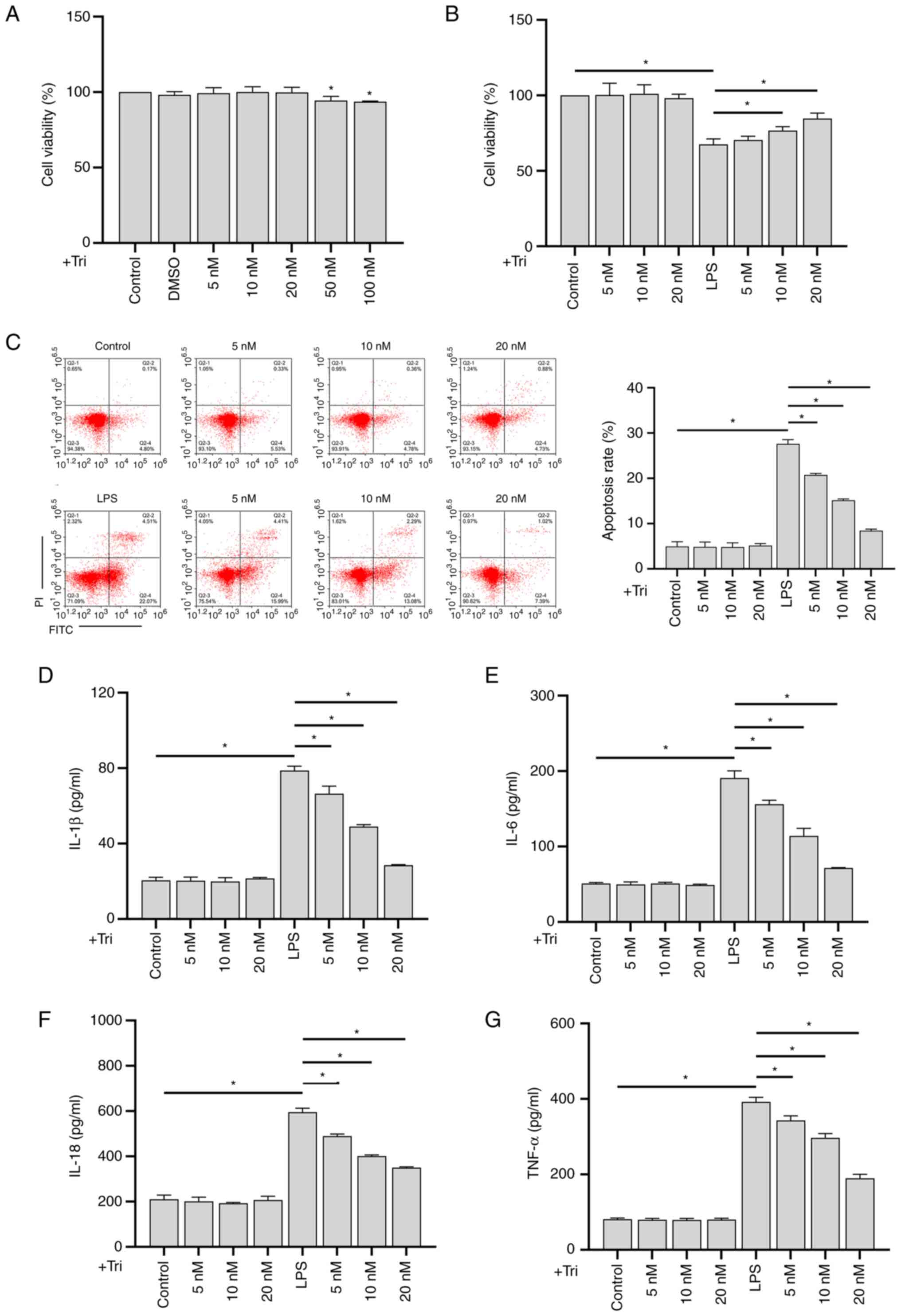
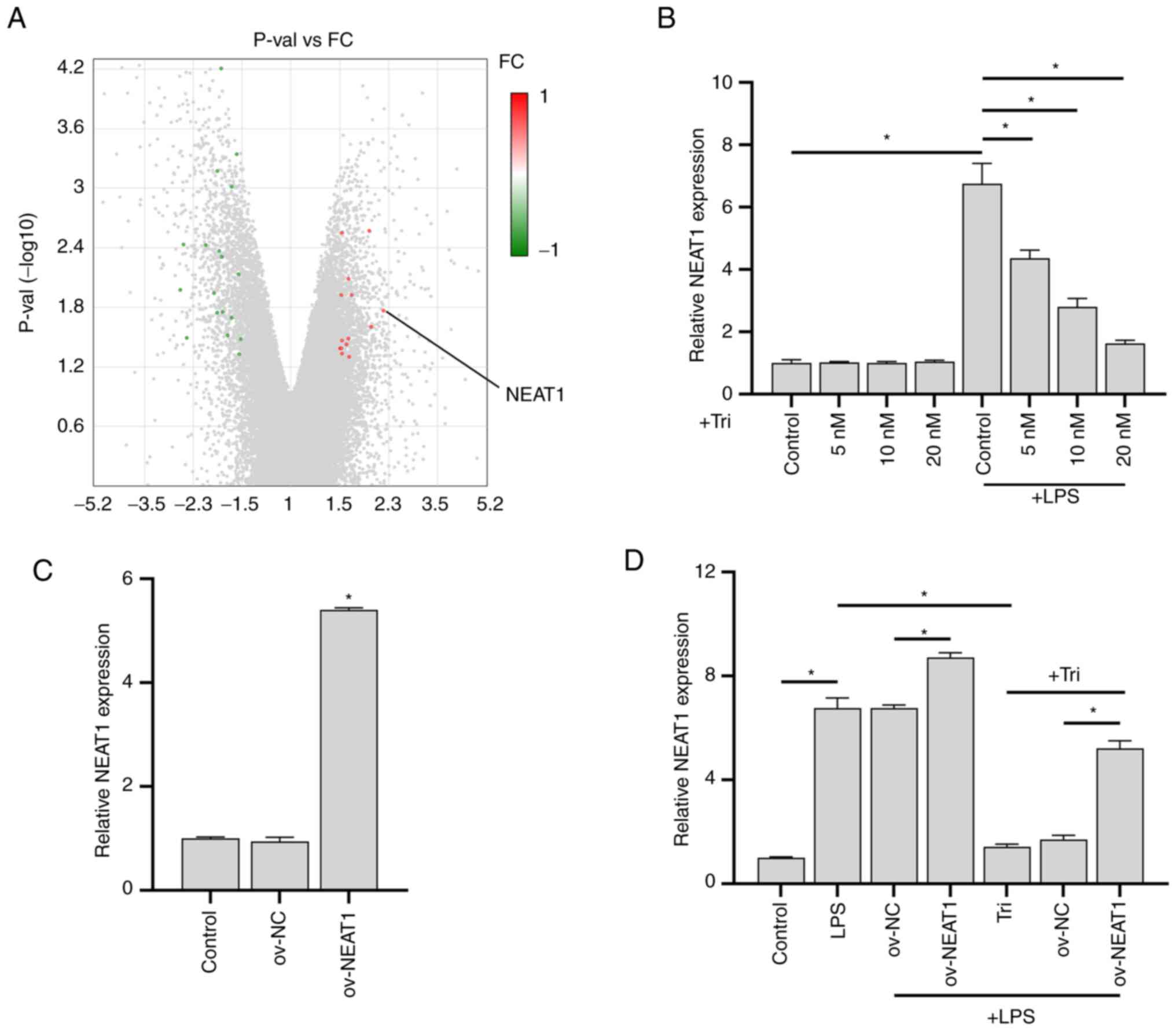
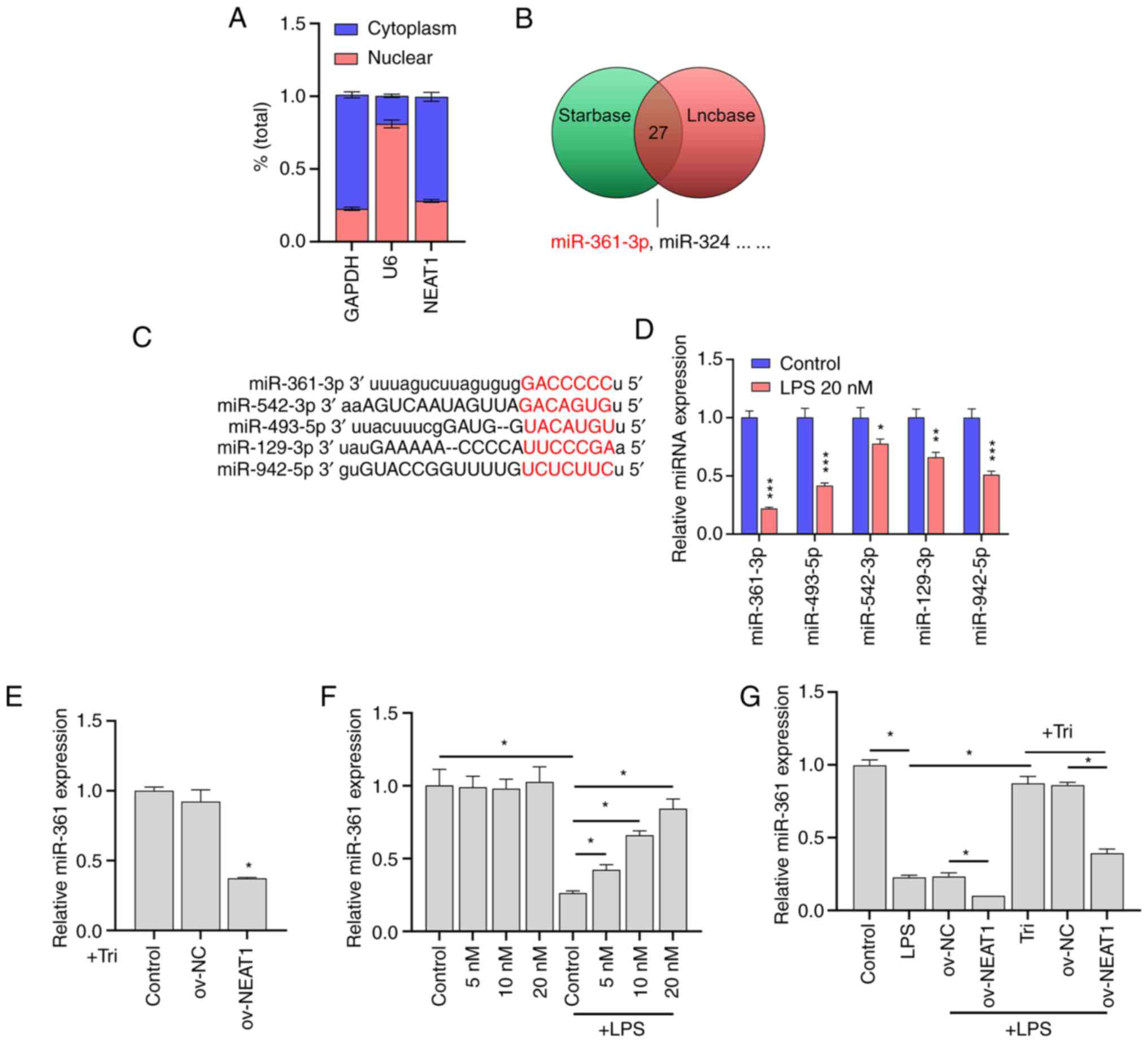
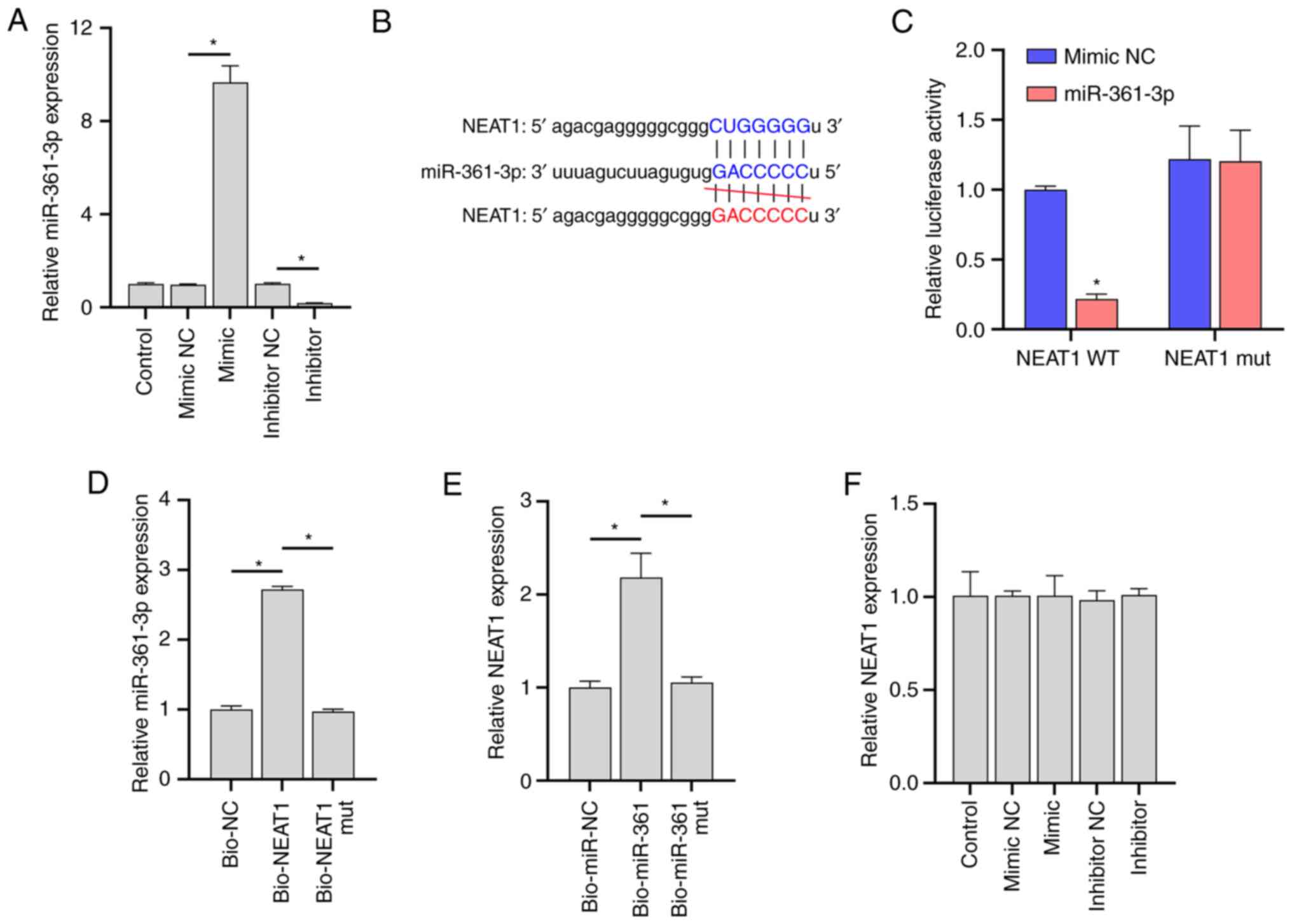
|
1
|
Tiwari S, Atluri V, Kaushik A, Yndart A
and Nair M: Alzheimer's disease: Pathogenesis, diagnostics, and
therapeutics. Int J Nanomedicine. 14:5541–5554. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang XX, Tian Y, Wang ZT, Ma YH, Tan L
and Yu JT: The epidemiology of Alzheimer's disease modifiable risk
factors and prevention. J Prev Alzheimers Dis. 8:313–321.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Serrano-Pozo A, Das S and Hyman BT: APOE
and Alzheimer's disease: Advances in genetics, pathophysiology, and
therapeutic approaches. Lancet Neurol. 20:68–80. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Frölich L and Hausner L: Disease-modifying
treatment approaches for Alzheimer's disease. Nervenarzt.
92:1239–1248. 2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
5
|
Athar T, Al Balushi K and Khan SA: Recent
advances on drug development and emerging therapeutic agents for
Alzheimer's disease. Mol Biol Rep. 48:5629–5645. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Knapskog AB, Engedal K, Selbæk G and
Øksengård AR: Alzheimer's disease-diagnosis and treatment. Tidsskr
Nor Laegeforen. 141:2021.PubMed/NCBI View Article : Google Scholar : (IN Norwegian).
|
|
7
|
Hao P, Jiang F, Cheng J, Ma L, Zhang Y and
Zhao Y: Traditional Chinese medicine for cardiovascular disease:
Evidence and potential mechanisms. J Am Coll Cardiol. 69:2952–2966.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Wang Q, Li C, Lu L, Zhang Q, Zhu R
and Wang W: A review of Chinese herbal medicine for the treatment
of chronic heart failure. Curr Pharm Des. 23:5115–5124.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pei H, Ma L, Cao Y, Wang F, Li Z, Liu N,
Liu M, Wei Y and Li H: Traditional Chinese medicine for Alzheimer's
disease and other cognitive impairment: A review. Am J Chin Med.
48:487–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ju Hwang C, Choi DY, Park MH and Hong JT:
NF-κB as a key mediator of brain inflammation in Alzheimer's
disease. CNS Neurol Disord Drug Targets. 18:3–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng S, LeBlanc KJ and Li L: Triptolide
preserves cognitive function and reduces neuropathology in a mouse
model of Alzheimer's disease. PLoS One. 9(e108845)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiao J, Xue B, Zhang L, Gong Y, Li K, Wang
H, Jing L, Xie J and Wang X: Triptolide inhibits
amyloid-beta1-42-induced TNF-alpha and IL-1beta production in
cultured rat microglia. J Neuroimmunol. 205:32–36. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pan XD and Chen XC: Advances in the study
of immunopharmacological effects and mechanisms of extracts of
Tripterygium wilfordii Hook. f. in neuroimmunologic disorders. Yao
Xue Xue Bao. 43:1179–1185. 2008.PubMed/NCBI(In Chinese).
|
|
14
|
Chen X, Ren G, Li Y, Chao W, Chen S, Li X
and Xue S: Level of LncRNA GAS5 and hippocampal volume are
associated with the progression of Alzheimer's disease. Clin Interv
Aging. 17:745–753. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yan XW, Liu HJ, Hong YX, Meng T, Du J and
Chang C: lncRNA XIST induces Aβ accumulation and neuroinflammation
by the epigenetic repression of NEP in Alzheimer's disease. J
Neurogenet. 36:11–20. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moreno-Garcia L, Lopez-Royo T, Calvo AC,
Toivonen JM, de la Torre M, Moreno-Martinez L, Molina N, Aparicio
P, Zaragoza P, Manzano R and Osta R: Competing endogenous RNA
networks as biomarkers in neurodegenerative diseases. Int J Mol
Sci. 21(9582)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma N, Tie C, Yu B, Zhang W and Wan J:
Identifying lncRNA-miRNA-mRNA networks to investigate Alzheimer's
disease pathogenesis and therapy strategy. Aging (Albany NY).
12:2897–2920. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duan R, Wang SY, Wei B, Deng Y, Fu XX,
Gong PY, E Y, Sun XJ, Cao HM, Shi JQ, et al: Angiotensin-(1-7)
analogue AVE0991 modulates astrocyte-mediated neuroinflammation via
lncRNA SNHG14/miR-223-3p/NLRP3 pathway and offers neuroprotection
in a transgenic mouse model of Alzheimer's disease. J Inflamm Res.
14:7007–7019. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dong LX, Zhang YY, Bao HL, Liu Y, Zhang GW
and An FM: LncRNA NEAT1 promotes Alzheimer's disease by down
regulating micro-27a-3p. Am J Transl Res. 13:8885–8896.
2021.PubMed/NCBI
|
|
20
|
Song Y, Wu Z and Zhao P: The protective
effects of activating Sirt1/NF-κB pathway for neurological
disorders. Rev Neurosci. 33:427–438. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Subhramanyam CS, Wang C, Hu Q and Dheen
ST: Microglia-mediated neuroinflammation in neurodegenerative
diseases. Semin Cell Dev Biol. 94:112–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cai M, Zhuang W, Lv E, Liu Z, Wang Y,
Zhang W and Fu W: Kaemperfol alleviates pyroptosis and
microglia-mediated neuroinflammation in Parkinson's disease via
inhibiting p38MAPK/NF-κB signaling pathway. Neurochem Int.
152(105221)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fu RH, Tsai CW, Chiu SC, Liu SP, Chiang
YT, Kuo YH, Shyu WC and Lin SZ: C9-ALS-associated proline-arginine
dipeptide repeat protein induces activation of NLRP3 inflammasome
of HMC3 microglia cells by binding of complement component 1 Q
subcomponent-binding protein (C1QBP), and syringin prevents this
effect. Cells. 11(3128)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Meng N, Pan P, Hu S, Miao C, Hu Y, Wang F,
Zhang J and An L: The molecular mechanism of γ-aminobutyric acid
against AD: The role of CEBPα/circAPLP2/miR-671-5p in regulating
CNTN1/2 expression. Food Funct. 14:2082–2095. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chia SY, Vipin A, Ng KP, Tu H, Bommakanti
A, Wang BZ, Tan YJ, Zailan FZ, Ng ASL, Ling SC, et al: Upregulated
blood miR-150-5p in Alzheimer's disease dementia is associated with
cognition, cerebrospinal fluid amyloid-β, and cerebral atrophy. J
Alzheimers Dis. 88:1567–1584. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu L, Liu L, Lu Y, Zhang T and Zhao W:
Serum aberrant expression of miR-24-3p and its diagnostic value in
Alzheimer's disease. Biomark Med. 15:1499–1507. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu SY, Fu CL, Liang L, Yang B, Shen W,
Wang QW, Chen Y, Chen YF, Liu YN, Zhu L, et al: miR-218-2 regulates
cognitive functions in the hippocampus through complement component
3-dependent modulation of synaptic vesicle release. Proc Natl Acad
Sci USA. 118(e2021770118)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Feng H, Gui Q, Zhu W, Wu G, Dong X, Shen
M, Luo H, Xue S and Cheng Q: Long-noncoding RNA Peg13 alleviates
epilepsy progression in mice via the miR-490-3p/Psmd11 axis to
inactivate the Wnt/β-catenin pathway. Am J Transl Res.
12:7968–7981. 2020.PubMed/NCBI
|
|
30
|
Fu Y, Hu X, Zheng C, Sun G, Xu J, Luo S
and Cao P: Intrahippocampal miR-342-3p inhibition reduces β-amyloid
plaques and ameliorates learning and memory in Alzheimer's disease.
Metab Brain Dis. 34:1355–1363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu X, Meng X, Tan F, Jiao Z, Zhang X, Tong
H, He X, Luo X, Xu P and Qu S: Regulatory mechanism of miR-543-3p
on GLT-1 in a mouse model of Parkinson's disease. ACS Chem
Neurosci. 10:1791–1800. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cai M, Wang YW, Xu SH, Qiao S, Shu QF, Du
JZ, Li YG and Liu XL: Regulatory effects of the long non-coding RNA
RP11-543N12.1 and microRNA-324-3p axis on the neuronal apoptosis
induced by the inflammatory reactions of microglia. Int J Mol Med.
42:1741–1755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mo JL, Liu Q, Kou ZW, Wu KW, Yang P, Chen
XH and Sun FY: MicroRNA-365 modulates astrocyte conversion into
neuron in adult rat brain after stroke by targeting Pax6. Glia.
66:1346–1362. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xiong YS, Liu FF, Liu D, Huang HZ, Wei N,
Tan L, Chen JG, Man HY, Gong CX, Lu Y, et al: Opposite effects of
two estrogen receptors on tau phosphorylation through disparate
effects on the miR-218/PTPA pathway. Aging Cell. 14:867–877.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang X, Tan L, Lu Y, Peng J, Zhu Y, Zhang
Y and Sun Z: MicroRNA-138 promotes tau phosphorylation by targeting
retinoic acid receptor alpha. FEBS Lett. 589:726–729.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sheinerman KS, Tsivinsky VG, Crawford F,
Mullan MJ, Abdullah L and Umansky SR: Plasma microRNA biomarkers
for detection of mild cognitive impairment. Aging (Albany NY).
4:590–605. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gao Y, Zhang N, Lv C, Li N, Li X and Li W:
lncRNA SNHG1 knockdown alleviates amyloid-β-induced neuronal injury
by regulating ZNF217 via sponging miR-361-3p in Alzheimer's
disease. J Alzheimers Dis. 77:85–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ji Y, Wang D, Zhang B and Lu H: MiR-361-3p
inhibits β-amyloid accumulation and attenuates cognitive deficits
through targeting BACE1 in Alzheimer's disease. J Integr Neurosci.
18:285–291. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang C, Chen S, Guo H, Jiang H, Liu H, Fu
H and Wang D: Forsythoside A mitigates Alzheimer's-like pathology
by inhibiting ferroptosis-mediated neuroinflammation via Nrf2/GPX4
axis activation. Int J Biol Sci. 18:2075–2090. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cai Y, Chai Y, Fu Y, Wang Y, Zhang Y,
Zhang X, Zhu L, Miao M and Yan T: Salidroside ameliorates
Alzheimer's disease by targeting NLRP3 inflammasome-mediated
pyroptosis. Front Aging Neurosci. 13(809433)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fang Z, Tang Y, Ying J, Tang C and Wang Q:
Traditional Chinese medicine for anti-Alzheimer's disease:
Berberine and evodiamine from Evodia rutaecarpa. Chin Med.
15(82)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Luo JF, Dong Y, Chen JY and Lu JH: The
effect and underlying mechanisms of garlic extract against
cognitive impairment and Alzheimer's disease: A systematic review
and meta-analysis of experimental animal studies. J Ethnopharmacol.
280(114423)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang H, Su Y, Sun Z, Chen M, Han Y, Li Y,
Dong X, Ding S, Fang Z and Li W and Li W: Ginsenoside Rg1
alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation
in APP/PS1 mice. J Ginseng Res. 45:665–675. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jiao H and Jia J: Ginsenoside compound K
acts via LRP1 to alleviate Amyloid β42-induced
neuroinflammation in microglia by suppressing NF-κB. Biochem
Biophys Res Commun. 590:14–19. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ning C, Mo L, Chen X, Tu W, Wu J, Hou S
and Xu J: Triptolide derivatives as potential multifunctional
anti-Alzheimer agents: Synthesis and structure-activity
relationship studies. Bioorg Med Chem Lett. 28:689–693.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li H, Zhang X, Chen M, Chen J, Gao T and
Yao S: Dexmedetomidine inhibits inflammation in microglia cells
under stimulation of LPS and ATP by c-Fos/NLRP3/caspase-1 cascades.
EXCLI J. 17:302–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Inoue E, Minatozaki S, Katsuta Y, Nonaka S
and Nakanishi H: Human β-defensin 3 inhibits porphyromonas
gingivalis lipopolysaccharide-induced oxidative and inflammatory
responses of microglia by suppression of cathepsins B and L. Int J
Mol Sci. 23(15099)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Garcia-Contreras M and Thakor AS: Human
adipose tissue-derived mesenchymal stem cells and their
extracellular vesicles modulate lipopolysaccharide activated human
microglia. Cell Death Discov. 7(98)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
He F, Liu Y, Liu S, Wang N, Song H, Xiong
G, Lu J, Yu C and Wang S: Celastrol inhibits neurotoxicity induced
by Cd2. Sheng Wu Gong Cheng Xue Bao. 38:3443–3452.
2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun Z, Du M, Lu Y and Zeng CQ: Effects of
triptolide on the expression of MHC II in microglia in kainic
acid-induced epilepsy. Mol Med Rep. 17:8357–8362. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wu YY and Kuo HC: Functional roles and
networks of non-coding RNAs in the pathogenesis of
neurodegenerative diseases. J Biomed Sci. 27(49)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Riva P, Ratti A and Venturin M: The long
non-coding RNAs in neurodegenerative diseases: Novel mechanisms of
pathogenesis. Curr Alzheimer Res. 13:1219–1231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen M, Lai X, Wang X, Ying J, Zhang L,
Zhou B, Liu X, Zhang J, Wei G and Hua F: Long non-coding RNAs and
circular RNAs: Insights into microglia and astrocyte mediated
neurological diseases. Front Mol Neurosci.
14(745066)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jiang S, Liu H, Zhang J, Zhang F, Fan J
and Liu Y: MMP1 regulated by NEAT1/miR-361-5p axis facilitates the
proliferation and migration of cutaneous squamous cell carcinoma
via the activation of Wnt pathway. Cancer Biol Ther. 22:381–391.
2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Culpan D, Cram D, Chalmers K, Cornish A,
Palmer L, Palmer J, Hughes A, Passmore P, Craig D, Wilcock GK, et
al: TNFR-associated factor-2 (TRAF-2) in Alzheimer's disease.
Neurobiol Aging. 30:1052–1060. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Detrait ER, Danis B, Lamberty Y and Foerch
P: Peripheral administration of an anti-TNF-α receptor fusion
protein counteracts the amyloid induced elevation of hippocampal
TNF-α levels and memory deficits in mice. Neurochem Int. 72:10–13.
2014.PubMed/NCBI View Article : Google Scholar
|