Introduction
Trigeminal neuralgia (TN) is one of the most common
causes of facial pain. It is characterized by intermittent
transient (lasting seconds to minutes), electrocution- or
needle-like pain in trigeminal nerve distribution area induced by
minor mechanical stimuli such as brushing and chewing (1). Despite the unclear pathogenesis in
TN, neurovascular compression is considered to be an important
cause of TN and microvascular decompression (MVD) is widely
recognized as the first-choice surgical treatment (2,3).
Compared with drug therapy, radiofrequency ablation, percutaneous
balloon compression (PBC) and other methods, MVD results in longer
pain-free periods and fewer side effects or complications, such as
postoperative facial spasms and hearing loss (2,3).
However, 3-31% of patients experience pain recurrence, which
affects the quality of life and prognosis of these patients
(4-6).
Neurosurgeons have studied prognostic prediction
systems for MVD, some of which originated from internationally
renowned medical centers (7,8).
Previous research has reported prognostic factors for MVD,
including sex, age, symptomatic side, trigeminal nerve branches,
sensitivity to carbamazepine, type 1 or 2 TN (TN1 or TN2), severity
and site of neurovascular compression, and type of vessels involved
(9-16).
To the best of our knowledge, however, most studies use univariate
or multivariate correlation analyses and there is no recognized
prognostic prediction system for MVD. More importantly, the
independent risk factors in the aforementioned MVD prognostic
models of TN all included TN type (TN1 or TN2), but the latest
international TN diagnostic guidelines [the International
Classification of Headache Disorders, 3rd edition (ICHD-3); the
11th Revision of the International Classification of Diseases
(ICD-11)] classify TN as classic, secondary and idiopathic, so
these models are not suiTable for the current status of clinical
diagnosis and treatment (17-19).
In addition, previous studies only provided static prognostic
models (7,8), which made it inconvenient for
clinicians or patients to access the predicted results of the model
at any time. It was hypothesized that a reliable and accurate
prediction model could be constructed based on preoperative
clinical and imaging factors. To test this hypothesis, univariate
and multifactor logistic regression statistical analysis methods
were used to analyze the included preoperative clinical and imaging
factors, and a model was constructed. The present study aimed to
combine the current prognostic factors for MVD and construct a new
practical predictive assessment systems to evaluate the risk of
pain recurrence in patients with TN after MVD based on preoperative
clinical and imaging factors. This may provide a reference for
patient consultation and choice of surgical plan.
Materials and methods
Clinical case data and
characteristics
Clinical data of 56 patients diagnosed with primary
unilateral TN at the Second Affiliated Hospital of Anhui Medical
University (Hefei, China) from August 2011 to October 2021 were
retrospectively reviewed. None of the patients underwent any
invasive intervention before surgery, such as MVD, PBC,
radiofrequency ablation or trigeminal sensory rhizotomy. The
characteristics of the study patients are shown in Table I. The retrospective study was
approved (approval no. 202115) by the Ethics Committee of the
Second Affiliated Hospital at Anhui Medical University. All
patients provided written informed consent. The diagnosis and
subtyping of TN were performed according to the latest criteria
[ICHD-3(17) and ICD-11(19)] established by the International
Headache Society and the World Health Organization. All patients
underwent uniform clinical and radiographic assessments, including
symptomatic and medical history, numeric rating scale, response to
medication and tolerability and retrospective analysis of
trigeminal magnetic resonance imaging (MRI) (20) before MVD, including conventional
3.0-Tesla MRI plain scans, three-dimensional time of light MR
angiography and 3D-FIESTA sequence. Based on these assessments,
surgical treatment was determined and performed by the same team of
experienced neurosurgeons. In addition, patients were excluded if
they had never been treated with carbamazepine or oxcarbazepine.
Patients were excluded if they had incomplete or missing data,
including medical history, MR images or long-term clinical
follow-up.
 | Table ICharacteristics of patients with MVD
(n=56). |
Table I
Characteristics of patients with MVD
(n=56).
| Characteristic | Value |
|---|
| Mean age of onset,
years | 54.81±11.09 |
| Mean age at
surgery, years | 58.67±10.82 |
| Mean duration
between onset and surgery, years | 3.86±3.26 |
| Sex, n (%) | |
|
Female | 33 (58.93) |
|
Male | 23 (41.07) |
| Symptomatic side, n
(%) | |
|
Left | 26 (46.43) |
|
Right | 30 (53.57) |
| Trigeminal nerve
branches, n (%) | |
|
V2 + V3 | 42 (75.00) |
|
Other | 14 (25.00) |
| Neurovascular
contact on MRI, n (%) | |
|
Vascular
deformity | 33 (58.93) |
|
Vascular
contact | 19 (33.93) |
|
Absent
vascular proximity | 4 (7.14) |
| NRS, n (%) | |
|
0-3 | 10 (17.86) |
|
4-10 | 46 (82.14) |
| REZ, n (%) | |
|
Yes | 52 (92.86) |
|
No | 4 (7.14) |
| Response to
neuroanalgesic drugs, n (%) | |
|
Yes | 41 (73.21) |
|
No | 15 (26.79) |
| Recurrence 1-year
post-MVD, n (%) | |
|
Yes | 13 (23.21) |
|
No | 43 (76.79) |
Postoperative follow-up
The outcome of MVD was assessed immediately after
surgery, before discharge and 1 year after surgery by outpatient or
telephone follow-up. This follow-up assessed postoperative pain,
degree of pain, need for medication, clinical improvement following
drug use and complications. Pain was assessed with the Barrow
Neurological Institute (BNI) pain intensity score: I indicated no
pain recurrence after MVD, whereas BNI pain score II-V (from BNI
level II to V, the pain severity gradually increases. BNI levels
III and IV require medication for pain relief, while BNI level V
indicates that medication is ineffective in alleviating the pain)
indicated pain recurrence after MVD (21).
Statistical analysis
Statistical analysis was performed using R language,
version 4.2.0 (http://www.Rproject.org). Data are presented as mean ±
standard deviation, percentages or odds ratio (OR) with 95%
confidence interval (CI). Univariate and multivariate logistic
regression analysis were used to determine risk factors of pain
recurrence and OR was calculated. According to these independent
risk factors, an online dynamic nomogram was constructed with R
language, version 4.2.0 (http://www.Rproject.org) At https://www.shinyapps.io, this nomogram was
transformed into a web server to facilitate use. Model performance
was assessed in terms of discrimination and calibration. The
concordance index (C-index) and receiver operating characteristic
(ROC) were used to measure model discrimination. Bootstrap
resampling was used for internal validation and calibration curve
was constructed, which graphically represents the association
between actual and predicted probabilities. Presenting the P-values
of the Hosmer-Lemeshow goodness-of-fit test on the calibration
curve can increase the rigor and objectivity of the model
evaluation by providing an additional quantitative assessment.
Decision curve analysis (DCA) was used to evaluate the clinical
applicability of the model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of 56 patients with
MVD
The present study included a total of 56 patients
who met the inclusion criteria and conducted follow-up evaluations.
Patients were aged 25-77 years with a mean age of onset 55 years
and mean age at surgery of 59 years. The mean disease duration was
4 years. Women were more common, accounting for 58.93% (n=33);
Right side pain was more common, accounting for 53.57% (n=30).
Among the 56 patients with MVD, pain involved the second and third
branches of trigeminal nerve (V2+V3) in 75.00% (n=42) of cases.
NoTable neurovascular compression or deformation on MRI was
observed in 58.93% (n=33) of cases, pain scores ranging from 4 to
10 accounted for 82.14% (n=46), vascular compression at the
trigeminal nerve root exiting the brainstem zone (REZ) occurred in
92.86% (n=52) of cases and neuroanalgesic drug treatment was
effective in 73.21% (n=41) of cases. One year after MVD, pain
recurrence occurred in 23.21% (n=13) of patients (Table I).
Establishment of the nomogram
Univariate and multivariate logistic regression
analysis showed that numeric rating scale (NRS; OR=58.50, 95%CI:
4.71-2,349.77, P=0.007), response to neuroanalgesic drugs
(OR=60.35, 95%CI: 4.84-2,661.18, P=0.007) and neurovascular contact
on MRI (OR=98.55, 95%CI: 5.39-10,642.56, P=0.014) were independent
risk factors for pain recurrence in patients with TN after MVD
(Table II). On the basis of these
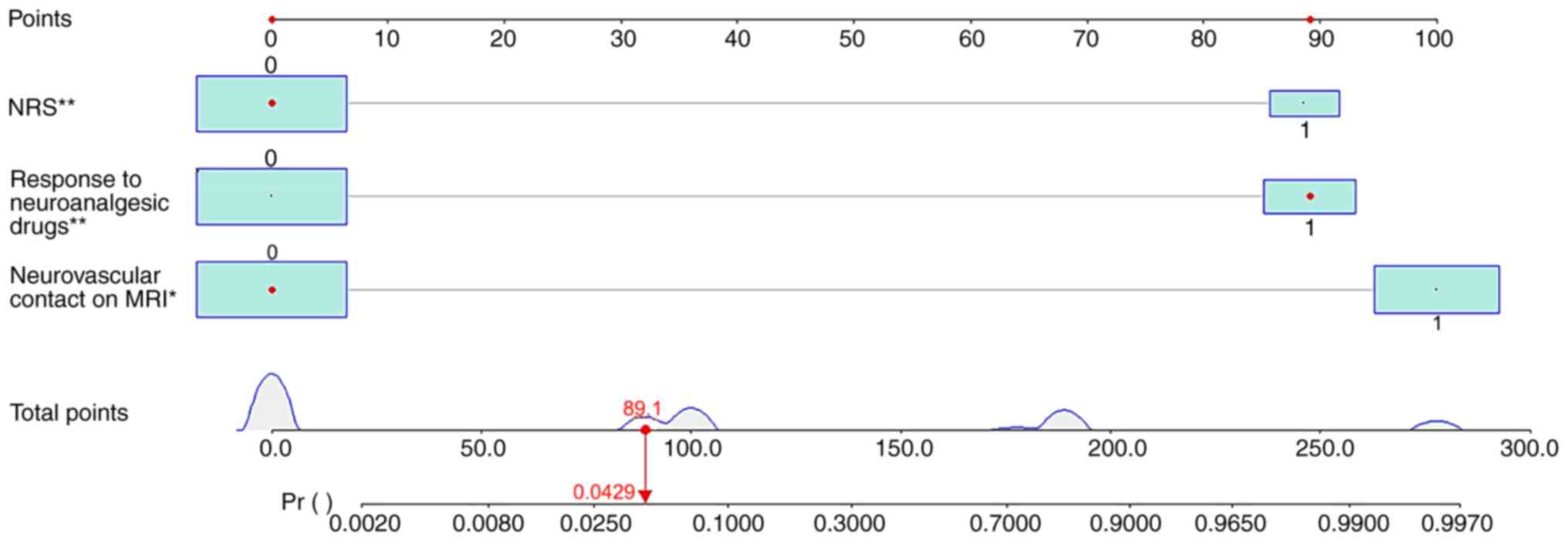
results, R software version 4.2.0 was used to develop an online
dynamic nomogram (Fig. 1). Each
clinical factor is given a score; total score represents
probability of pain recurrence in patients with TN after MVD. To
visualize the nomogram and make the clinical application more
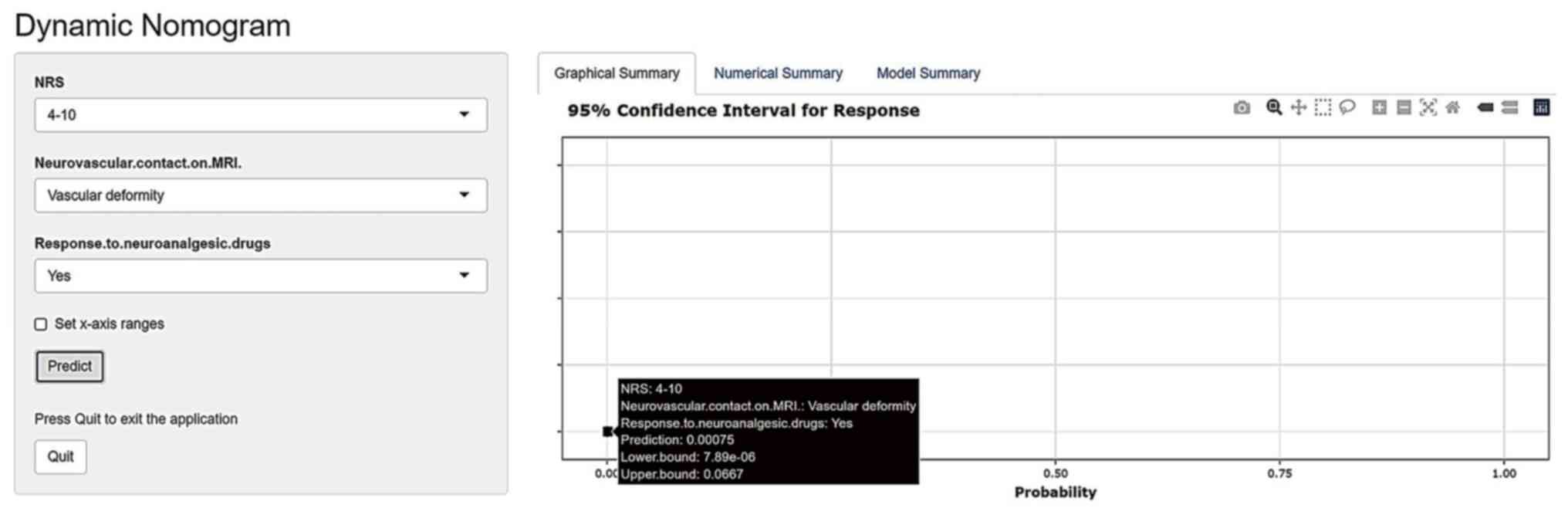
convenient, this nomogram was created by web server (ppramvdftn.shinyapps.io/DynNomapp/).
After entering the predictive variables of the model in the left
panel, the predicted value of pain recurrence in patients with TN
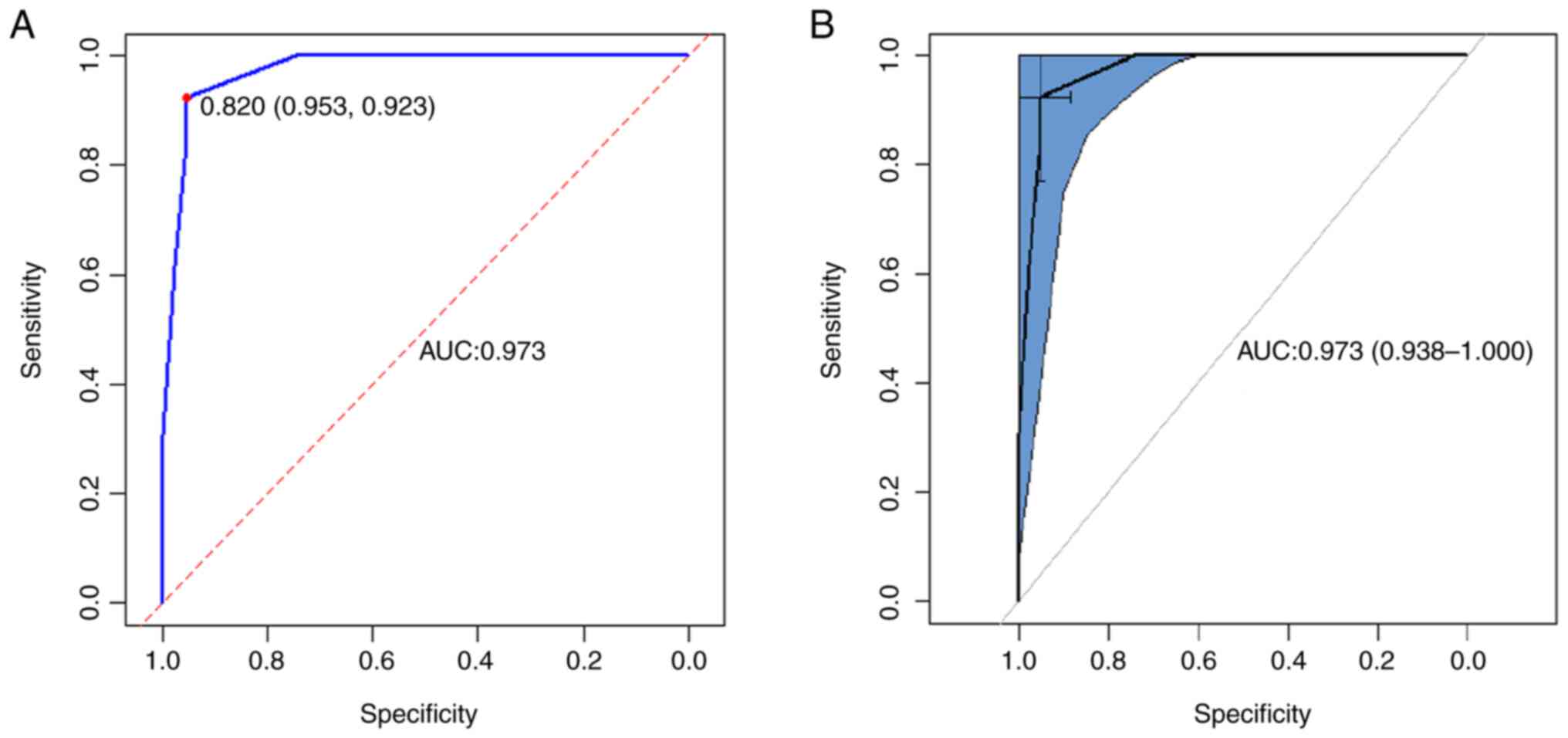
after MVD will be displayed in the right panel (Fig. 2). The prediction model had good
discriminative ability and area under the ROC curve was 0.973
(Fig. 3A). When the optimal cutoff
value for model scoring was 0.820, the sensitivity was 0.923 and
the specificity was 0.953 (Fig.
3A). Furthermore, the ROC curve demonstrated a 95% confidence
interval of 0.938-1.000 (Fig. 3B).
The C-index was 0.973 (95% CI, 0.938-1.000).
 | Table IIUnivariate and multivariate logistic
regression analysis. |
Table II
Univariate and multivariate logistic
regression analysis.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristic | OR | 95%CI | P-value | OR | 95%CI | P-value |
|---|
| Age at onset,
years | | | | | | |
|
<55 | 1.00 | | | - | | |
|
≥55 | 0.24 | 0.06-0.86 | 0.035 | - | - | - |
| Duration of
illness | 0.99 | 0.78-1.19 | 0.889 | - | - | - |
| Sex | | | | | | |
|
Female | 1.00 | | | - | - | - |
|
Male | 1.31 | 0.37-4.62 | 0.671 | - | - | - |
| Symptomatic
side | | | | | | |
|
Left | 1.00 | | | - | - | - |
|
Right | 0.29 | 0.07-1.04 | 0.068 | - | - | - |
| Trigeminal nerve
branches | | | | | | |
|
V2 + V3 | 1.00 | | | - | - | - |
|
Other | 0.19 | 0.01-1.14 | 0.131 | - | - | - |
| Neurovascular
contact on MRI | | | | | | |
|
Vascular
deformity | 1.00 | | | 1.00 | | |
|
Absent
vascular proximity or vascular contact only | 34.91 | 5.89-674.28 | 0.001 | 98.55 | 5.39-10642.56 | 0.014 |
| NRS | | | | | | |
|
4-10 | 1.00 | | | 1.00 | | |
|
0-3 | 32.80 | 6.29-265.27 | <0.001 | 58.50 | 4.71-2349.77 | 0.007 |
| REZ | | | | | | |
|
Yes | 1.00 | | | - | | |
|
No | 12.60 | 1.45-270.27 | 0.036 | - | - | - |
| Response to
neuroanalgesic drugs | | | | | | |
|
Yes | 1.00 | | | 1.00 | | |
|
No | 13.88 | 3.45-66.90 | <0.001 | 60.35 | 4.84-2661.18 | 0.007 |
Validation of the nomogram
The bootstrap verification method was used to verify
the generated model internally and the C-index of internal
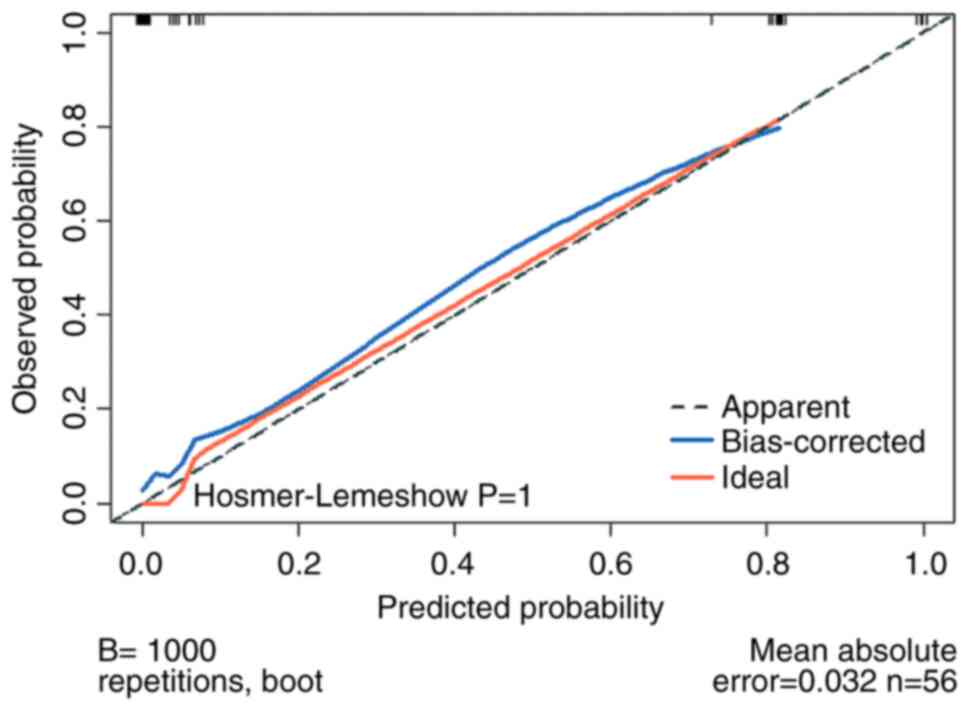
validation was 0.97. Calibration curve (Fig. 4) showed that predicted and actual
probability of pain recurrence was close to x=y. Hosmer-Lemeshow
goodness-of-fit test yielded P=1, which also indicates that the
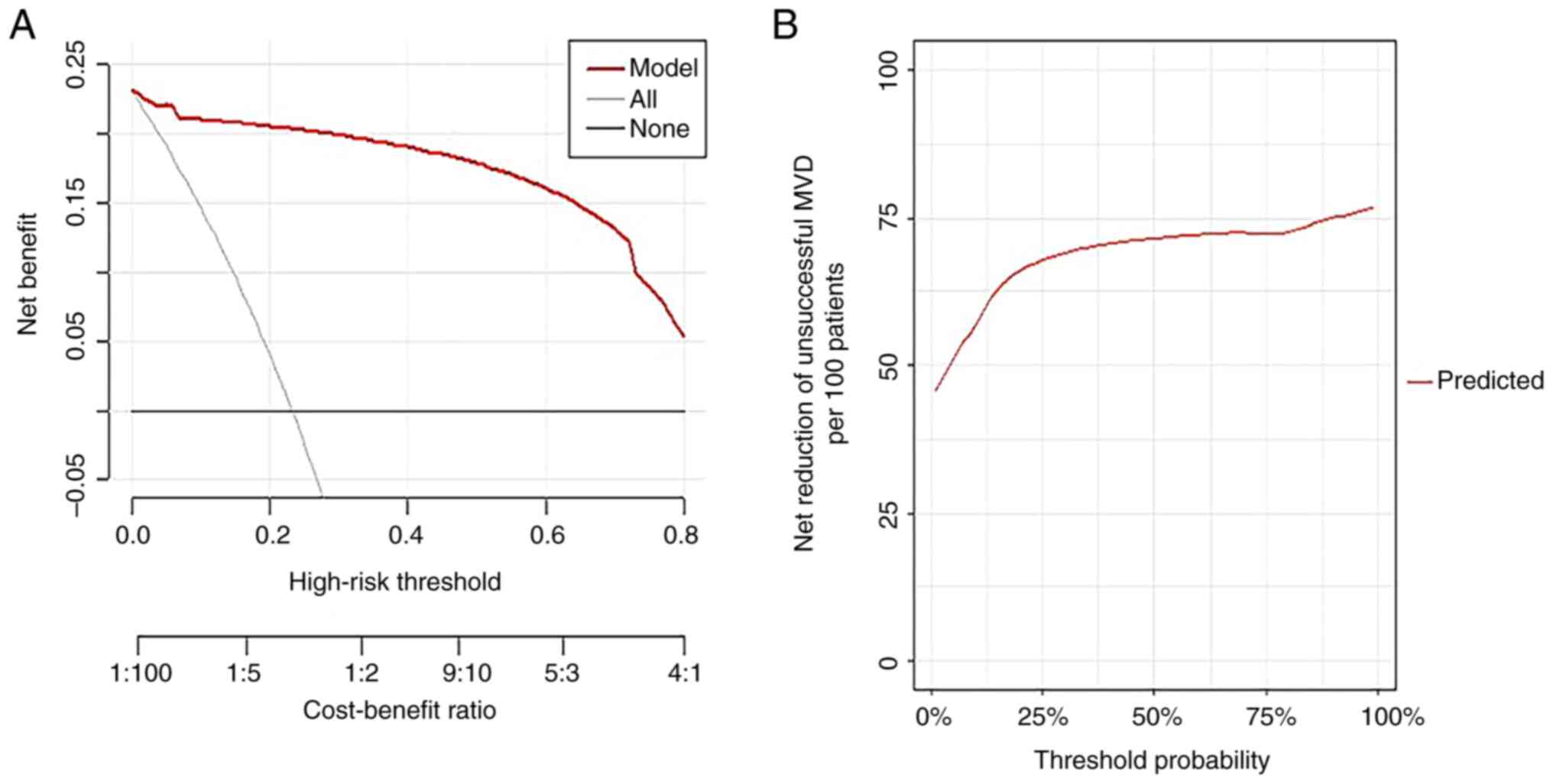
model had a good calibration degree. DCA (Fig. 5) indicated the clinical usefulness.
When the probability of high-risk threshold was between 0 and 100%,
the model can achieve a greater net benefit than if all patients
had surgery or none had surgery (Fig.
5A). The net reduction of MVD cases likely to be unsuccessful
increased to >75 per 100 patients when applied to patients with
a perceived likelihood of success after MVD of 87.5% (Fig. 5B).
Discussion
MVD is currently recognized as the first choice for
treatment of TN and its effectiveness has been confirmed (2). Nevertheless, 3-31% of patients with
TN experience pain recurrence (4-6).
The present study showed a 1-year recurrence rate of 23.21%.
Independent risk factors associated with prognosis of MVD have been
reported (7,8,22),
such as TN1 or TN2 status, sensitivity to carbamazepine and
severity of neurovascular compression. Prognostic prediction
systems for MVD have been developed based on these independent risk
factors by different methods (7,8). The
aforementioned studies all reported TN1 or TN2 as an independent
risk factor related to the prognosis of MVD. However, the latest
classification of TN by the International Headache Association in
2019 abandoned the traditional TN1 and TN2 classification method
(18,19). According to the latest
international pain classification guidelines (ICHD-3 and ICD-11)
(17-19),
previous evaluation and prediction models are not suiTable for the
current clinical diagnosis and treatment. Therefore, the present
study was based on previous literature and the latest international
pain guidelines. The present study developed a novel practical
prognosis prediction system in patients with TN after MVD.
R language was used to program the model and the
experimental design method was more novel, the technology was more
comprehensive and the results were more reliable than other
statistical analysis software such as SPSS. Univariate and
multivariate logistic regression analysis showed that NRS, response
to neuroanalgesic drugs and neurovascular contact on MRI were
associated with good prognosis of MVD. Other factors, including
sex, the side of pain, age of onset and disease duration, did not
affect the prognosis of MVD. After long-term follow-up, the
effectiveness of MVD as a surgical method for treating
drug-refractory TN has been proven, and these findings are
consistent with the previous literature (17,23,24).
In the present study, no significant difference was seen between
sexes; however, there were more female than male patients and women
still account for the majority of patients with pain recurrence
following MVD. Therefore, based on these findings, it can be
concluded that men may have a more favorable prognosis. At present,
the causal connection between sex and the rate of pain recurrence
in patients with TN after MVD remains unclear and it is not known
if sex is a risk factor for TN. Female patients exhibit a higher
incidence of TN, which may be due to hormone secretion and gene
expression differences. For example, migraine and menstrual pain
are more common in female patients, and female patients are more
sensitive to pain (9,17,25).
NRS is a more detailed numerical pain score that accurately
represents the preoperative pain degree of patients with TN
compared with other pain scores such as the McGill Pain
Questionnaire (23,24). Generally, the more severe
trigeminal nerve compression and stimulation response, the more
severe the pain and relief of neurovascular compression is
associated with patient prognosis. Studies have reported that the
molecular mechanisms underlying the pathogenesis of TN involve
changes in various pain-associated neuropeptides, inflammatory
mediators and ion channels (26,27).
The neuroanalgesic drugs, such as carbamazepine, have
membrane-stabilizing effects, which decrease permeability of nerve
cell membrane to Na+ and Ca2+, thereby
decreasing the cell excitability and prolonging the refractory
period. They may also enhance synaptic transmission function of
γ-aminobutyric acid. Neuroanalgesic drugs are widely accepted as a
classical conservative treatments of TN (1,17).
Patients who respond to drug therapy have better prognosis.
Effective drug treatment may be influenced by factors such as
vascular compression and compression deformation. When the vascular
compression is relieved, the effect of MVD is excellent. However,
if the nerve has no obvious vascular compression, stimulation
injury is severe or the postoperative compression is relieved but
the function does not recover due to severe injury, MVD does not
improve pain and the prognosis is poor. The presence of
neurovascular contact on MRI is identified as a standalone risk
factor for pain recurrence following MVD. The level of
neurovascular compression indicated by this independent risk factor
plays a key role in classical theories of etiology. Basic and
clinical evidence support the hypothesis of demyelination (1,28),
which suggests the trigeminal nerve is compressed by blood vessels,
especially the superior and anterior inferior cerebellar arteries
and the trigeminal nerve is stimulated by pulsating blood vessels
(26,27). Therefore, demyelination of the
sensory branches of the trigeminal nerve is the primary driver of
the pathogenesis and pathophysiology of TN. This pathological
demyelination can result from physical compression of the
trigeminal ganglion or other primary demyelinating diseases.
Studies in patients with TN and animal models have revealed
significant molecular changes, channel lesions and
electrophysiological abnormalities in affected trigeminal nerves
(12,26-28).
Therefore, neurovascular contact on MRI is an independent risk
factor for pain recurrence after MVD.
Based on the aforementioned independent risk
factors, an online dynamic nomogram was developed. C-index and ROC
were used to measure model discrimination. Bootstrap resampling was
used for internal validation and calibration curve was constructed
to assess calibration of the proposed model. DCA was used to
evaluate the clinical applicability of the model. The
aforementioned indexes showed that the model had good
discrimination and calibration and clinical applicability. Patient
data was input into the web version of the model to obtain the
predicted recurrence probability. According to preoperative
clinical and imaging findings, patients with TN with high NRS,
response to neuroanalgesic drugs and severe neurovascular
compression had the lowest probability of pain recurrence after
MVD. The present prediction model demonstrated a net benefit
compared with if all patients had surgery or none had surgery when
the high-risk threshold probability was between 0 and 100%. The
present model would reduce the risk of surgical failure by >75%,
even in cases where the neurosurgeon believes likelihood of success
is >90%. Other studies have included more clinical features in
the assessment of pain outcomes in patients with TN after MVD to
improve overall management (7,8).
Among them, the classical typing method of TN (TN1 and TN2) was not
included in this study (15)
because the International Headache Society published a new
classification in its 2019 guidelines (19). The most representative studies in
assessing pain outcomes with TN after MVD are those by Hardaway
et al (7) and Panczykowski
et al (8). The
aforementioned studies provide important guidance and reference the
study of a prediction pain recurrence model in patients with TN
after MVD. However, the aforementioned models all adopted the
classical TN classification (15)
and the latest TN classification (19) was not used, which could not well
apply to the current clinical diagnosis and treatment practices.
The present study used a numerical rating scale to assess severity
of TN, which is the primary symptom of this condition. There are
four categories of digital pain score: 0, no; 1-3, mild; 4-6,
moderate; and 7-10, severe pain. A previous study showed that when
the NRS score iss >4, the quality of life, sleep and diet are
affected. In such cases, the standard clinical diagnosis and
treatment protocols typically involve the use of medications or
surgical interventions (29).
Based on this, the present study divided patients into NRS0-3 and
NRS4-10 categories. Univariate and multivariate analyses
demonstrated that NRS was associated with the risk of recurrence
after MVD, as previously reported (23,24).
NRS is commonly used in chronic pain scoring systems and is similar
to Visual Analog Scale, being less affected by non-pain intensity
factors than VRS or Faces Pain Scale-Revised (24). Therefore, the present study
simulated clinical practice. The present study showed that NRS,
response to neuroanalgesic drugs and neurovascular contact on MRI
predicted the recurrence rate of pain following MVD in patients
with TN.
Nomogram is used to analyze the prognosis of
patients with brain injury (30)
and cancer (31,32) and replace traditional prediction
models. In previous studies, such as that by Hardaway et al
(7) and Panczykowski et al
(8), it was suggested that
prognostic models incorporating multiple independent risk factors
can effectively predict the outcome of patients undergoing MVD.
This approach helps provide valuable guidance to clinicians and
patients in making preoperative decisions and reduce the incidence
of unnecessary surgeries. However, the previous studies only used
univariate or multivariate analysis, or the key factors in the
model did not use the latest trigeminal pain diagnosis and
treatment guidelines (such as TN classification), or only provided
a static prognostic scoring system which is not helpful for
clinicians or patients to refer to (7,8,33)
The present study used R language software, to establish a
user-friendly prediction model web version, more convenient to use,
more accurate data display. As shown in Fig. 2 (available at ppramvdftn.shinyapps.io/DynNomapp/pages), the
personalized information allows for the selection of appropriate
independent risk factors based on individual patients. The
prognostic system then provides real-time probability predictions
accordingly. For example, for a patient with 4-10 based on NRS,
response to neuroanalgesic drugs and vascular deformity, the
predicted value of the system is 0.00075, the recurrence rate is
low and surgery is recommended. The system is easy to use and the
data is comprehensible and accurate.
The present study has limitations. First, a
single-center study with a relatively small sample size introduces
selection bias. In addition, the present study did not elucidate
whether race, location, lifestyle habits, depression and anxiety or
other factors influence the likelihood of better pain improvement
(34,35). Future studies should expand the
single-center sample size to refine the model and conduct
multicenter studies that will allow random selection of patients
with TN from other centers for external validation. In addition,
because of the retrospective cohort nature of the study, biases
during follow-up are inevitable. Physicians should consider other
relevant factors, including patient overall health, comorbidities
and preferences, alongside the predictive model results when
deciding on the appropriate course of treatment. The present cohort
was followed up for 1 year; longer follow-up is needed to test the
prognostic risk model and effect of MVD on the risk of pain
recurrence in patients with TN.
In summary, the present study developed an online
dynamic nomogram to predict the likelihood of pain recurrence in
patients with TN after MVD. The analysis of ROC, calibration and
DCA curve showed that nomogram had good prediction and calibration
performance. The model is valuable for predicting pain improvement
in patients with TN after MVD, reducing the incidence of
unnecessary MVD; it is a new practical prognostic prediction system
for TN MVD, which is worthy of being used by clinical doctors and
patients, providing them with valuable references when making
decisions regarding treatment options.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW designed the study, collected data and wrote the
manuscript. SL analyzed the data and constructed the model. ZW
collected clinical case data, conducted follow-ups with patients
after discharge, and performed preliminary organization and
analyzed of case data. BZ, DW and ZG performed MVD. JW designed the
study. HW and SL confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Second Affiliated Hospital at Anhui Medical
University (Hefei, China; approval no. 202115). The Helsinki
Declaration was followed. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cruccu G, Di Stefano G and Truini A:
Trigeminal neuralgia. N Engl J Med. 383:754–762. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cruccu G, Gronseth G, Alksne J, Argoff C,
Brainin M, Burchiel K, Nurmikko T and Zakrzewska JM: American
Academy of Neurology Society and European Federation of
Neurological Society. AAN-EFNS guidelines on trigeminal neuralgia
management. Eur J Neurol. 15:1013–1028. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barker FG II, Jannetta PJ, Bissonette DJ,
Larkins MV and Jho HD: The long-term outcome of microvascular
decompression for trigeminal neuralgia. N Engl J Med.
334:1077–1083. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zheng JH, Sun K, Zhang HT, Xie YJ,
Wang-Yang LX, Chen HY and Wang C: A study on the recurrence rate of
trigeminal neuralgia after MVD and the related factors. J Neurol
Surg B Skull Base. 81:572–578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee SH, Levy EI, Scarrow AM, Kassam A and
Jannetta PJ: Recurrent trigeminal neuralgia attribuTable to veins
after microvascular decompression. Neurosurgery. 46:356–361;
discussion 361-362. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Meybodi AT, Habibi Z, Miri M and
Tabatabaie SA: Microvascular decompression for trigeminal neuralgia
using the ‘Stitched Sling Retraction’ technique in recurrent cases
after previous microvascular decompression. Acta Neurochir (Wien).
156:1181–1187; discussion 1187. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hardaway FA, Gustafsson HC, Holste K,
Burchiel KJ and Raslan AM: A novel scoring system as a preoperative
predictor for pain-free survival after microsurgery for trigeminal
neuralgia. J Neurosurg. 1–8. 2019.PubMed/NCBI View Article : Google Scholar : (Online ahead of
print).
|
|
8
|
Panczykowski DM, Jani RH, Hughes MA and
Sekula RF: Development and evaluation of a preoperative trigeminal
neuralgia scoring system to predict long-term outcome following
microvascular decompression. Neurosurgery. 87:71–79.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Katusic S, Beard CM, Bergstralh E and
Kurland LT: Incidence and clinical features of trigeminal
neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 27:89–95.
1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang WB, Min LZ, Tao BB, Sun QY, Li ST
and Wang XQ: Prognosis comparison of different branches of
trigeminal neuralgia. World Neurosurg. 133:e1–e5. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Poshataev VK, Konovalov AN and Shimanskiy
VN: [Surgical management of venous compression causing trigeminal
neuralgia]. Zh Vopr Neirokhir Im N N Burdenko. 81:48–55.
2017.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
12
|
Kumar K, Das KK, Singh S, Khatri D, Deora
H, Singh J, Bhaisora K, Srivastava AK, Jaiswal AK and Behari S:
Vascular offenders in trigeminal neuralgia: A unified
classification and assessment of the outcome of microvascular
decompression. World Neurosurg. 127:e366–e375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu J, Wu G, Jiang Y, Li L, Wang D and Liu
R: Relationship between arterial blood pressure during trigeminal
nerve combing and surgical outcome in patients with trigeminal
neuralgia. World Neurosurg. 137:e98–e105. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zakrzewska JM, Wu N, Lee JYK, Werneburg B,
Hoffman D and Liu Y: Characterizing treatment utilization patterns
for trigeminal neuralgia in the United States. Clin J Pain.
34:691–699. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eller JL, Raslan AM and Burchiel KJ:
Trigeminal neuralgia: Definition and classification. Neurosurg
Focus. 18(E3)2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maarbjerg S, Wolfram F, Gozalov A, Olesen
J and Bendtsen L: Significance of neurovascular contact in
classical trigeminal neuralgia. Brain. 138:311–319. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Headache Classification Committee of the
International Headache Society (IHS) The International
Classification of Headache Disorders, 3rd edition. Cephalalgia.
38:1–211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bendtsen L, Zakrzewska JM, Abbott J,
Braschinsky M, Di Stefano G, Donnet A, Eide PK, Leal PRL, Maarbjerg
S, May A, et al: European academy of neurology guideline on
trigeminal neuralgia. Eur J Neurol. 26:831–849. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Scholz J, Finnerup NB, Attal N, Aziz Q,
Baron R, Bennett MI, Benoliel R, Cohen M, Cruccu G, Davis KD, et
al: The IASP classification of chronic pain for ICD-11: Chronic
neuropathic pain. Pain. 160:53–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leal PR, Hermier M, Souza MA,
Cristino-Filho G, Froment JC and Sindou M: Visualization of
vascular compression of the trigeminal nerve with high-resolution
3T MRI: A prospective study comparing preoperative imaging analysis
to surgical findings in 40 consecutive patients who underwent
microvascular decompression for trigeminal neuralgia. Neurosurgery.
69:15–25; discussion 26. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sandhu SK and Lee JY: Measurement of
trigeminal neuralgia pain: Penn facial pain scale. Neurosurg Clin N
Am. 27:327–336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ishaque AH, Xie H, Danyluk H, Wheatley BM,
Broad R, Kong L and Sankar T: Comparison of prognostic scoring
systems to predict durable pain relief after microvascular
decompression for trigeminal neuralgia. World Neurosurg.
157:e432–e440. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Farrar JT, Young JP Jr, LaMoreaux L, Werth
JL and Poole MR: Clinical importance of changes in chronic pain
intensity measured on an 11-point numerical pain rating scale.
Pain. 94:149–158. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hawker GA, Mian S, Kendzerska T and French
M: Measures of adult pain: Visual Analog Scale for Pain (VAS Pain),
Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire
(MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain
Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS),
and measure of Intermittent and Constant Osteoarthritis Pain
(ICOAP). Arthritis Care Res (Hoboken). 63 (Suppl 11):S240–S252.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Holste K, Chan AY, Rolston JD and Englot
DJ: Pain outcomes following microvascular decompression for
drug-resistant trigeminal neuralgia: A systematic review and
meta-analysis. Neurosurgery. 86:182–190. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen Q, Yi DI, Perez JNJ, Liu M, Chang SD,
Barad MJ, Lim M and Qian X: The molecular basis and pathophysiology
of trigeminal neuralgia. Int J Mol Sci. 23(3604)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Smith CA, Paskhover B and Mammis A:
Molecular mechanisms of trigeminal neuralgia: A systematic review.
Clin Neurol Neurosurg. 200(106397)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Araya EI, Claudino RF, Piovesan EJ and
Chichorro JG: Trigeminal neuralgia: Basic and clinical aspects.
Curr Neuropharmacol. 18:109–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang CC, Lee MH, Yang JT, Chen KT, Huang
WC, Tsai PJ, Kao CH, Liao CW and Lin MH: Percutaneous
radiofrequency trigeminal rhizotomy benefits in patients with
refractory trigeminal neuralgia. Medicine (Baltimore).
101(e29543)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen M, Li Z, Yan Z, Ge S, Zhang Y, Yang
H, Zhao L, Liu L, Zhang X, Cai Y and Qu Y: Predicting neurological
deterioration after moderate traumatic brain injury: Development
and validation of a prediction model based on data collected on
admission. J Neurotrauma. 39:371–378. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen Z, Zhong M, Xu Z, Ye Q, Xie W, Gao S,
Chen L, Qiu L, Jiang J, Wu H, et al: Development and validation of
a nomogram based on geriatric nutritional risk index to predict
surgical site infection among gynecologic oncology patients. Front
Nutr. 9(864761)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guo X, Liu Y, Liu LJ, Li J, Zhao L, Jin
XR, Yan W, Lin BQ, Shi S, Li ZY, et al: Development and validation
of survival nomograms in colorectal cancer patients with
synchronous liver metastases underwent simultaneous surgical
treatment of primary and metastatic lesions. Am J Cancer Res.
11:2654–2669. 2021.PubMed/NCBI
|
|
33
|
Theodosopoulos PV, Marco E, Applebury C,
Lamborn KR and Wilson CB: Predictive model for pain recurrence
after posterior fossa surgery for trigeminal neuralgia. Arch
Neurol. 59:1297–1302. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reinard K, Nerenz DR, Basheer A, Tahir R,
Jelsema T, Schultz L, Malik G, Air EL and Schwalb JM: Racial
disparities in the diagnosis and management of trigeminal
neuralgia. J Neurosurg. 126:368–374. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang B, Zhu W and Li S: Effects of
depression and anxiety on microvascular decompression outcome for
trigeminal neuralgia patients. World Neurosurg. 128:e556–e561.
2019.PubMed/NCBI View Article : Google Scholar
|