Introduction
Proteus is a gram-negative bacterium, which
is widely distributed in a range of settings, including water
sources, soil and sewage, but it is primarily a flora member of the
gastrointestinal systems of humans and animals (1). Swarming motility, and the production
of urease, hemolysin and numerous fimbrial adhesions are the
phenotypic characteristics of this bacterium (2). Proteus mirabilis is the most
prevalent cause of human infections among all Proteus
species (3). P. mirabilis
is the most frequently detected bacteria in long-term urinary
catheterization and is a significant cause of complicated urinary
tract infections (UTIs), wound infections, gastroenteritis, and, in
rare cases, bacteremia (2,3). A distinctive feature of P.
mirabilis is that it produces crystalline biofilms, leading to
encrusted and clogged catheters in long-term urinary
catheterization, which aggravates catheter-associated urinary tract
infections (CAUTIs) (4).
Therefore, urine retention and reflux, as well as severe bladder
distension and pyelonephritis, may develop. Furthermore,
crystalline biofilms have been reported to be associated with the
persistence of P. mirabilis in the urinary system via
shielding it from antimicrobial agents and the host immune
mechanisms (5).
Colistin exhibits a broad spectrum of antibacterial
activity, mostly against gram-negative bacteria since its
antibacterial activity occurs on the outer membrane (6). However, some gram-negative bacteria
are naturally resistant to colistin, such as Neisseria
meningitidis, Burkholderia species and P.
mirabilis (7). Antimicrobial
resistance is a major public health issue and antimicrobial
resistance in numerous bacterial species has an impact on a number
of facets of medical practice, from treatment of infections in
primary healthcare to the clinical management of patients with
severe diseases in tertiary care (8). The worldwide spread of
antibiotic-resistant bacterial strains poses a considerable
obstacle to appropriate treatment, as there are few clinically
available antibiotics that maintain adequate action against these
strains (9).
Iron is a vital element for growth, and is necessary
for the activity of numerous proteins and enzymes participating in
various physiological pathways, such as oxygen transportation, gene
regulation and nitrogen fixation (10). In the mammalian host, the majority
of intracellular iron is stored in ferritin or bound to haem or
haem-containing substances, whereas extracellular iron is bound to
transferrin, lactoferrin, haemopexin and haptoglobin, making it
unavailable for bacterial uptake (11). Bacterial pathogens that have the
ability to colonize humans use several strategies to scavenge
essential elements, such as iron and zinc; therefore, there is a
perpetual competition between bacteria and the host for
micronutrients (12). Pathogenic
bacteria have evolved multiple iron transportation pathways,
intracellular iron stores, redox stress resistance systems and
iron-sensitive regulatory sequences to control the expression of
genes involved in several cellular activities to counterbalance
iron-deficient situations and sustain iron homeostasis (10). A number of pathogenic bacteria use
siderophores to overcome the iron-limiting environment in the host.
Siderophores are low molecular weight iron-binding substances that
are secreted and imported by microorganisms for iron acquisition
(13). The iron is released from
the siderophore after cellular uptake to aid microbial metabolism
and multiplication. During infection with bacterial and fungal
pathogens, siderophores are thought to be important virulence
components (14). The existence of
these regulatory and competing mechanisms underlines the
significance of iron in the survival of bacteria.
Given the critical function of iron in the growth
and survival of numerous pathogenic bacteria, minimizing the amount
of iron available at the infection site may help to improve the
treatment outcome. Using chelator compounds that sequester various
metals and impede bacterial iron uptake is one of the approaches
for attaining iron limitation (8).
Iron chelating agents can form complexes with iron in both its
ferrous (Fe2+) and ferric (Fe3+) states,
although chelators generally exhibit different levels of affinity
for the different states (15,16).
Deferoxamine (DFO) is a semi-synthetic drug derived from the
bacterial siderophore desferrioxamine B, which has been licensed
for medical use in the treatment of iron excess in patients
(17). DFO also has an impact on
the amount of iron available to microorganisms, this feature
constitutes the founding of new microbial control strategies, such
as novel treatment regimens or even preservation systems (18,19).
Considering the ongoing occurrence and spread of antibiotic
resistance, and the limited prospect that the current development
process will be able to meet the demand for new antimicrobial
agents with novel mechanisms of action, it is necessary to
investigate the potential of alternatives (8). Iron chelators that have already
withstood toxicity and preclinical testing in animals may provide
an alternative therapeutic technique in the case of
multidrug-resistant bacteria, for which entire classes of
antibiotics are no longer considered treatment options (20). Siderophores may also serve as a
facilitator for antibiotics across the cell membrane because of
increased cell membrane permeability induced by iron deprivation
(21). We hypothesize that iron
deprivation or interactions with cell membranes caused by DFO or
increased siderophore synthesis may cause inhibition or
inactivation of proteins and enzymes necessary for critical
processes of bacteria, as well as synergy with membrane-active
antibiotics, such as colistin.
Materials and methods
Bacterial strains
Clinical P. mirabilis isolates (n=11)
recovered from blood culture samples between July and December 2021
in the Research and Application Hospital of Gaziantep University
(Gaziantep, Turkey), a tertiary care center, were used in the
present study. The isolates were collected from 11 individual
patients. Escherichia coli ATCC25922 (American Type Culture
Collection) and E. coli NCTC 13846 (National Collection of
Type Cultures) strains were provided by the Microbiology Laboratory
of Gaziantep University's Medical Faculty Hospital (Gaziantep,
Turkey) and employed as quality control in the broth microdilution
tests. Prior to testing, the isolates were cultured from frozen
stocks onto Columbia Agar with 5% sheep blood (BD Biosciences) and
incubated overnight at 35˚C.
Determination of minimum inhibitory
concentration (MIC)
MIC values of DFO and colistin against P.
mirabilis were determined using the reference broth
microdilution method according to the International Organization
for Standardization (ISO) standards (ISO 20776-1:2019) (22). Colistin (Biosynth Ltd.) stock
solutions were prepared in sterile distilled water
(dH2O) and stored in aliquots at -20˚C. DFO
(Desferal®; Novartis Corporation) was freshly prepared
as a 50 mg/ml stock solution in sterile dH2O prior to
the study. Test solutions of DFO and colistin were prepared
immediately before use. Briefly, serial two-fold dilutions of DFO
and colistin in cation-adjusted Mueller-Hinton Broth (CAMHB; Oxoid;
Thermo Fisher Scientific, Inc.) were prepared in a 96-well plate.
The inoculum to be tested was prepared from overnight cultures by
dilution in MHB to provide a final bacterial density of
5x105 colony-forming unit (CFU)/ml. Colistin was tested
over a range from 0.062 to 128 µg/ml, and DFO was tested over a
range from 0.062 to 10 mg/ml. Each well was loaded with bacterial
suspensions, and the plates were incubated at 35˚C for 24 h.
Controls for positive growth and negative sterility were also
performed. The lowest concentrations of DFO and colistin with no
visible signs of turbidity were defined as MIC values.
Checkerboard assay
Fractional inhibitory concentration index (FICI)
values of combinations of DFO and colistin against a total of 11
P. mirabilis isolates were determined using the checkerboard
technique (23,24). In brief, serial two-fold dilutions
of the first compound (colistin) were prepared across the columns
and the second compound (DFO) dilutions were prepared across the
rows of a 96-well plate. Individual wells were inoculated with
suspensions of overnight cultures in MHB to provide a final
inoculum density of 5x105 CFU/ml. The plates were
incubated for 24 h at 35˚C. FIC values were defined by broth
microdilution on separate checkerboard panels containing increasing
concentrations of DFO (rows G through A, 8 to 512 µg/ml) and
colistin (columns 1 through 11, 0.06 to 64 µg/ml). The FIC of both
drugs was calculated using the formula: FIC=MIC of Drug in
combination/MIC of Drug alone. The FICI was then calculated from
the sum of the FIC values using the following formula: FICI=(MIC of
colistin in combination/MIC of colistin alone) + (MIC of DFO in
combination/MIC of DFO alone). The interpretation of the findings
was as follows: Synergy if FICI ≤0.5; no interaction if FICI 0.5-4;
and antagonism if FICI >4(23).
On an occasion where a MIC for one of the test compounds was
off-scale (greater than the highest concentration tested), the MIC
was set to the next highest two-fold concentration for calculation
of the FIC (e.g. if the highest MIC was tested 32 µg/ml, the FIC
was calculated based on a MIC of 64 µg/ml) (25).
Time-kill assay
In accordance with the results from the checkerboard
assays, two isolates were randomly selected for further evaluation
of the DFO-colistin combination using a time-kill assay. Colistin
was tested alone and in combination with DFO for the selected
isolates at 0.5X, 1X and 2X the MIC concentrations of colistin. For
isolates where the DFO MIC was >512 µg/ml, a DFO concentration
of 128 µg/ml (0.25X the highest concentration tested) was used
during testing. In preparation for the study, bacterial suspensions
were prepared in MHB from freshly-grown blood agar plates, diluted
and grown to the log-phase. The turbidity of bacterial cultures was
adjusted to form a final inoculum density of 5x105
CFU/ml, as verified by viable count, and added to flasks containing
20 ml MHB. Then, treatments were added to broth cultures to yield
desired concentrations. Growth controls without any treatment were
also included. Test and control flasks were incubated at 35˚C and
viable counts were performed at 0, 1, 3, 6, 9, 12 and 24 h by
serial dilution plating. Samples were spread onto Mueller-Hinton
Agar (MHA; Difco; BD Biosciences) plates, because swarming ability
of Proteus makes colony counting difficult on media other
than MHA. All plates were incubated for 20-24 h at 35˚C. Colonies
were counted manually, and the CFU/ml was determined from the
average count of the duplicate plates, followed by the calculation
of the log10 CFU/ml. Antimicrobial activity was calculated for each
isolate as the change in the bacterial count of (Δlog10
CFU/ml) obtained in 24 h compared with the count at the start and
defined as Δlog10 CFU24. Bactericidal
activity was evaluated as a ≥3 log10 decrease in CFU/ml
over the time period examined, whereas synergy was considered a ≥2
log10 decrease in CFU/ml for the antibiotic combination
in comparison with the most effective monotherapy (26,27).
Time-kill studies were performed as three independent replicates
and graphs displaying the results were generated using Excel
(version 16; Microsoft Corp.).
Results
Broth microdilution and checkerboard
method
MICs, detected by broth microdilution method, were
8-16 µg/ml for colistin. However, DFO did not affect bacterial
growth even at a concentration of 10 mg/ml (Table I). Because the DFO may easily
transfer iron to bacteria with a homologous siderophore receptor
and because CAMHB is a rich broth with abundant iron, the outcome
was not surprising. Using EUCAST criteria, all P. mirabilis
strains were classified as resistant to colistin (28). After combination with DFO, the MIC
values of colistin were reduced. The results of a checkerboard
assay showed synergy (i.e. FICI ≤0.5) between colistin and DFO for
all of the isolates (Table I). No
antagonism was observed for the combination.
 | Table IAntimicrobial activity of colistin
with DFO against PM strains. |
Table I
Antimicrobial activity of colistin
with DFO against PM strains.
| | MIC value,
µg/ml | |
|---|
| Isolate | Colistin | DFO | Colistin MIC in
combination with DFO | FICI | Interpretation |
|---|
| PM1 | 16 | >512 | 0.5 | 0.06 | Synergy |
| PM2 | 16 | >512 | 4 | 0.5 | Synergy |
| PM3 | 16 | >512 | 0.5 | 0.06 | Synergy |
| PM4 | 16 | >512 | 1 | 0.12 | Synergy |
| PM5 | 16 | >512 | 0.5 | 0.06 | Synergy |
| PM6 | 16 | >512 | 0.5 | 0.06 | Synergy |
| PM7 | 16 | >512 | 4 | 0.5 | Synergy |
| PM8 | 16 | >512 | 4 | 0.5 | Synergy |
| PM9 | 16 | >512 | 0.5 | 0.06 | Synergy |
| PM10 | 8 | >512 | 0.5 | 0.09 | Synergy |
| PM11 | 16 | >512 | 0.5 | 0.06 | Synergy |
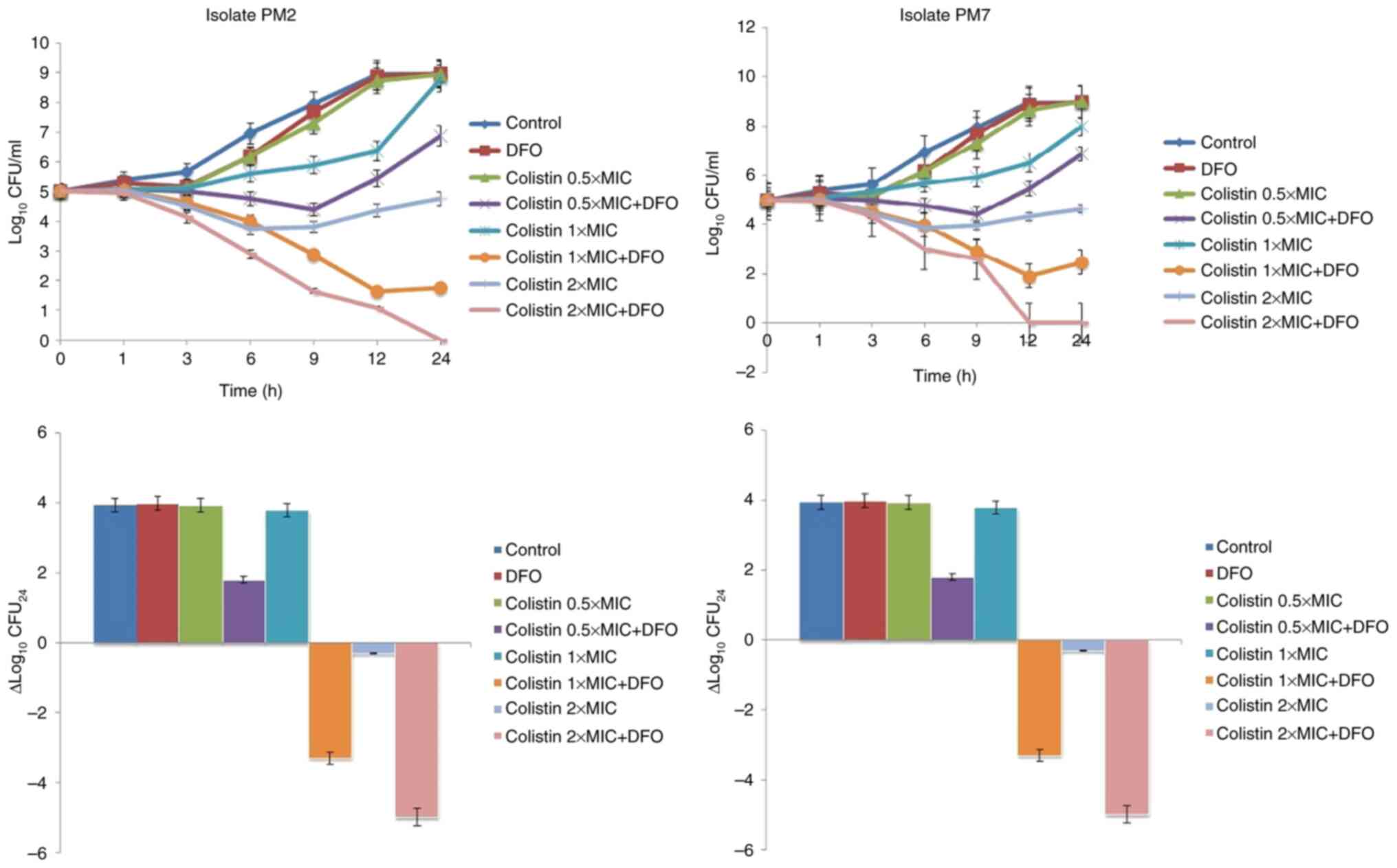
Time-kill method
The two randomly selected P. mirabilis
isolates (PM2 and PM7) were used in the time-kill assays. The
time-kill profiles of isolates and changes in log10
CFU/ml from the initial inoculum to 24 h are shown in Fig. 1. DFO monotherapy (512 µg/ml) and
colistin monotherapy at a concentration of 0.5xMIC produced little
or no bacterial killing at any timepoint, with bacterial growth
close to control values across the 24 h. Colistin monotherapy at
concentrations of 1xMIC and 2xMIC showed bacteriostatic activity
maintained for 9 h, with subsequent re-growth in varying amounts.
Colistin + DFO showed synergistic activity against all strains at
most time intervals and concentrations tested. Colistin + DFO
showed bactericidal activity at colistin concentrations of 1xMIC
and 2xMIC although a degree of re-growth was observed in isolate
PM7 at 12-24 h (Fig. 1).
Discussion
A limitation of the present study is that there were
no animal experiments conducted, in which the physiological
environment could be better evaluated, since they were not included
in the initial research design and ethics committee approvals.
Excess iron has been shown to aggravate the situation in various
infections, including tuberculosis, malaria, invasive bacterial
infections, cystitis, keratitis and wound infections (29-31).
P. mirabilis is a significant causative agent of UTIs,
especially CAUTIs. Bacterial capability to import Fe3+,
Zn2+ and Ni2+, and export Cu+ is
essential for efficacious colonization of the urinary system
(32). In the course of a UTI, it
has been shown that iron overload in the bladder or in the urine
can exacerbate inflammation and escalate urothelial cell death
(33). Urine samples from patients
with acute UTIs have markedly higher iron levels and sloughed
epithelial cells than urine samples from the healthy population
(32). In addition, it has been
reported that, for infants, having higher basal iron serum
concentrations or increased iron supplementation is associated with
an increased risk of UTIs (34-36).
Similarly, iron concentrations in the urine of postmenopausal women
who are prone to recurrent and chronic UTIs have been shown to be
higher than those in the general postmenopausal female population
(37-39).
Increased ambient iron has also been reported to lead to enhanced
growth and a significant increase in the intracellular bacterial
load of E. coli, another frequent causative agent of UTIs,
in bladder epithelial cells (33).
Given that high iron levels enhance bacterial colonization,
infection development and infection chronicity in specific systems,
limiting ambient iron appears to be an appropriate strategy to
battle pathogenic microorganisms.
Sequestration of iron by chelation may be a
beneficial adjunct for the treatment of infections, given the
relationship between iron excess or dietary iron supplementation
and infection (40). For a number
of years, iron chelators have been used to treat iron excess
conditions, and their pharmacological and safety profiles have been
widely investigated (41).
Depletion of iron through synthetic iron chelators can be effective
in inhibiting bacterial growth (29). DFO, as an iron chelator, aids the
host's intrinsic iron-withholding systems and appears as a
promising treatment option for local infections (21). Iron chelation with DFO has been
reported to improve host cell survival, reduce bacterial
proliferation in urothelial cells and reduce autophagy (33). Furthermore, DFO has shown
antibacterial activity as a single agent, and can inhibit the
growth of Acinetobacter baumannii, Pseudomonas
aeruginosa and Staphylococcus aureus at varying
concentrations (21). Furthermore,
impaired iron acquisition can reduce the urinary tract colonization
of P. mirabilis (42). To the best of our knowledge,
the present study is the first investigation to determine DFO MIC
values against P. mirabilis isolates using the standard
broth microdilution method. However, the results revealed that DFO
does not have the potential to be an antibacterial agent on its
own.
An overabundance of iron is hazardous to the host,
not just because it enhances bacterial growth, but also because it
induces increased inflammatory activity and epithelial cell stress
(33,43). Furthermore, local iron deprivation
may have an effect on the host immune system response by reducing
local reactive oxygen species (ROS) formation (29). It has been demonstrated that the
cumulation of iron causes an elevated inflammatory response in
local bladder tissue, as well as increased bacterial colonization,
whereas these findings can be reversed by a low-iron diet (44). During the course of infection,
unbound labile iron accumulates in the plasma and induces a variety
of responses, including elevated cell proliferation rates,
persistent suppression of cell proliferation, and apoptotic or
necrotic cell death via ROS. Therefore, pharmacological regulation
of iron by chelation therapy is crucial for achieving a balance
between inhibiting cell damage and supplying the cellular demands
(29). Although the most concrete
indicator of the success of medical treatment against infections is
the discontinuation of bacterial growth, since limiting ambient
iron reduces inflammation, iron chelators combined with antibiotics
can reduce patient complaints and symptoms.
Medical device-associated infections are frequently
related to indwelling objects, such as urinary catheters and
prosthetic joints, and are responsible for ~50% of all nosocomial
infections (45). These objects
provide an abiotic surface and facilitate the formation of biofilms
(46). Biofilm production is an
important virulence factor in the emergence of CAUTIs (42). Biofilm-related infections are
notoriously difficult to treat due to the adjustments enabled by
biofilm development, which result in increased antibiotic
resistance as well as increased resistance to host immune
mechanisms (46). Higher levels of
iron are required for the production of biofilms than for bacterial
proliferation (47,48). A recent study showed that iron can
trigger biofilm formation in P. mirabilis (42). Similarly, biofilm production
is decreased when lactoferrin, a physiological iron chelator, is
added to P. aeruginosa cultures (49). A previous study also observed that
DFO can improve the ability of tobramycin to dissolve formed
biofilms grown on human airway epithelial cells (50). Iron chelation may be useful in
infection treatment due to the crucial function of iron in biofilm
development and bacterial pathogenicity (29). Inhibition of bacterial iron
acquisition via catheters made of iron-scavenging materials or
coated with chelators may decrease biofilm development and further
infection, but this approach has yet to be tested in a clinical
setting (29,42). However, generally, it has been
assumed that the local administration of iron-chelating agents
represents a safe pharmacological therapy with a low risk of side
effects (29). Iron chelation may
be employed as a prophylactic strategy to minimize medical
device-associated infections, especially for certain systems, such
as the urinary system.
Bacteria sense an iron-deficient environment and
react correspondingly by upregulating iron-acquisition pathways as
well as virulence genes (51). A
β-barrel receptor on the outer membrane of gram-negative bacteria
recognizes iron-bound siderophores. The iron-bound siderophore is
translocated into the periplasm after ligand engagement causes a
conformational alteration (29).
An ATP-binding cassette transporter in the inner membrane mediates
conformational alterations in transportation into the cytoplasm and
iron reduction. This complex will then attach to certain receptor
proteins on the bacterial cell surface, allowing it to be taken
through active transport (29). It
is hypothesized that iron limitation conditions may result in
increased production of siderophores, which are specific molecules
for transporting iron. Siderophore secretion has the physical
outcome of allowing molecules to diffuse away from producers while
possibly preventing diffused molecules from returning producer
cells. Diffusion can still result in significant siderophore loss,
putting bacterial fitness at risk (52). In the present study, in addition to
discovering a synergistic interaction between DFO and colistin in
all P. mirabilis isolates using the checkerboard method, the
MIC values of the DFO and colistin combination were in the
susceptible ranges of colistin against Enterobacterales for the
vast majority of the isolates, according to EUCAST guidelines
(28). However, in vivo
experiments would be necessary to confirm the in vitro
findings. According to data not shown, no significant synergy was
detected in E. coli, K. pneumoniae, P.
aeruginosa and A. baumannii isolates in the subsequent
investigation to assess whether DFO can exhibit synergistic effects
when used with colistin against other gram-negative bacteria. From
this point of view, we hypothesize that the ability of all bacteria
to produce siderophores and their responses to iron restriction is
not the same, and the synergy of DFO and colistin detected in the
present study is based on a specific mechanism. Effective
siderophore production was previously thought to be a feature of
aerobic gram-negative bacteria (53). However, it has been discovered that
some gram-positive bacteria may generate siderophores (54). The identification of bacterial
species that lack effective siderophore production will become more
relevant if iron chelators are combined with antimicrobial
treatment.
Colistin is a polymyxin antibiotic with rapid
bactericidal activity against gram-negative bacteria (55). Similarly, the bactericidal action
of the DFO-colistin combination (colistin 1xMIC + DFO, colistin
2xMIC+ DFO) was evident from the onset of the present study,
according to our time-kill assay results. As a result, it was
hypothesized that several intrinsic mechanisms related to colistin
resistance of P. mirabilis were rapidly eliminated via the
addition of DFO. Furthermore, the fact that the bactericidal effect
of the DFO-colistin combination was maintained for ≥12 h in the
present experiments indicated that it could be beneficial without
the requirement for frequent dose repetitions since colistin has a
long half-life (14.4 h) (56).
This insight can be beneficial for optimizing doses and improving
treatment approaches when combined with the pharmacokinetic profile
of the targeted patient population. Conformational changes in the
outer membrane of the bacteria during both increased secretion and
uptake of siderophores may be responsible for vulnerabilities
against colistin activity. Similarly, vancomycin, which is not
preferred in P. aeruginosa infections due to its low
gram-negative activity, has been reported to be effective when used
with the DFO-gallium complex due to disturbance of the outer
membrane via a combination of electrostatic and hydrophobic
interactions coupled with selective binding and resulting in
enhanced permeability of vancomycin as a result of the membrane
damage (57). Under low iron
concentrations, several physiological changes may occur in the
bacterial pathogens, including a shift to a planktonic state
(58,59). Bacteria in a planktonic state are
known to be more susceptible to certain antimicrobials, suggesting
a potential mechanism of iron chelation-induced sensitization to
antimicrobials (8). Because of the
increased permeability induced by iron deprivation, siderophores
may potentially serve as a facilitator for colistin across the cell
membrane. The use of antivirulence compounds combined with
antibiotics may be a promising approach for virulence attenuation
and pathogen elimination (60). In
addition to binding iron, DFO may also have an affinity for zinc.
The ability to sequestrate zinc is thought to be responsible for
making metallo-β lactamase producers susceptible to β-lactams
(61). Similarly, deprivation of
iron reduces the activity of key proteins and enzymes, such as
cytochromes, which are examples of iron-dependent proteins that are
crucial for energy metabolism and ribonucleotide reductase, which
is involved in DNA synthesis. If any of these are disrupted, the
multiplication of the microorganism may be halted (21). Briefly, inhibition of bacterial
growth, reducing biofilm formation, membrane disruption,
inactivation of specific enzymes or proteins that are essential for
cell replication, and reducing oxidant stress on host cells may
constitute potential pathways for the enhanced bactericidal effect
of colistin via iron chelation. We are currently investigating
whether the synergistic interaction discovered between DFO and
colistin extends to other antibiotics. Identifying patient groups
and explaining the mechanisms underlying the synergistic
interaction will be aided by determining the interactions between
different drug groups and iron chelators.
In conclusion, the present study suggested that DFO
has the potential for use as an adjunct to colistin through iron
sequestration, thus providing synergistic activity to an antibiotic
that is not normally considered a treatment option against P.
mirabilis. In vivo experiments will provide useful
information on DFO-colistin efficacy, since these models are better
in terms of reflecting physiological conditions such as metal ion
levels. Also, in vivo models account for parameters, such as
compound biodistribution, pH and the presence of host factors.
Because CAMHB is a rich broth with substantially higher iron,
carbon sources and other cofactors than the levels in the human
body, the synergy that was detected in vitro may be greater
in vivo.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Scientific Research
Programme of Gaziantep University (grant no. TF.UT.21.28).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ME and YZ contributed to the study conception and
design, and performed material preparation, data collection and
analysis. The first draft of the manuscript was written by ME and
both authors commented on previous versions of the manuscript. ME
and YZ confirm the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of The Declaration of Helsinki. Approval was granted by
the Gaziantep University Clinical Research Ethics Committee (date,
January 27, 2021; approval no. 2021/11; Gaziantep, Turkey).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Drzewiecka D: Significance and roles of
proteus spp. Bacteria in natural environments. Microb Ecol.
72:741–758. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mobley HLT: Proteus mirabilis
overview. Methods Mol Biol. 2021:1–4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Armbruster CE, Mobley HLT and Pearson MM:
Pathogenesis of Proteus mirabilis infection. EcoSal Plus.
8(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wasfi R, Hamed SM, Amer MA and Fahmy LI:
Proteus mirabilis Biofilm: Development and therapeutic
strategies. Front Cell Infect Microbiol. 10(414)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yuan F, Huang Z, Yang T, Wang G, Li P,
Yang B and Li J: Pathogenesis of Proteus mirabilis in
catheter-associated urinary tract infections. Urol Int.
105:354–361. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
El-Sayed Ahmed MAE, Zhong LL, Shen C, Yang
Y, Doi Y and Tian GB: . Colistin and its role in the Era of
antibiotic resistance: An extended review (2000-2019). Emerg
Microbes Infect. 9:868–885. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gogry FA, Siddiqui MT, Sultan I and Haq
QMR: Current update on intrinsic and acquired colistin resistance
mechanisms in bacteria. Front Med (Lausanne).
8(677720)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vinuesa V and McConnell MJ: Recent
advances in iron chelation and gallium-based therapies for
antibiotic resistant bacterial infections. Int J Mol Sci.
22(2876)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cassini A, Högberg LD, Plachouras D,
Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar
ME, Devleesschauwer B, Cecchini M, et al: Attributable deaths and
disability-adjusted life-years caused by infections with
antibiotic-resistant bacteria in the EU and the European economic
area in 2015: A population-level modelling analysis. Lancet Infect
Dis. 19:56–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Andrews SC, Robinson AK and
Rodríguez-Quiñones F: Bacterial iron homeostasis. FEMS Microbiol
Rev. 27:215–237. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sousa Gerós A, Simmons A, Drakesmith H,
Aulicino A and Frost JN: The battle for iron in enteric infections.
Immunology. 161:186–199. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seyoum Y, Baye K and Humblot C: Iron
homeostasis in host and gut bacteria-a complex interrelationship.
Gut Microbes. 13:1–19. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Palmer LD and Skaar EP: Transition metals
and virulence in bacteria. Annu Rev Genet. 50:67–91.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sassone-Corsi M, Chairatana P, Zheng T,
Perez-Lopez A, Edwards RA, George MD, Nolan EM and Raffatellu M:
Siderophore-based immunization strategy to inhibit growth of
enteric pathogens. Proc Natl Acad Sci USA. 113:13462–13467.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou T, Winkelmann G, Dai ZY and Hider RC:
Design of clinically useful macromolecular iron chelators. J Pharm
Pharmacol. 63:893–903. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu ZD and Hider RC: Design of clinically
useful iron(III)-selective chelators. Med Res Rev. 22:26–64.
2022.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Byrne SL, Krishnamurthy D and
Wessling-Resnick M: Pharmacology of iron transport. Annu Rev
Pharmacol Toxicol. 53:17–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kontoghiorghes GJ, Kolnagou A, Skiada A
and Petrikkos G: The role of iron and chelators on infections in
iron overload and non iron loaded conditions: Prospects for the
design of new antimicrobial therapies. Hemoglobin. 34:227–239.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Holbein BE and Mira de Orduña R: Effect of
trace iron levels and iron withdrawal by chelation on the growth of
Candida albicans and Candida vini. FEMS Microbiol
Lett. 307:19–24. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Thompson MG, Corey BW, Si Y, Craft DW and
Zurawski DV: Antibacterial activities of iron chelators against
common nosocomial pathogens. Antimicrob Agents Chemother.
56:5419–5421. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gokarn K and Pal RB: Activity of
siderophores against drug-resistant Gram-positive and Gram-negative
bacteria. Infect Drug Resist. 11:61–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
International Organization for
Standardization (ISO): ISO 20776-1:2019(en) Susceptibility testing
of infectious agents and evaluation of performance of antimicrobial
susceptibility test devices. Part 1: Broth micro-dilution reference
method for testing the in vitro activity of antimicrobial agents
against rapidly growing aerobic bacteria involved in infectious
diseases. ISO, Geneva, Switzerland, 2019. https://www.iso.org/obp/ui/en/#iso:std:iso:20776:-1:ed-2:v2:en.
Accessed March 24, 2022.
|
|
23
|
Xu X, Xu L, Yuan G, Wang Y, Qu Y and Zhou
M: Synergistic combination of two antimicrobial agents closing each
other's mutant selection windows to prevent antimicrobial
resistance. Sci Rep. 8(7237)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stein C, Makarewicz O, Bohnert JA, Pfeifer
Y, Kesselmeier M, Hagel S and Pletz MW: Three dimensional
checkerboard synergy analysis of colistin, meropenem, tigecycline
against multidrug-resistant clinical Klebsiella pneumonia
isolates. PLoS One. 10(e0126479)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thwaites M, Hall D, Stoneburner A,
Shinabarger D, Serio AW, Krause KM, Marra A and Pillar C: Activity
of plazomicin in combination with other antibiotics against
multidrug-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis.
92:338–345. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Clinical and Laboratory Standards
Institute (CLSI): M26-A Methods for determining bactericidal
activity of antimicrobial agents; Approved guideline. Volume 19.
CLSI, Wayne, PA, USA, 1999.
|
|
27
|
Gómez-Junyent J, Benavent E, Sierra Y, El
Haj C, Soldevila L, Torrejón B, Rigo-Bonnin R, Tubau F, Ariza J and
Murillo O: Efficacy of ceftolozane/tazobactam, alone and in
combination with colistin, against multidrug-resistant
Pseudomonas aeruginosa in an in vitro biofilm
pharmacodynamic model. Int J Antimicrob Agents. 53:612–619.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
The European Committee on Antimicrobial
Susceptibility Testing (EUCAST): Breakpoint tables for
interpretation of MICs and zone diameters Version 12.0, 2022.
https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf.
Accessed July 15, 2022.
|
|
29
|
Scott C, Arora G, Dickson K and Lehmann C:
Iron chelation in local infection. Molecules.
26(189)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Soofi S, Cousens S, Iqbal SP, Akhund T,
Khan J, Ahmed I, Zaidi AK and Bhutta ZA: Effect of provision of
daily zinc and iron with several micronutrients on growth and
morbidity among young children in Pakistan: A cluster-randomised
trial. Lancet. 382:29–40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Isanaka S, Aboud S, Mugusi F, Bosch RJ,
Willett WC, Spiegelman D, Duggan C and Fawzi WW: Iron status
predicts treatment failure and mortality in tuberculosis patients:
A prospective cohort study from Dar es Salaam, Tanzania. PLoS One.
7(e37350)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Subashchandrabose S and Mobley HL: Back to
the metal age: battle for metals at the host-pathogen interface
during urinary tract infection. Metallomics. 7:935–942.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bauckman KA and Mysorekar IU:
Ferritinophagy drives uropathogenic Escherichia coli
persistence in bladder epithelial cells. Autophagy. 12:850–863.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mava Y, Ambe JP, Bello M, Watila I and
Nottidge VA: Urinary tract infection in febrile children with
sickle cell anaemia. West Afr J Med. 30:268–272. 2011.PubMed/NCBI
|
|
35
|
Collard KJ: Iron homeostasis in the
neonate. Pediatrics. 123:1208–1216. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Arshad M and Seed PC: Urinary tract
infections in the infant. Clin Perinatol. 42:17–28. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Matsumoto T: Urinary tract infections in
the elderly. Curr Urol Rep. 2:330–333. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pfrimer K, Micheletto RF, Marchini JS,
Padovan GJ, Moriguti JC and Ferriolli E: Impact of aging on urinary
excretion of iron and zinc. Nutr Metab Insights. 7:47–50.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Detweiler K, Mayers D and Fletcher SG:
Bacteruria and urinary tract infections in the elderly. Urol Clin
North Am. 42:561–568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Carver PL: The battle for iron between
humans and microbes. Curr Med Chem. 25:85–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kovacevic Z, Kalinowski DS, Lovejoy DB,
Quach P, Wong J and Richardson DR: Iron chelators: Development of
novel compounds with high and selective anti-tumour activity. Curr
Drug Deliv. 7:194–207. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Iribarnegaray V, González MJ, Caetano AL,
Platero R, Zunino P and Scavone P: Relevance of iron metabolic
genes in biofilm and infection in uropathogenic Proteus
mirabilis. Curr Res Microb Sci. 2(100060)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Silva B and Faustino P: An overview of
molecular basis of iron metabolism regulation and the associated
pathologies. Biochim Biophys Acta. 1852:1347–1359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bauckman KA, Matsuda R, Higgins CB,
DeBosch BJ, Wang C and Mysorekar IU: Dietary restriction of iron
availability attenuates UPEC pathogenesis in a mouse model of
urinary tract infection. Am J Physiol Renal Physiol. 316:F814–F822.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
VanEpps JS and Younger JG: Implantable
device-related infection. Shock. 46:597–608. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vestby LK, Grønseth T, Simm R and Nesse
LL: Bacterial Biofilm and its role in the pathogenesis of disease.
Antibiotics (Basel). 9(59)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Singh PK, Tack BF, McCray PB Jr and Welsh
MJ: Synergistic and additive killing by antimicrobial factors found
in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol.
279:L799–L805. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rogan MP, Taggart CC, Greene CM, Murphy
PG, O'Neill SJ and McElvaney NG: Loss of microbicidal activity and
increased formation of biofilm due to decreased lactoferrin
activity in patients with cystic fibrosis. J Infect Dis.
190:1245–1253. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
49
|
Sharma P, Dube D, Sinha M, Yadav S, Kaur
P, Sharma S and Singh TP: Structural insights into the dual
strategy of recognition by peptidoglycan recognition protein,
PGRP-S: Structure of the ternary complex of PGRP-S with
lipopolysaccharide and stearic acid. PLoS One.
8(e53756)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Moreau-Marquis S, O'Toole GA and Stanton
BA: Tobramycin and FDA-approved iron chelators eliminate
Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am
J Respir Cell Mol Biol. 41:305–313. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zughaier SM and Cornelis P: Editorial:
Role of iron in bacterial pathogenesis. Front Cell Infect
Microbiol. 8(344)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kramer J, Özkaya Ö and Kümmerli R:
Bacterial siderophores in community and host interactions. Nat Rev
Microbiol. 18:152–163. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ito A, Sato T, Ota M, Takemura M,
Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto
S, et al: In Vitro Antibacterial Properties of Cefiderocol, a Novel
Siderophore Cephalosporin, against Gram-Negative Bacteria.
Antimicrob Agents Chemother. 62:e01454–e01417. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sheldon JR and Heinrichs DE: Recent
developments in understanding the iron acquisition strategies of
gram positive pathogens. FEMS Microbiol Rev. 39:592–630.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sabnis A, Hagart KL, Klöckner A, Becce M,
Evans LE, Furniss RCD, Mavridou DA, Murphy R, Stevens MM, Davies
JC, et al: Colistin kills bacteria by targeting lipopolysaccharide
in the cytoplasmic membrane. Elife. 10(e65836)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Plachouras D, Karvanen M, Friberg LE,
Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I,
Poulakou G, Kontopidou F, Armaganidis A, et al: Population
pharmacokinetic analysis of colistin methanesulfonate and colistin
after intravenous administration in critically ill patients with
infections caused by gram-negative bacteria. Antimicrob Agents
Chemother. 53:3430–3436. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Qiao J, Liu Z, Cui S, Nagy T and Xiong MP:
Synthesis and evaluation of an amphiphilic deferoxamine:
Gallium-conjugated cationic random copolymer against a murine wound
healing infection model of Pseudomonas aeruginosa. Acta
Biomater. 126:384–393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Banin E, Brady KM and Greenberg EP:
Chelator-induced dispersal and killing of Pseudomonas
aeruginosa cells in a biofilm. Appl Environ Microbiol.
72:2064–2069. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hancock V, Dahl M and Klemm P: Abolition
of biofilm formation in urinary tract Escherichia coli and
Klebsiella isolates by metal interference through
competition for fur. Appl Environ Microbiol. 76:3836–3841.
2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang Y, Lin Y, Zhang X, Chen L, Xu C, Liu
S, Cao J, Zheng X, Jia H, Chen L, et al: Combining Colistin with
furanone C-30 rescues Colistin resistance of gram-negative bacteria
in vitro and in vivo. Microbiol Spectr. 9(e0123121)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mojica MF, Bonomo RA and Fast W:
B1-Metallo-β-Lactamases: Where do we stand? Curr Drug Targets.
17:1029–1050. 2016.PubMed/NCBI View Article : Google Scholar
|