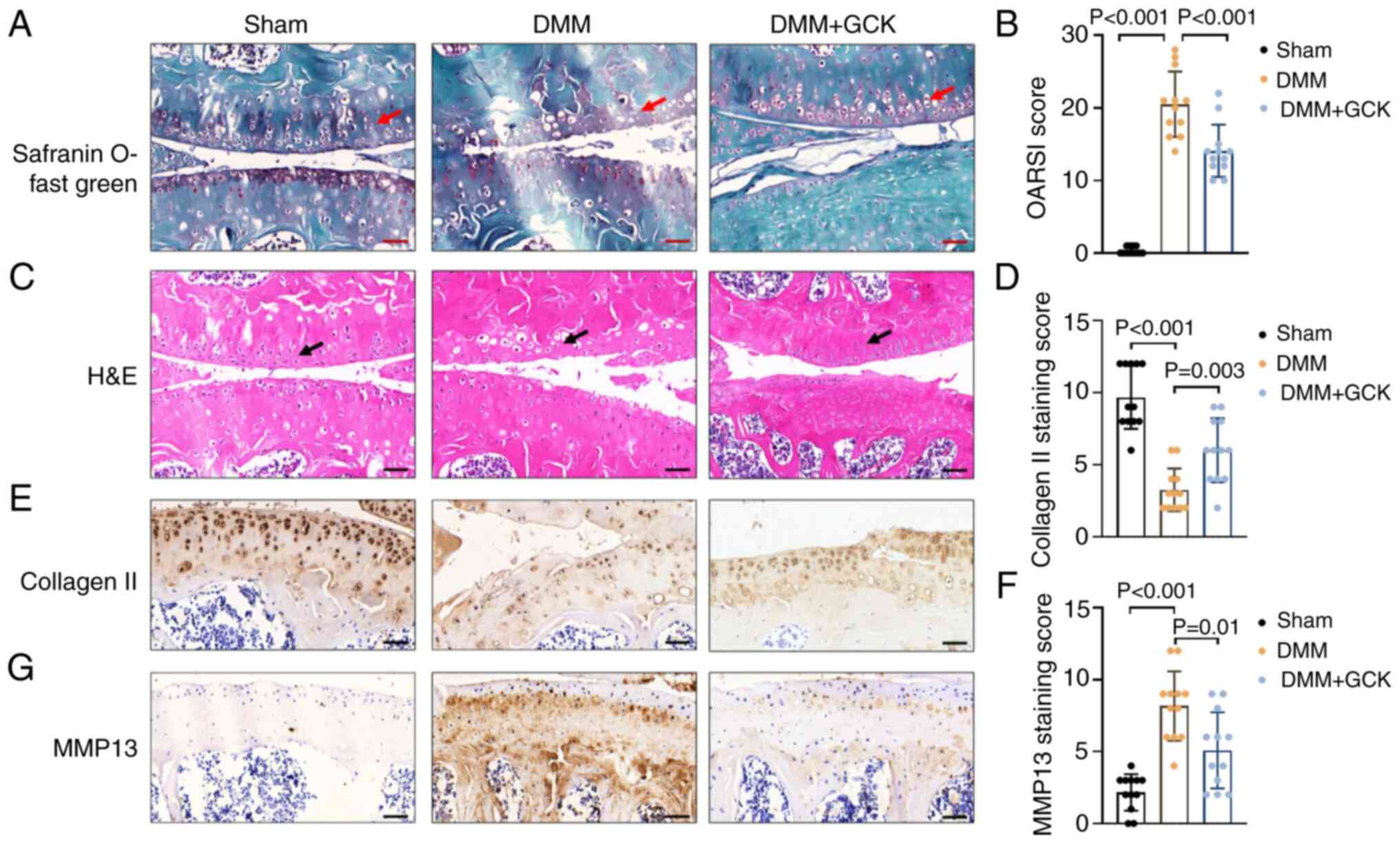
GCK reduces cartilage degradation in
vivo in a surgically-induced model of osteoarthritis
To explore the potential effects of GCK on
osteoarthritis in vivo, an osteoarthritis model was
established in mice by DMM surgery, without significant adverse
effects among the animals, such as significant loss of body weight
and consistent bleeding. Knee joints were collected,
paraffin-embedded, sectioned and stained. Safranin O-Fast Green and
H&E staining revealed that DMM surgery resulted in cartilage
erosion in the femur and tibia, loss of the superficial zone and
reduced uncalcified cartilage (red and black arrows; .. 2A and C).
However, these pathological changes were mitigated by GCK
supplementation (Fig. 2A and
C).
To quantify the aforementioned changes, the sections
were scored using the Osteoarthritis Research Society International
(OARSI) semi-quantitative grading system, which assesses the lesion
severity and the affected area in both the femur and tibia
(26). The DMM group had a
significantly increased OARSI score compared with that in the sham
group (Fig. 2B). By contrast, the
DMM mice treated with GCK had a significantly lower OARSI score
compared with that in the DMM-only group (Fig. 2B). Protein expression was then
assessed using immunohistochemistry. The mice treated with GCK
exhibited significantly increased collagen II (Fig. 2E and D) and significantly decreased MMP13
expression (Fig. 2G and F) compared with that in the DMM-only
group.
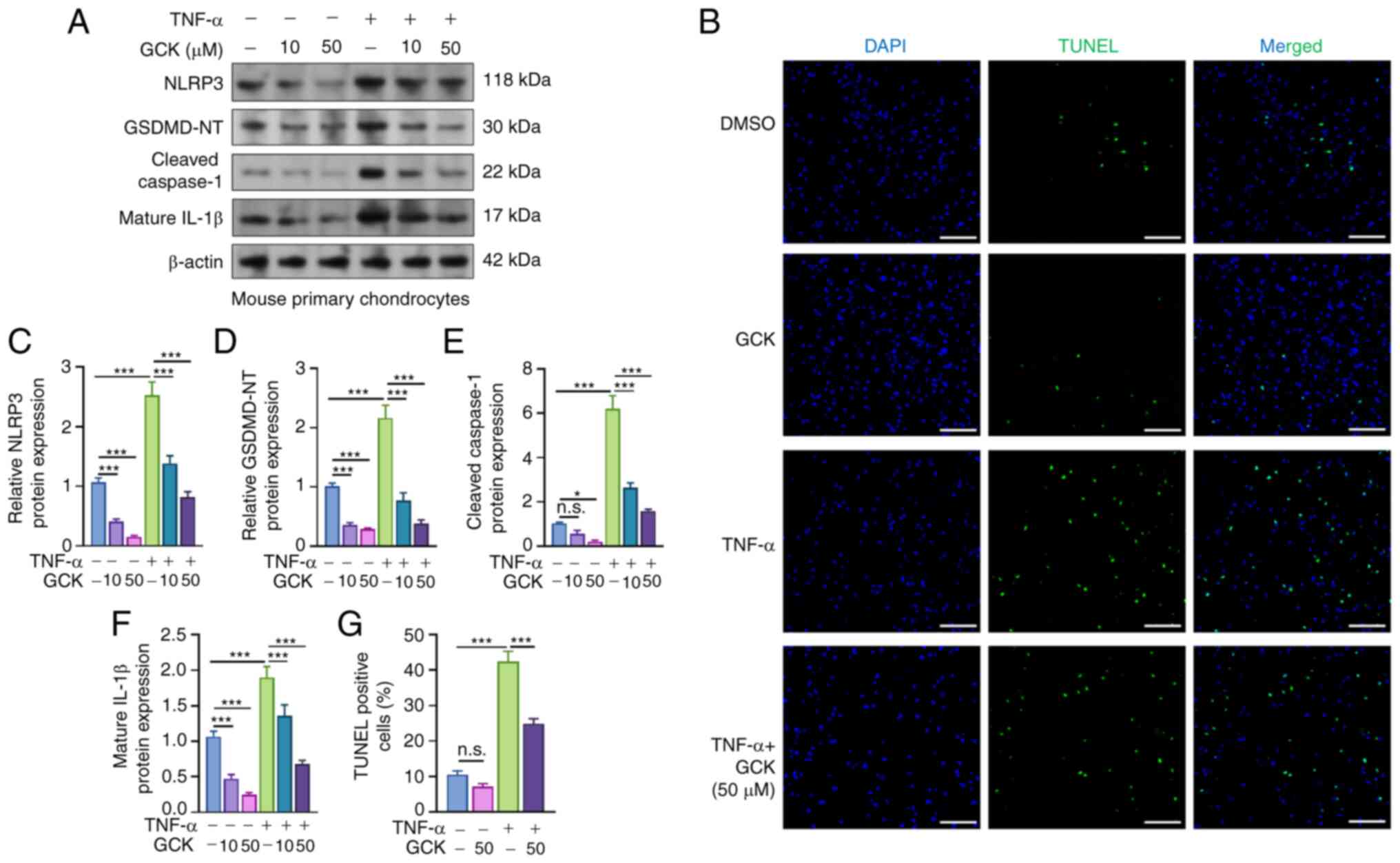
GCK suppresses
osteoarthritis-associated NLRP3 inflammasome activation and
pyroptosis
In PMCs, TNF-α treatment stimulated a significant
increase in the levels of NLRP3, GSDMD-NT, cleaved caspase-1 and
mature IL-1β, the markers indicating the occurrence of pyroptosis
(5) (Fig. 3A and C-F). However, GCK treatment suppressed
the increase of these proteins in a dose-dependent manner (Fig. 3A and C-F). TUNEL assay was then used to examine
the extent of cell death of PMCs. The TNF-α treatment group had a
significantly higher level of TUNEL-positive cells compared with
that in the DMSO group (Fig. 3B
and G). However, GCK treatment
decreased the percentage of TUNEL-positive PMCs induced by TNF-α
(Fig. 3B and G).
GCK suppresses
osteoarthritis-associated pyroptosis in vivo
IHC analysis of the knee joint tissue sections from
DMM mice with or without GCK treatment confirmed that NLRP3 and
GSDMD-NT expression was significantly increased in the cartilage of
the DMM mice compared with that in the sham control group (Fig. 4A-C). Compared with that in the DMM
mice without GCK treatment, the DMM mice that received GCK
treatment exhibited significantly reduced NLRP3 and GSDMD-NT
expression (Fig. 4A-C).
Discussion
During the pathological development of
osteoarthritis, the expression of inflammatory and catabolic
factors is upregulated. Among these factors, IL-1β and TNF-α serve
critical roles and can induce the expression of cartilage-degrading
enzymes, such as MMP1, MMP3, MMP13, ADAMTS4 and ADAMTS5 (2,27).
These factors can all contribute to the degradation of aggrecans
and type II collagen, leading to cartilage matrix damage (2). Furthermore, IL-1β and TNF-α can
trigger inflammatory-associated chondrocyte cell death (2,27).
Pyroptosis has recently been characterized as an
important component in osteoarthritis (28). Although it remains unclear whether
pyroptosis serves as a cause or the result of cartilage
degeneration, osteoarthritis-related risk factors, such as
cholesterol, oxidized low-density lipoprotein and
lipopolysaccharide, have been reported to initiate pyroptosis
(29,30). Osteoarthritis-related risk factors
mainly trigger chondrocyte pyroptosis through the NLRP3
inflammasome pathway to cause the upregulation of IL-1β and TNF-α
(31,32). Therefore, the initiation of
chondrocyte pyroptosis can disrupt the balance between the
anabolism and catabolism of the chondrocyte ECM, resulting in ECM
degradation (28). Inhibiting
chondrocyte pyroptosis may prove to be a viable strategy to slow
the progression of osteoarthritis (3,28).
The anti-inflammatory properties of GCK have been
characterized in previous studies (14,16,33).
GCK has been found to reduce the synthesis of proinflammatory
cytokines IL-6, IL-1β, TNF-α, COX-2 and inducible nitric oxide
synthase (14). Furthermore, GCK
has been observed to exert anti-inflammatory and bone-protective
effects in rheumatoid arthritis by inhibiting the production of
MMP1 and MMP3 whilst suppressing the JNK and ERK pathways (16). GCK can also inhibit TNF receptor 2
expression to weaken TNF-α downstream signaling (16). In addition, GCK has been reported
to promote the osteogenic differentiation of rat bone marrow
mesenchymal stem cells by activating the Wnt/β-catenin signaling
pathway (34). In the present
study, it was demonstrated that GCK could alleviate the
TNF-α-induced imbalance of ECM homeostasis in PMCs in vitro,
in addition to reducing cartilage degradation in vivo in a
surgery-induced model of osteoarthritis. These results support the
presence of chondrocyte protective effects of GCK.
Although the parental forms of GCK have demonstrated
anti-pyroptotic effects in various human cells, such as human
induced pluripotent stem cell-derived cardiomyocytes and adipocytes
(19,20), whether GCK can exert
anti-pyroptotic effects in chondrocytes remain unclear. In high-fat
diet/streptozotocin-induced diabetic mice, GCK has been documented
to protect against diabetic nephropathy by suppressing NLRP3
inflammasome activation and the NF-κB/p38 signaling pathway
(18). Using PMCs, the present
study demonstrated that GCK suppressed osteoarthritis-associated
NLRP3 inflammasome activation and pyroptosis. IHC staining of the
knee joint tissue sections from DMM mice found that GCK attenuated
the NLRP3 and GSDMD-NT expression that was induced by the DMM
surgery. These findings suggest that GCK can alleviate
osteoarthritis by inhibiting NLRP3-mediated pyroptosis in
chondrocytes (Fig. 4D).
The present study has a number of limitations. Due
to the absence of detection devices, whether GCK could alleviate
the severe joint pain associated with osteoarthritis was not
assessed. In addition, GCK as a natural product may have a series
of docking proteins, which was not explored in the present study.
Future studies are needed to resolve these issues.
In conclusion, the present study revealed that GCK
could reduce cartilage degradation in a mouse model of
osteoarthritis by inhibiting NLRP3-inflammasome activation and
subsequent pyroptosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Department of
Orthopedics (The Affiliated Hospital of Southwest Medical
University, China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL was involved in study conceptualization, data
curation and formal analysis, investigation, developing the
methodology, providing resources and software analysis. JW was
involved in developing the methodology, providing resources,
software analysis, data validation and visualization. NZ performed
the conceptualization of the study, project administration,
supervision, data validation and writing of the manuscript. YL and
JW confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal-related experiments were approved by the
Institutional Animal Care and Use Committee of Chinese People's
Liberation Army General Hospital [Beijing, China; approval no.
SCXK(JING)2019-0010].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coryell PR, Diekman BO and Loeser RF:
Mechanisms and therapeutic implications of cellular senescence in
osteoarthritis. Nat Rev Rheumatol. 17:47–57. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
An S, Hu H, Li Y and Hu Y: Pyroptosis
plays a role in osteoarthritis. Aging Dis. 11:1146–1157.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang J, Hu S, Bian Y, Yao J, Wang D, Liu
X, Guo Z, Zhang S and Peng L: Targeting cell death: Pyroptosis,
ferroptosis, apoptosis and necroptosis in osteoarthritis. Front
Cell Dev Biol. 9(789948)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu Y, Zhang J, Yu S, Li Y, Zhu J, Zhang K
and Zhang R: Cell pyroptosis in health and inflammatory diseases.
Cell Death Discov. 8(191)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miao EA, Leaf IA, Treuting PM, Mao DP,
Dors M, Sarkar A, Warren SE, Wewers MD and Aderem A:
Caspase-1-induced pyroptosis is an innate immune effector mechanism
against intracellular bacteria. Nat Immunol. 11:1136–1142.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Yi YS: Ameliorative effects of ginseng and
ginsenosides on rheumatic diseases. J Ginseng Res. 43:335–341.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang N, Liu D, Zhang X, Li J, Wang M, Xu T
and Liu Z: Effects of ginsenosides on bone remodelling for novel
drug applications: A review. Chin Med. 15(42)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheng W, Jing J, Wang Z, Wu D and Huang Y:
Chondroprotective effects of ginsenoside Rg1 in human
osteoarthritis chondrocytes and a rat model of anterior cruciate
ligament transection. Nutrients. 9(263)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aravinthan A, Hossain MA, Kim B, Kang CW,
Kim NS, Hwang KC and Kim JH: Ginsenoside Rb1 inhibits
monoiodoacetate-induced osteoarthritis in postmenopausal rats
through prevention of cartilage degradation. J Ginseng Res.
45:287–294. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Luan J, Che G, Man G and Xiao F:
Ginsenoside Rb1 from Panax ginseng attenuates
monoiodoacetate-induced osteoarthritis by inhibiting
miR-21-5p/FGF18-mediated inflammation. J Food Biochem.
46(e14340)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang W, Zeng L, Wang ZM, Zhang S, Rong XF
and Li RH: Ginsenoside Rb1 inhibits matrix metalloproteinase 13
through down-regulating Notch signaling pathway in osteoarthritis.
Exp Biol Med (Maywood). 240:1614–1621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hossain MA, Alam MJ, Kim B, Kang CW and
Kim JH: Ginsenoside-Rb1 prevents bone cartilage destruction through
down-regulation of p-Akt, p-P38, and p-P65 signaling in rabbit.
Phytomedicine. 100(154039)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang R, Huang XM, Yan HJ, Liu XY, Zhou Q,
Luo ZY, Tan XN and Zhang BL: Highly selective production of
compound K from Ginsenoside Rd by hydrolyzing glucose at C-3
glycoside using beta-Glucosidase of Bifidobacterium breve ATCC
15700. J Microbiol Biotechnol. 29:410–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sharma A and Lee HJ: Ginsenoside compound
K: Insights into recent studies on pharmacokinetics and
health-promoting activities. Biomolecules. 10(1028)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Baik IH, Kim KH and Lee KA: Antioxidant,
anti-inflammatory and antithrombotic effects of ginsenoside
compound K enriched extract derived from ginseng sprouts.
Molecules. 26(4102)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang M, Xie X, Yang Y and Li F:
Ginsenoside compound K-a potential drug for rheumatoid arthritis.
Pharmacol Res. 166(105498)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Song W, Wei L, Du Y, Wang Y and Jiang S:
Protective effect of ginsenoside metabolite compound K against
diabetic nephropathy by inhibiting NLRP3 inflammasome activation
and NF-κB/p38 signaling pathway in high-fat
diet/streptozotocin-induced diabetic mice. Int Immunopharmacol.
63:227–238. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yi YS: Roles of ginsenosides in
inflammasome activation. J Ginseng Res. 43:172–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang M, Wang R, Sun H, Sun G and Sun X:
Ginsenoside Rb1 ameliorates cardiotoxicity triggered by aconitine
via inhibiting calcium overload and pyroptosis. Phytomedicine.
83(153468)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin Y, Hu Y, Hu X, Yang L, Chen X, Li Q
and Gu X: Ginsenoside Rb2 improves insulin resistance by inhibiting
adipocyte pyroptosis. Adipocyte. 9:302–312. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gosset M, Berenbaum F, Thirion S and
Jacques C: Primary culture and phenotyping of murine chondrocytes.
Nat Protoc. 3:1253–1260. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen J, Wang Q, Wu H, Liu K, Wu Y, Chang Y
and Wei W: The ginsenoside metabolite compound K exerts its
anti-inflammatory activity by downregulating memory B cell in
adjuvant-induced arthritis. Pharm Biol. 54:1280–1288.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu KK, Wang QT, Yang SM, Chen JY, Wu HX
and Wei W: Ginsenoside compound K suppresses the abnormal
activation of T lymphocytes in mice with collagen-induced
arthritis. Acta Pharmacol Sin. 35:599–612. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu CY, Li L, Chen SL, Yang X, Zhang CZ and
Cao Y: A Zic2/Runx2/NOLC1 signaling axis mediates tumor growth and
metastasis in clear cell renal cell carcinoma. Cell Death Dis.
12(319)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Y, Chen S, Zhang X and Zhuo N: U2 small
nuclear RNA auxiliary factor 2, transcriptionally activated by the
transcription factor Dp-1/E2F transcription factor 1 complex,
enhances the growth and aerobic glycolysis of leiomyosarcoma cells.
Bioengineered. 13:10200–10212. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Moskowitz RW: Osteoarthritis cartilage
histopathology: Grading and staging. Osteoarthritis Cartilage.
14:1–2. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Haseeb A and Haqqi TM: Immunopathogenesis
of osteoarthritis. Clin Immunol. 146:185–196. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chang X, Kang Y, Yang Y, Chen Y and Shen
Y, Jiang C and Shen Y: Pyroptosis: A novel intervention target in
the progression of osteoarthritis. J Inflamm Res. 15:3859–3871.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang Z and Kraus VB: Does
lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev
Rheumatol. 12:123–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tall AR and Westerterp M: Inflammasomes,
neutrophil extracellular traps, and cholesterol. J Lipid Res.
60:721–727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Lin Z, Chen D and He Y: CY-09
attenuates the progression of osteoarthritis via inhibiting NLRP3
inflammasome-mediated pyroptosis. Biochem Biophys Res Commun.
553:119–125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang L, Ma S, Su H and Cheng J:
Isoliquiritigenin Inhibits IL-1β-Induced production of matrix
metalloproteinase in articular chondrocytes. Mol Ther Methods Clin
Dev. 9:153–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bai L, Gao J, Wei F, Zhao J, Wang D and
Wei J: Therapeutic potential of ginsenosides as an adjuvant
treatment for diabetes. Front Pharmacol. 9(423)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ding L, Gu S, Zhou B, Wang M, Zhang Y, Wu
S, Zou H, Zhao G, Gao Z and Xu L: Ginsenoside compound K enhances
fracture healing via promoting osteogenesis and angiogenesis. Front
Pharmacol. 13(855393)2022.PubMed/NCBI View Article : Google Scholar
|