Introduction
Intracranial aneurysm (IA) is a common
cerebrovascular disease, and its rupture may lead to massive
intracranial and subarachnoid hemorrhage (1). Therefore, patients who are at a high
risk of aneurysm rupture need to be diagnosed at an early stage in
order for this to be prevented or actively treated and to reduce
severe complications.
However, even with different diagnostic procedures
available for identifying and predicting the aneurysmal rupture
(2,3), the majority of these patients have no
evident clinical symptoms before the rupture occurs, and 16-65%
consequently develop ischemia (2,4-6).
At present, the mechanisms responsible for the
formation and rupture of IA are not yet clinically clear. Research
has indicated that microRNAs (miRNAs/miRs) play a key role in
processing the majority of proteins, can be identified in
biological fluids, and may be potential early biomarkers for
various cerebrovascular diseases (7,8).
It has been demonstrated that miRNAs are involved in
the development of IAs (9).
Although reports exist of protein biomarkers in IAs, including
tumor necrosis factor receptor (TNFR)-1 and S100B (10,11),
studies on circulating miRNAs as biomarkers for ruptured IAs are
limited.
The present meta-analysis aimed to identify the
circulating miRNA-126 (miR-126) in ruptured IAs and evaluate their
potential function as biomarkers for predicting aneurysmal
rupture.
Data and methods
Literature research strategy
The present study searched the comparative articles
involving circulating miR-126 levels and IAs through electronic
databases, including the Cochrane Library, MEDLINE (1980 to
February, 2023), PubMed (1980 to February, 2023) and Embase (1980
to February, 2023). The Preferred Reporting Items for Systematic
reviews and Meta-Analyses (PRISMA) guidelines were applied for
establishing the study protocol and design. The keywords ‘aneurysm
and genes’, ‘microRNA’ or ‘miRNA’ or ‘miR-126’, and ‘intracranial
aneurysm and miR-126’ were used in the MeSH list.
Selection of studies
Two of the authors (GF and VEG) independently
extracted data from the included articles, following the guidelines
for the epidemiology of meta-analysis. The following essential
information was obtained: The main authors, year of publication,
total case number in the IA rupture and non-rupture or/+ controls
(healthy individuals) groups, study type, outcome indicator, etc.
The extracted data were input into a designed, standardized table
according to the Cochrane Handbook. The flow of the study selection
process is depicted in Fig. 1.
When there is disagreement, another author with authority has the
final say.
Inclusion and exclusion criteria
If an article satisfied the following population,
intervention, comparison, outcomes and study (PICOS) design
criteria, it was considered for inclusion in the present
meta-analysis: i) Population: Limited to patients with IA rupture
and non-rupture or/+ controls (healthy individuals); ii)
intervention: The use of the expression levels of circulating
miR-126 at the IA rupture and non-rupture or/+ controls (healthy
individuals); iii) comparison: The expression levels of circulating
miR-126 were compared between patients with IA rupture and
non-rupture or/+ controls (healthy individuals); iv) The detailed
data of these articles are presented in Table I. To avoid publication bias, the
final aim was to collect a homogeneous pool of manuscripts,
including articles that compare only two modalities: The expression
levels of circulating miR-126 between patients with IA rupture and
non-rupture or/+ controls (healthy individuals).
 | Table IDesign and baseline characteristics
of the trials included in the present meta-analysis. |
Table I
Design and baseline characteristics
of the trials included in the present meta-analysis.
| | Sample size | Mean age (mean ±
SD) | No. of males | BMI
(kg/m2) >22 | Smoking | Location: Anterior
circulation | Location: Posterior
circulation | Size: <5 mm | Size: 5-10 mm | Size: >10
mm | miR-126 expression
>5 | |
|---|
| Author, year | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | Exp | Cont | (Refs.) |
|---|
| Yang et al,
2020 | 79 | 23 | 53.6±4.9 | 52.8±4 | 30 | 11 | 39 | 5 | 43 | 7 | 58 | 12 | 39 | 17 | 23 | 4 | 17 | 2 | 80 | 23 | (16) |
| Wu et al,
2021 | 62 | 47 | 54.3±5.1 | 51.4±4.7 | 23 | 22 | NR | NR | 25 | 19 | 26 | 0 | 32 | 0 | 26 | 0 | 4 | 0 | 19 | 8 | (14) |
| Luo et al,
2022 | 85 | 83 | 50.3±3.9 | 50.3±3.9 | 45 | 42 | 42 | 40 | NR | NR | NR | NR | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 22 | (15) |
All prospective and retrospective studies that
evaluated at least one of the two modalities were included.
Editorials, reviews, case reports and articles focusing on the
pediatric population, unrelated outcomes, co-morbidities,
experimental techniques, or one of the two modalities from that
article pool were excluded. In addition, in the case of mixed or
unclear results, the data were included in either the IA rupture,
the non-rupture, or/+ controls (healthy individuals) group. In
addition, to determine the association with levels of circulating
miR-126 between patients with IA rupture and non-rupture or/+
controls (healthy individuals), information about the patient's age
was collected. A body mass index (BMI) >22 kg/m2,
smoking, aneurysm location (anterior or posterior circulation);
aneurysm size (<5, 5-10 and >10 mm); and the expression
levels of circulating miR-126 >5 were detected in different time
periods from 3 to 14 days. The expression levels of circulating
miR-126 reported by the included articles were assessed after the
IA rupture or in non-rupture or/+ controls (healthy individuals).
Additionally, to decrease the risk of bias in the included
articles, a quality assessment tool [the Newcastle-Ottawa Scale
(NOS)] was used (Table II)
(12).
 | Table IINewcastle-Ottawa Scale (NOS) quality
assessment of final article pool. |
Table II
Newcastle-Ottawa Scale (NOS) quality
assessment of final article pool.
| | Newcastle-Ottawa
Scale |
|---|
| Author, year | Study design | Selection | Comparability | Exposure | Total scores | (Refs.) |
|---|
| Yang et al,
2020 | Prosp | 3 | 3 | 3 | 9 | (16) |
| Wu et al,
2021 | Prosp | 3 | 3 | 3 | 9 | (14) |
| Luo et al,
2022 | Retro | 3 | 2 | 2 | 7 | (15) |
Procedure for determining circulating
miR-126 levels
As previously described (14-16),
plasma was selected at a range of time points (1, 3, 7 and 14 days
post-event) from each patient with IA rupture and from each patient
with non-rupture or/+ controls (healthy individuals) (fasting
state). All plasma samples were extracted from
ethylene-diamine-tetra-acetic acid (EDTA) tubes and centrifuged as
previously described (14-16).
The serum miR-126 levels were examined using reverse
transcription-quantitative PCR (RT-qPCR). Fasting venous blood (5
ml) was drawn from subjects [in the research group (RG)] on the
first day after admission and 1 week after surgery, and in [the
control group (CG)] during the morning physical examination], and
then centrifuged for 10 min at 1,500 x g and 4˚C. The supernatant
was obtained in the refrigerator at -80˚C for preservation. Total
RNA in serum (200 µl) was extracted using TRIzol reagent, and the
concentration and purity of the RNA solution were examined using a
Narodrop spectrophotometer. The OD260/OD280 was between 1.8 and
2.1. The total RNA was applied as a template, and cDNA was
synthesized by reverse transcription. The total reaction system of
RT-qPCR was 20 µl, including template cDNA (1 µl), Taq polymerase
(0.2 µl), forward primer and reverse primer (each 1 µl), 2X
SYBR-Green mix (1 µl), 20 mmol/l dNTPs (1 µl). Finally, the
RNase-free water was supplemented to 20 µl. The reaction conditions
were 95˚C for 2 min, 95˚C for 15 sec, 60˚C for 30 sec, and 70˚C for
10 sec, for a total of 40 cycles. The forward primer of miR-126 was
5'-ACACTCCAGCTGGGTCGTACCGTGAGTAAT-3', and the reverse primer was
5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCATTAT-3'. The forward
primer of internal reference gene U6 was 5'-CTCGCTTCGGCAGCACA-3',
and the reverse primer was 3'-AACGCTTCACGAATTTGCGT-5'. The results
were represented by the relative quantitative method and calculated
using the 2-ΔΔCt method. The RT-qPCR protocol does not
correspond to any analysis performed during the present study
(14-16).
The RT-qPCR protocol described herein is related to the included
articles which constitute the article pool of the present
meta-analysis. Thus, this protocol is described herein in order to
present the procedure for determining circulating miR-126 levels
used in the included articles.
Evaluation of the risk of bias
The Cochrane Collaboration tool was used to assess
the risk of bias and was used by two authors (GF and VEG) for each
study (13). The evaluation
included random sequence generation, allocation concealment, the
blinding of participants and assessors, the blinding of outcome
assessment, incomplete outcome data, selective reporting and other
biases. The assessment results were classified into three levels:
Low risk, high risk and unclear risk. A third author was designated
to arbitrate any disagreements.
Statistical analysis and assessment of
heterogeneity
All analyses were carried out using Review Manager
Software (RevMan), version 5.4. Heterogeneity across trials was
identified using I2 statistics; considering
I2 >50% as high heterogeneity, a meta-analysis was
conducted using a random-effect model according to the Cochrane
Handbook for Systematic Reviews of Interventions (version 5.1.0).
Otherwise, the fixed-effect model was performed. The continuous
outcomes were expressed as a weighted mean difference with 95%
confidence intervals (CIs). For discontinuous variables, odds
ratios (OR) with 95% CIs were applied for the assessment. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Included studies
In total, three articles (14-16)
fulfilled the eligibility requirements. The total number of
patients was 379 [226 with IA rupture and 153 with non-rupture or/+
controls (healthy individuals)]. The study sample was based on
three studies (Table I). Of these
three studies, two were retrospective and one was prospective.
Epidemiological and clinical
features
The mean age of the patients was 52.1 (52.7 years
for the IA rupture sample and 51.5 years for the non-rupture or/+
controls (healthy individuals sample). The male-to-female ratio was
1:1.9 (Table I).
Age
Information regarding age was available in three
articles (14-16).
No significant difference in age was observed between the patients
with IA rupture and the non-rupture or/+ controls (healthy
individuals) (OR, 1.14; 95% CI, -0.60 to 2.88; and P=0.20), but
with heterogeneity (P=0.03 and I2=70%) (Fig. 2A). For testing the sensitivity, the
‘leave out one’ model was used and one study was removed at a time
(Table III). No heterogeneity
(P=0.49 and I2=0%) was achieved only after removing the
article by Wu et al (14);
again, no statistically significant difference was found (OR, 0.21;
95% CI, -0.80 to 1.22; P=0.68) (Fig.
2B). When examining the funnel plot of the same parameter, it
was found that the study results without the study by Wu et
al (14) displayed better
dispersion, with a low publication bias (Fig. 2C and D).
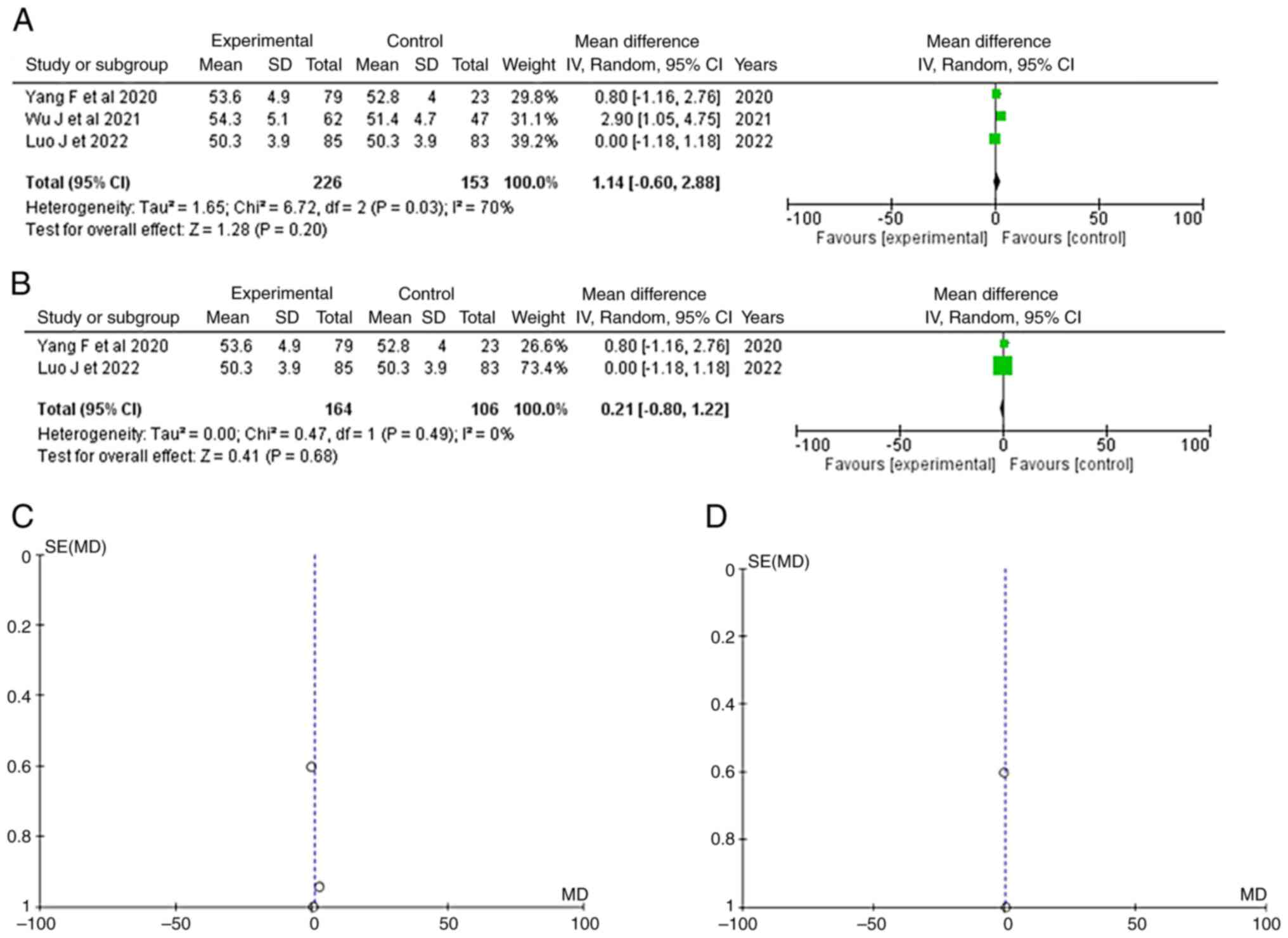 | Figure 2(A) Forest plot for age: The results
demonstrate no statistically significant difference groups (OR,
1.14; 95% CI, -0.60 to 2.88; P=0.20). (B) Forest plot for age
without the study by Wu et al (14). The results again demonstrate no
statistically significant difference (OR, 0.21; 95% CI, -0.80 to
1.22; P=0.68). (C and D) Funnel plots of the age between groups,
with (left) or without (right) Wu et al (14), and with (left) heterogeneity
(P=0.03 and I2=70%) or without (right) heterogeneity
(P=0.49 and I2=0 %). The studies depicted are as
follows: Wu et al (14),
Luo et al (15) and Yang
et al (16). I2,
the percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval; P,
P-value; OR, odds ratio. |
 | Table IIIParameters for the results of the
meta-analysis. |
Table III
Parameters for the results of the
meta-analysis.
| | Groups | Overall effect | Heterogeneity |
|---|
| Parameter | ‘Leave out one’
model | Trial, n=3 | Exper | Control | Effect
estimate | 95% CI | P-value | I2
(%) | P-value |
|---|
| Age (years) | - | 3 | 226 | 153 | 1.14 | (-0.60-2.88) | 0.20 | 70 | <0.05 |
| | Yang et al,
2020(16) | 2 | 147 | 130 | 1.36 | (-1.48-4.20) | 0.35 | 85 | <0.05 |
| | Wu et al,
2021(14) | 2 | 164 | 106 | 0.21 | (-0.80-1.22) | 0.68 | 0 | 0.49 |
| | Luo et al,
2022(15) | 2 | 141 | 70 | 1.88 | (-0.18-3.93) | 0.07 | 57 | 0.13 |
| Sex (male) | - | 3 | 98 | 75 | 0.85 | (056-1.30) | 0.46 | 0 | 0.52 |
| BMI
(kg/m2) >22 | | 2 | 81 | 45 | 1.76 | (0.55-5.67) | 0.34 | 72 | 0.06 |
| Alcohol use | | 2 | 43 | 37 | 0.88 | (0.51-1.52) | 0.64 | 0 | 0.33 |
| Smoking | | 2 | 68 | 26 | 1.57 | (0.59-4.19) | 0.37 | 60 | 0.12 |
| Location | | | | | | | | | |
|
Anterior
circulation | | 2 | 84 | 12 | 9.99 | (0.41-243.1) | 0.16 | 79 | <0.05 |
|
Posterior
circulation | | 2 | 38 | 11 | 3.09 | (0.04-256.6) | 0.62 | 89 | <0.05 |
| Size | | | | | | | | | |
|
<5
mm | | 2 | 71 | 17 | 5.03 | (0.02-1310.1) | 0.57 | 93 | <0.05 |
|
5-10 mm | - | 2 | 49 | 4 | 9.11 | (0.29-290.4) | 0.21 | 81 | 0.02 |
|
>10
mm | - | 2 | 21 | 2 | 3.52 | (0.90-13.85) | <0.05 | 0 | 0.58 |
| miR-126 | | 3 | 132 | 53 | 1.88 | (1.10-3.21) | <0.05 | 0 | 0.73 |
BMI >22(kg/m2)
As regards information on BMI, it was available in
two articles (15,16). No significant difference was found
between the groups (OR, 1.76; 95% CI, 0.55 to 5.67; P=0.34), but
with heterogeneity (P=0.06 and I2=72%) (Table III and Fig. S1).
Smoking
Information regarding smoking was available in two
articles (14,16). No significant difference was found
between the IA rupture and non-rupture or/+ control (healthy)
groups (OR, 1.57; 95% CI, 0.59 to 4.19; P=0.37), but with
heterogeneity (P=0.12 and I2=60%) (Table III and Fig. S2).
Location. Anterior circulation
As regards anterior circulation, information was
available in two articles (14,16).
No significant difference was found between groups (OR, 9.99; 95%
CI, 0.41-243.1; P=0.16) and with no heterogeneity (P<0.05 and
I2=79%) (Table III and Fig. S3).
Posterior circulation. Information regarding
posterior circulation was available in two articles (14,16).
Again, no significant difference was found between the IA rupture
and non-rupture or/+ control (healthy) groups (OR, 3.09; 95% CI,
0.04 to 256.6; P=0.57), but with heterogeneity (P<0.05 and
I2=93%) (Table III and Fig. S4).
Aneurysm size. <5 mm
As regards an aneurysm size <5 mm, information
was available in two articles (14,16).
No significant difference was found between groups (OR, 5.03; 95%
CI, 0.02 to 1310.1; P=0.57) with no heterogeneity (P<0.05 and
I2=93%) (Table III and Fig. S5).
5-10 mm. Information regarding an aneurysm
size 5-10 mm was available in two articles (14,16).
No significant difference was found between the IA rupture and
non-rupture or/+ control (healthy) groups (OR, 9.11; 95% CI, 0.29
to 290.4; P=0.21), but with heterogeneity (P<0.05 and I2=81%)
(Table III and Fig. S6).
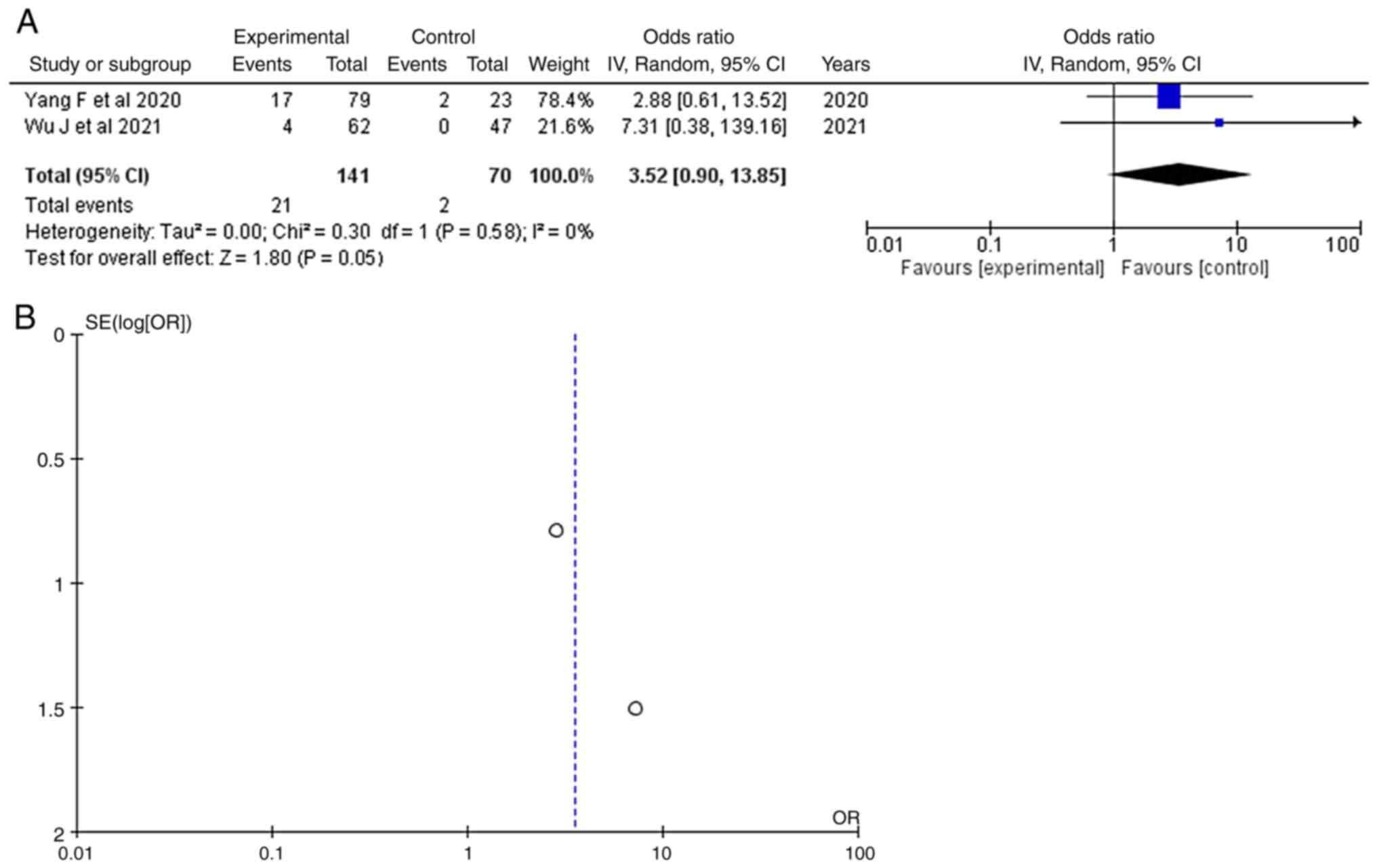
>10 mm. As regards an aneurysm size >10
mm, information was available in two articles (14,16)
and this demonstrated a statistically significant result (OR, 3.52;
95% CI, 0.90 to 13.8; P<0.05), with no heterogeneity (P=0.58;
I2=0%) (Table III and Fig. 3). An aneurysm size >10 mm was
found in 21 of 141 (14.8%) patients diagnosed with an IA ruptured
aneurysm and in 2 of 70 (2.8%) non-rupture or/+ control (healthy)
patients. When examining the funnel plot of the same parameter, no
publication bias was found.
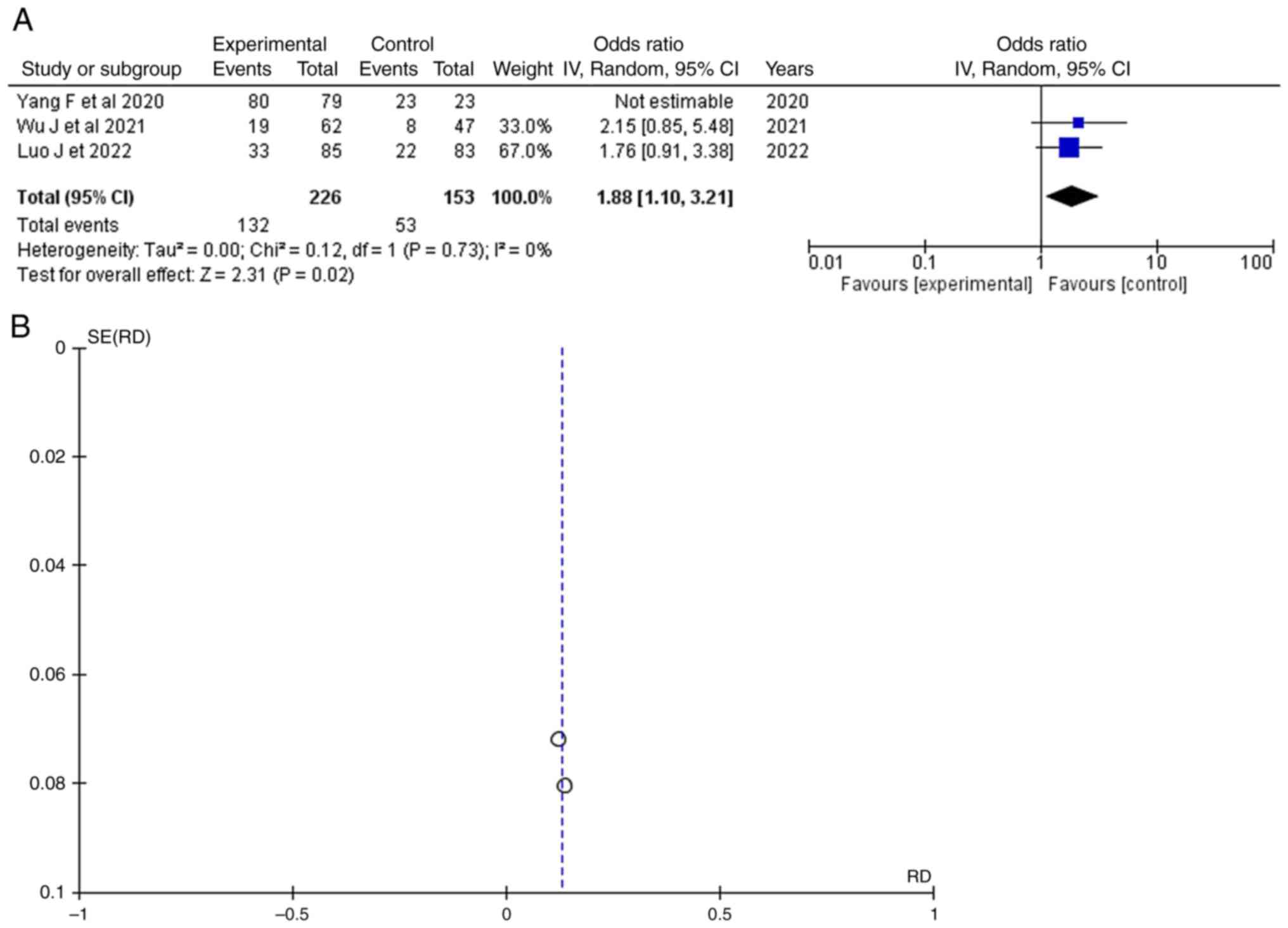
miR-126 expression >5
Information regarding miR-126 expression was
available in three articles (14-16)
and this demonstrated a statistical result (OR, 1.88; 95% CI,
1.10-3.21; P<0.05) with no heterogeneity (P=0.73 and I2=0%)
(Table III and Fig. 4). A miR-126 expression >5 was
found in 132 of 226 (58.4%) patients diagnosed with IA ruptured
aneurysms and in 53 of 153 (34.6%) non-rupture or/+ controls
(healthy) patients. When examining the funnel plot of the same
parameter, no publication bias was found.
Discussion
The present study suggests that the circulating
miR-126 levels may be used as a biomarker for predicting aneurysmal
rupture. More precisely, a miR-126 expression >5 was the
only statistically significant parameter related to IA bleeding
compared with non-rupture or/+ control (healthy) patients. Of note,
an aneurysmal size >10 mm was also associated with an IA
rupture.
miRNAs constitute a varied class of small (18-25
nucleotides in length) non-coding RNA molecules (17). miRNAs balance numerous genes,
various biological pathways and regulatory networks inside cells by
unifying various regulatory mechanisms, whether in a type of
transcriptional input or by their operating regulatory output on
different pathways (18). Defects
in miRNA regulation may often impair cellular and biological
activity and, ultimately, contribute to disease progression. Since
miRNAs are involved in disease evolution, circulating miRNAs have
potential diagnostic value (19).
The miR-126 gene is located on human chromosome 9 and is mostly
expressed in vascular endothelial cells. Mature miR-126 controls
the propagation of vascular endothelial cells (20). In the present study, the expression
of serum miR-126 was higher in patients with IA rupture compared
with non-rupture or/+ control (healthy) patients.
Circulating miR-126 levels have been formerly
established to be increased in the serum of patients with
unruptured IAs compared to healthy controls (16). However, further analysis has
indicated that levels of circulating miR-126 can be increased in
several pathways, such as erythroblastic leukemia viral oncogene
homolog signaling and mitogen-activated protein kinase signaling
pathways, which are related to IA, but have higher levels in
ruptured IAs compared with unruptured IAs. Thus, the present
meta-analysis included a miR-126 level of expression >5 to
evaluate its potential role as a biomarker for predicting
aneurysmal ruptures.
The underlying mechanisms responsible for the
creation, enlargement and rupture of IAs are complex. It is
considered that under conditions of continuous hemodynamic
pressure, the cerebral artery walls turn fragile and becomes unable
to resist these types of pressure, and structural modifications and
pathological development are conducted in these walls. Therefore,
intimal hyperplasia and the appearance of blood clots serve to
distinguish the barriers of unruptured aneurysms (21).
Some researchers have reported that larger aneurysms
are significantly associated with an increased risk of rupture
(22). Although the difference in
size between the ruptured and unruptured aneurysms decreases with
an increasing age, the mean size of all ruptured aneurysms is
significantly larger than the mean size of unruptured aneurysms
(23). However, although size is
one of the strongest predictors, small aneurysms often rupture
(24,25). In the present meta-analysis, an
aneurysmal size >10 mm was associated with an IA rupture.
The present study has several limitations however,
which should be mentioned. The expression levels of circulating
miR-126 were detected over a different time period of 3-14 days,
and the value of miR-126 in the prognosis of patients remains
uncertain. In addition, the possible association between aneurysm
size and other parameters such as C-protein, and the association
between miR-126 and varying degrees of severity of vasospasm and
the small sample size constitute the main limitation of the present
study.
In conclusion, the present study proposes that the
circulating miR-126 levels may be used as biomarkers for predicting
aneurysmal ruptures. The change in the circulating levels of
miR-126 in plasma between patients with IA bleeding and non-rupture
or/+ controls (healthy) may have a marked effect on IA ruptures.
Furthermore, an aneurysmal size >10 mm in patients with
unruptured aneurysms is associated with a high risk of bleeding and
may thus help physicians confirm the level of therapy accordingly.
Future studies are required to examine the circulating levels of
miR-126, which were recognized in the present study as a potential
biomarker for IA rupture. These levels may be relevant as a
diagnostic tool in clinical practice for distinguishing between
patients with severe and mild vasospasm.
Supplementary Material
(A) Forest plot for body mass index.
The results demonstrated no statistically significant results (OR,
1.76; 95% CI, 0.55 to 5.67; P=0.34). (B) Funnel plot of the body
mass index in the groups, demonstrating high heterogeneity (P=0.06
and I2=72%). The studies depicted are as follows: Luo
et al (15) and Yang et
al (16). I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval; P,
P-value; OR, odds ratio.
(A) Forest plot for smoking: The
results demonstrated no statistically significant difference (OR,
1.57; 95% CI, 0.59 to 4.19; P=0.37). (B) Funnel plot of the same
parameter, demonstrating high heterogeneity (P=0.12 and
I2=60%). The studies depicted are as follows: Wu et
al (14) and Yang et al
(16). I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval; P,
P-value; OR, odds ratio.
(A) Forest plot for anterior
circulation. The Results demonstrated no statistically significant
difference (OR, 9.99; 95% CI, 0.41 to 243.1; P=0.16). (B) Funnel
plot of the same parameter, demonstrating high heterogeneity
(P=0.03 and I2=79%). The studies depicted are as
follows: Wu et al (14) and
Yang et al (16).
I2, the percentage of total variation across studies
that is due to heterogeneity rather than chance; CI, confidence
interval; P, P-value; OR, odds ratio.
(A) Forest plot for posterior
circulation. The results demonstrated no statistically significant
difference (OR, 3.09; 95% CI, 0.04 to 256.6; P=0.62). (B) Funnel
plot of the same parameter, demonstrating high heterogeneity
(P=0.003 and I2=89%). The studies depicted are as
follows: Wu et al (14) and
Yang et al (16).
I2, the percentage of total variation across studies
that is due to heterogeneity rather than chance; CI, confidence
interval; P, P-value; OR, odds ratio.
(A) Forest plot for an aneurism size
<5 mm. The results demonstrated no statistically significant
difference (OR, 5.03; 95% CI, 0.02 to 1310.1; P=0.57). (B) Funnel
plot of the same parameter, demonstrating high heterogeneity
(P<0.05 and I2=93%). The studies depicted are as
follows: Wu et al (14) and
Yang et al (16).
I2, the percentage of total variation across studies
that is due to heterogeneity rather than chance; CI, confidence
interval; P, P-value; OR, odds ratio.
(A) Forest plot for an aneurism size
5-10 mm. The results demonstrated no statistically significant
difference (OR, 9.11; 95% CI, 0.29-290.4; P=0.21). (B) Funnel plot
of the same parameter, demonstrating high heterogeneity (P<0.05
and I2=81%). The studies depicted are as follows: Wu
et al (14) and Yang et
al (16). I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; CI, confidence interval; P,
P-value; OR, odds ratio.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF and VEG conceptualized the study. VEG, PS, GF,
NM, PP, KP, DAS and NT analyzed the data, and wrote and prepared
the draft of the manuscript. VEG and GF provided critical
revisions. All authors contributed to manuscript revision, and have
read and approved the final version of the manuscript. VEG and GF
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Han H, Guo S, Jiang H and Wu X:
Feasibility and efficacy of enhanced recovery after surgery
protocol in Chinese elderly patients with intracranial aneurysm.
Clin Interv Aging. 14:203–207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsolaki V, Aravantinou-Fatorou A,
Georgakopoulou VE, Spandidos DA, Papalexis P, Mathioudakis N,
Tarantinos K, Trakas N, Sklapani P and Fotakopoulos G: Early
diagnosis of cerebral vasospasm associated with cerebral ischemia
following subarachnoid hemorrhage: Evaluation of computed
tomography perfusion and transcranial doppler as accurate methods.
Med Int (Lond). 2(34)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fotakopoulos G, Makris D, Kotlia P,
Kapsalaki E, Papanikolaou J, Georgiadis I, Zakynthinos E and
Fountas K: The value of computed tomography perfusion &
transcranial doppler in early diagnosis of cerebral vasospasm in
aneurysmal & traumatic subarachnoid hemorrhage. Future Sci OA.
4(FSO313)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ko NU, Rajendran P, Kim H, Rutkowski M,
Pawlikowska L, Kwok PY, Higashida RT, Lawton MT, Smith WS, Zaroff
JG and Young WL: Endothelial nitric oxide synthase polymorphism
(-786T->C) and increased risk of angiographic vasospasm after
aneurysmal subarachnoid hemorrhage. Stroke. 39:1103–1108.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dumont AS, Dumont RJ, Chow MM, Lin CL,
Calisaneller T, Ley KF, Kassell NF and Lee KS: Cerebral vasospasm
after subarachnoid hemorrhage: Putative role of inflammation.
Neurosurgery. 53:123–135. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Starke RM, Kim GH, Komotar RJ, Hickman ZL,
Black EM, Rosales MB, Kellner CP, Hahn DK, Otten ML, Edwards J, et
al: Endothelial nitric oxide synthase gene single-nucleotide
polymorphism predicts cerebral vasospasm after aneurysmal
subarachnoid hemorrhage. J Cereb Blood Flow Metab. 28:1204–1211.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT
and Juo SHH: Serum microRNA-21 and microRNA-221 as potential
biomarkers for cerebrovascular disease. J Vasc Res. 50:346–354.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fotakopoulos G, Georgakopoulou VE,
Spandidos DA, Papalexis P, Angelopoulou E, Aravantinou-Fatorou A,
Trakas N, Trakas I and Brotis AG: Role of miR-200 family in brain
metastases: A systematic review. Mol Clin Oncol.
18(15)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meeuwsen JAL, van T Hof FNG, van Rheenen
W, Rinkel GJE, Veldink JH and Ruigrok YM: Circulating microRNAs in
patients with intracranial aneurysms. PLoS One.
12(e0176558)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Torres R, Mancha F, Bustamante A,
Canhao P, Fragata I and Montaner J: Usefulness of TNFR1 as
biomarker of intracranial aneurysm in patients with spontaneous
subarachnoid hemorrhage. Future Sci OA. 6(FSO431)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jung CS, Lange B, Zimmermann M and Seifert
V: CSF and serum biomarkers focusing on cerebral vasospasm and
ischemia after subarachnoid hemorrhage. Stroke Res Treat.
2013(560305)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wells GA, Shea B, O'Connell D, et al: The
Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses, 2014. Available from:
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
|
|
13
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu J, Gareev I, Beylerli O, Mukhamedzyanov
A, Pavlov V, Khasanov D and Khasanova G: Circulating miR-126 as a
potential non-invasive biomarker for intracranial aneurysmal
rupture: A pilot study. Curr Neurovasc Res. 18:525–534.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo J, Zhu X, Liu F, Zhao L, Sun Z, Li Y,
Ye L and Li W: Expression of serum miR-126 in patients with
intracranial aneurysm and its relationship with postoperative
cerebral vasospasm. Am J Transl Res. 14:4372–4379. 2022.PubMed/NCBI
|
|
16
|
Yang F, Xing WW, Shen DW, Tong MF and Xie
FM: Effect of miR-126 on intracranial aneurysms and its predictive
value for rupture of aneurysms. Eur Rev Med Pharmacol Sci.
24:3245–3253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9(402)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fernández-Hernando C and Moore KJ:
MicroRNA modulation of cholesterol homeostasis. Arterioscler Thromb
Vasc Biol. 31:2378–2382. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Supriya M, Christopher R, Indira Devi B,
Bhat DI and Shukla D: Circulating MicroRNAs as potential molecular
biomarkers for intracranial aneurysmal rupture. Mol Diagn Ther.
24:351–364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu D, Han L, Wu X, Yang X, Zhang Q and
Jiang F: Genome-wide microRNA changes in human intracranial
aneurysms. BMC Neurol. 14(188)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fukuda M and Aoki T: Molecular basis for
intracranial aneurysm formation. Acta Neurochir Suppl. 120:13–15.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nguyen TN, Hoh BL, Amin-Hanjani S, Pryor
JC and Ogilvy CS: Comparison of ruptured vs unruptured aneurysms in
recanalization after coil embolization. Surg Neurol. 68:19–23.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Weir B, Disney L and Karrison T: Sizes of
ruptured and unruptured aneurysms in relation to their sites and
the ages of patients. J Neurosurg. 96:64–70. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rinaldo L, Nesvick CL, Rabinstein AA and
Lanzino G: Differences in size between unruptured and ruptured
saccular intracranial aneurysms by location. World Neurosurg.
133:e828–e834. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim BJ, Kang HG, Kwun BD, Ahn JS, Lee J,
Lee SH, Kang DW, Kim JS and Kwon SU: Small versus large ruptured
intracranial aneurysm: Concerns with the site of aneurysm.
Cerebrovasc Dis. 43:139–144. 2017.PubMed/NCBI View Article : Google Scholar
|