Introduction
Multiple myeloma (MM) is a malignant neoplasm of the
blood system caused by the malignant proliferation of plasma cells
(PC) within the bone marrow. Its clinical symptoms are associated
with end-organ damage, with an annual incidence of 2.1 per 100,000
population (1). With the passage
of time and the continuous emergence of new drugs such as
immunomodulatory agents, proteasome inhibitors and monoclonal
antibodies, the survival rate of patients has been greatly improved
(2), and the highest 5-year total
survival rate can reach 60% (3).
However, despite the rapid development in the treatment of MM, it
remains an incurable type of tumor to which almost all patients
will eventually succumb. With each relapse, malignant plasma cells
undergo clonal evolution, acquire new mutations, and lead to a
rapid deterioration of the disease and drug resistance (4).
The emergence of chimeric antigen receptor T (CAR-T)
cell immunotherapy has created a new era in tumor treatment,
especially for malignant tumors of the hematopoietic system, which
has greatly improved the remission rate. Its high remission rate
has stimulated the desire of medical scholars to explore for
relapsed and refractory acute lymphoblastic leukemia, chronic
lymphocytic leukemia and B-cell non-Hodgkin lymphoma (5-7).
B-cell maturation antigen (BCMA) is a cell surface
receptor primarily expressed by PC and belongs to the tumor
necrosis factor receptor superfamily. Its function is to maintain
the intracellular homeostasis of long-lived PC. BCMA is almost
exclusively expressed on the surface of plasma blasts and PCs, but
not expressed on memory B cells, naïve B cells, CD34+
hematopoietic stem cells and other normal histiocytes. The
expression of mRNA and protein on malignant cells is higher than
that on normal PC (8,9), making it a potential therapeutic
target for MM. CAR-T cells have strong inhibitory activity against
MM cell lines in vitro, and anti-CAR-T cell therapy in MM
mouse model can rapidly and continuously eliminate tumor cells,
resulting in 100% survival of mice (10). Numerous domestic clinical trials
have also confirmed the effectiveness of anti-CAR-T cell therapy
for MM (11). Based on these
findings, the present study launched a new clinical protocol for
anti-CAR-T cell immunotherapy against relapsed or refractory
multiple myeloma (RRMM).
Materials and methods
Patients
Between June 2020 and November 2021, 10 patients
with RRMM who successfully received CAR-T cell transfusion therapy
in the Department of Hematology of Handan Central Hospital were
included in the present study. Inclusion criteria included: i)
Patients aged between 18-69 years; ii) patients who met the RRMM
diagnostic criteria established by the International Myeloma
Working Group (IMWG) (12); iii)
patients without current infection; iv) patients without major
organ failure; and, v) patients with no history of bone marrow
transplantation within 100 days before enrollment. All patients had
a definite previous diagnosis of MM and had undergone at least two
treatments, including proteasome inhibitors and immunomodulators,
or both. Patients included in this study were those who relapsed
after remission, or did not reached remission and were resistant to
at least one drug. The assessable abnormal indicators are as
follows: Serum monoclonal M protein ≥5g/l or urine light chain ≥200
mg/24 h, or the increase of the affected serum-free light chain
>100 mg/l, or >25% of bone marrow plasma cells, or the
emergence of new extramedullary lesions. This study was approved by
the Ethics Committee of Handan Central Hospital [ethics no. (2020),
ethics review no. 007] and informed consent was obtained from all
patients.
Research route
Humanized anti-BCMA CAR-T cells were genetically
modified by autologous T lymphocytes expressing the anti-human
single-chain antibody 4C8A against BCMA in the extracellular region
and the intracellular domain attached to the single-stranded
antibody is composed of a sequentially linked 41BB: CD3ζ signaling
domain. Plasmids, lentiviral vectors and clinical-grade CAR-T cells
of CAR-BCMA single-chain antibody were prepared by Shandong Kaiti
Biological Products Co., Ltd. All patients received standard
lymphodepleting chemotherapy regimen with fludarabine
25/mg/(m2/d) and cyclophosphamide 300
mg/(m2/d) for 3 days starting on day -6, followed by
infusion of CAR-T cells on day 0. Patients were grouped according
to the concentration of CAR-T cell infusion: Two cases in the
0.5x106/kg group, three cases in the
2.0x106/kg group, and five cases in the
4.0x106/kg group. Non-steroidal anti-inflammatory drugs
(NSAIDs), diphenhydramine and promethazine were given 1 h before
transfusion to prevent allergic reactions. Indicator monitoring and
condition assessment were performed before treatment, weekly for
the first three weeks after treatment, one month, two months, and
every three months thereafter, with follow-up for up to two years.
The indexes tested included: Routine blood test, biochemical tests,
cytokine level, copy number of CAR-T cells, coagulation function,
proportion of bone marrow plasma cells, the quantification of
immunoprotein in serum and urine, serum and urine immunofixation
electrophoresis, quantification of free light chain in serum and
urine and minimal residual disease (MRD).
Detection of DNA copy numbers by
reverse transcription-quantitative (RT-q)PCR
hBCMA scFv-4-1BB-CD3 CAR (Shandong Kaiti Biological
Products Co., Ltd.) copy numbers were assessed through RT-qPCR
analysis to evaluate BCMA CAR-T cell expansion and persistence.
Genomic DNA was extracted with Omega DNA Blood Mini Kit (Omega
Bio-Tek, Inc.) from 250 µl fresh peripheral blood samples following
CAR-T cell infusion. PCR was performed using the SYBR Green
PremixPro TaqHS qPCR Kit (cat. no. AG11701; Eric Biotechnology Co.
Ltd.) following the instructions of the manufacturer. PCR was
performed by heating at 95˚C for 10 min, followed by 40 cycles of
95˚C for 10 sec, 60˚C for 20 sec, and 72˚C for 15 sec, with a final
step for 10 min at 72˚C. β-actin was used as an internal control.
Relative gene expression was analyzed by the 2-ΔΔCq
method (13) and normalized to
β-actin level. The primer sequences are shown in Table I. Reactions were run on Roche
LightCycler 96 RT-PCR system (Roche Diagnostics). The experiments
were repeated three times.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primers (5'-3') | Size, bp |
|---|
| BCMA-CAR-T | Forward:
TGAAAGTGAGCTGCAAAGCG | 181 |
| | Reverse:
GTGCTGGTGCTTTTATCCGC | |
| β-actin | Forward:
GAGCTACGAGCTGCCTGAC | 121 |
| | Reverse:
GGTAGTTTCGTGGATGCCACAG | |
Study endpoints
The primary endpoint was disease response rate and
progression-free survival (PFS), and secondary endpoints were
adverse events and duration of efficacy. The severity of adverse
events was graded according to the National Cancer Institute Common
Terminology Criteria for Adverse Events (NCI-CTCAE) grading
standard. The diagnosis and treatment criteria for cytokine release
syndrome (CRS) were based on the NCI CTCAE 4.0 standard (14). Within 90 days after infusion,
immune effector cell-associated neurotoxicity syndrome (ICANS) was
graded according to the highest grade of any event. Clinical
response and disease progression were evaluated according to the
IMWG uniform response standard for multiple myeloma (12).
Results
Demographics of patients
Between June 2020 and November 2021, 10 RRMM
patients (six males and four females) were enrolled in this study
with a median age of 54 (36-73) years. The median time from
diagnosis to CAR-T cell therapy was 24 (6-72) months, with a median
number of prior chemotherapy courses of 11 (4-20) and a median
number of treatment lines of three (2-10). All patients received at
least two treatment regimens with poor efficacy, and two patients
received five-line and above treatment. The medical history of
patients who had previously received drug treatment were as
follows: Bortezomib in eight (80%) cases, ixazomib in six (60%)
cases, carfilzomib in one (10%) case, pomalidomide in one (10%)
case, lenalidomide in four (40%) cases, thalidomide in four (40%)
cases, CD38 monoclonal antibody in one (10%) case, autologous stem
cell transplantation in one (10%) case, anthracyclines in six (60%)
cases, and cyclophosphamide in six (60%) cases. The Eastern
Cooperative Oncology Group score of patients was 0-1 in eight (80%)
cases and 4 in two (20%) cases (Table
II).
 | Table IIBaseline characteristics and
therapeutic efficacy. |
Table II
Baseline characteristics and
therapeutic efficacy.
| Baseline
characteristic | Value |
|---|
| Male sex, n (%) | 6(60) |
| Median age (range),
year | 54 (36-73) |
| Median time since
diagnosis (range), months | 24 (6-72) |
| Median no. of
therapies (range), months | 11 (4-20) |
| Number of prior lines
of treatment (range) | 3 (2-10) |
| Extramedullary
disease, n (%) | 3(30) |
| Previous therapies, n
(%) | |
|
Bortezomib | 8(80) |
|
Ixazomib | 6(60) |
|
Carfilzomib | 1(10) |
|
Pomalidomide | 1(10) |
|
Lenalidomide | 4(40) |
|
Thalidomide | 4(40) |
|
Daratumumab | 1(10) |
| Prior ASCT, n
(%) | 1(10) |
|
Anthracyclines | 6(60) |
|
Cyclophosphamide | 6(60) |
| ECOG
performance-status score, n (%) | |
|
0 | 5(50) |
|
1 | 3(30) |
|
2 | 0 (0) |
|
3 | 0 (0) |
|
4 | 2(20) |
| Therapeutic
efficacy, % (n/total n) | |
|
ORR | 88.9 (8/9) |
|
sCR | 77.8 (7/9) |
|
PR | 11.1 (1/9) |
|
SD | 11.1 (1/9) |
Efficacy evaluation
Among the 10 patients, one patient succumbed 24 days
after the infusion of CAR-T cells due to rapidly progressing
disease caused by heavy tumor burden. The direct cause of death was
pneumonia and gastrointestinal bleeding and the treatment efficacy
could not be evaluated. The remaining nine RRMM patients did not
receive any treatment for MM after infusion of CAR-T cells and were
followed up regularly. The overall response rate (ORR) of nine
patients was 88.9%, with eight (88.9%) patients achieving partial
remission (PR) or above. Among them, seven (77.8%) patients
achieved complete remission (CR) with negative MRD, one (11.1%)
patient achieved PR and one (11.1%) patient had stable condition
(SD; Table II). The median
follow-up time of nine patients were 337 (253-504) days, with a
median time to response of 43 (22-169) days, and a median PFS of
337 (253-504) days. Among the eight patients who achieved PR or
above, the duration of disease control exceeded six months, and
three of them maintained disease remission state for more than a
year. The infusion dose of CAR-T cells in one SD patient was
0.5x106/kg. At present, among the nine patients, only
one patient with SD experienced disease progression 337 days after
receiving CAR-T infusion, while the disease status of the remaining
patients remained stable at a median follow-up time of 337
(253-504) days. Among the three patients with extramedullary
involvement, two patients had disappearance of extramedullary
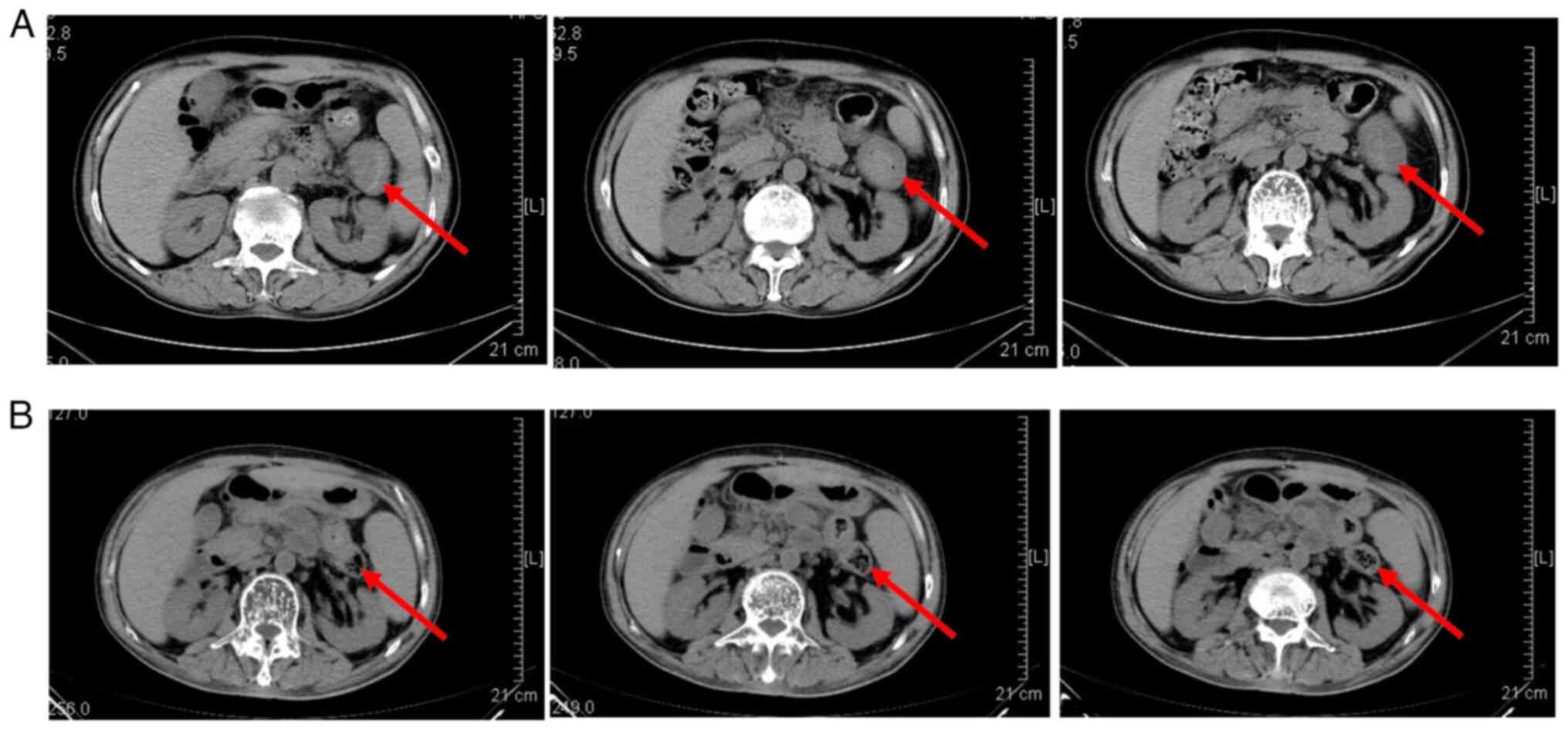
lesions and one patient had a stable disease status (Fig. 1).
Safety assessment
Of the 10 patients, nine (90%) had grade II or lower
CRS after infusion of CAR-T cells, and no ICANS occurred. The
median time to the onset of CRS was six (1-8) days post-infusion
and the median duration was five (2-15) days, with four (44.4%)
patients improving with NSAIDs and five (55.6%) patients improving
with one time tocilizumab treatment. The dose of CAR-T cell
infusion in one patient without CRS was 4.0x106/kg, and
the disease remission status was CR. There were nine (90%) patients
with hematological adverse reactions, six (60%) patients with
severe anemia, five (50%) patients with grade III and above
leukopenia, five (50%) patients with granulocytopenia, four (40%)
patients with grade III and above thrombocytopenia, three (30%)
patients with grade III and above pancytopenia, and eight (80%)
patients with hemocytopenia before infusion of CAR-T cells
(Table III). After 1 month of
CAR-T cell infusion, the hemoglobin of nine patients was higher
than 60 g/l, only one (10%) patient still had agranulocytosis
combined with grade IV thrombocytopenia, and the remaining patients
had granulocytes higher than 1.0x109/l and platelets
>50x109/l. The other adverse reactions were as
follows: Five (50%) patients had grade III pulmonary infection,
which improved after anti-infection treatment; two (20%) patients
had elevated glutamic oxaloacetic transaminase, which improved
within 14 days after liver protection treatment (Table III). A patient with a dose of
4.0x106/kg of CAR-T cell infusion had grade IV
pancytopenia, bacteremia and grade III pulmonary infection. One
month after CAR-T cell infusion, there was still granulocytopenia
combined with grade IV thrombocytopenia. One patient with CAR-T
cell infusion dose of 4.0x106/kg developed grade IV
pancytopenia, bacteremia and grade III lung infection, and there
was still agranulocytosis with grade IV thrombocytopenia after 1
month of CAR-T cell transfusion. After 2 months of treatment with
hormones plus tocilizumab, he was discontinued from blood
transfusion. One patient succumbed to lung infection and
gastrointestinal bleeding on the 24th day after infusion due to
rapid progression.
 | Table IIIAdverse events. |
Table III
Adverse events.
| Number of
patients | Any grade, n
(%) | Grade III, n
(%) | Grade IV, n
(%) |
|---|
| CRS | 9(90) | 0 (0) | 0 (0) |
| ICANS | 0 (0) | 0 (0) | 0 (0) |
| Anemia | 8(80) | 4(40) | 2(20) |
| Leukopenia | 7(70) | 2(20) | 3(30) |
| Neutropenia | 7(70) | 2(20) | 3(30) |
|
Thrombocytopenia | 5(50) | 2(20) | 2(20) |
| Pancytopenia | 4(40) | 1(10) | 2(20) |
| Pulmonary
infection | 5(50) | 5(50) | 0 (0) |
| Glutamic
oxaloacetic transaminase increased | 2(20) | 1(10) | 0 (0) |
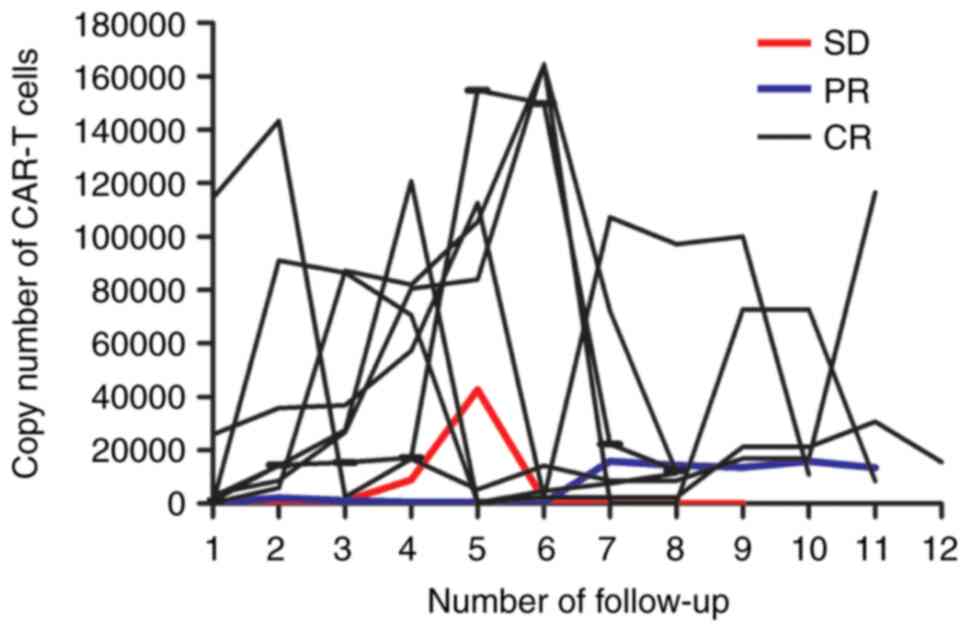
Copy number of CAR-T cells
During the follow-up, the highest copy number of
CAR-T cells in seven patients with CR was >1x105
copies/µl gDNA, and all patients achieved the best treatment effect
within 30 (7-30) days after the copy number of CAR-T cells reached
1x105 copies/µl gDNA (Fig.
2). One SD patient with the highest copy number of CAR-T cells
(4x104) had the number reduced to 84 copies/µl gDNA 30
days before relapse. The remaining eight patients who remained in
remission had a median copy number of 2.5x104
(8,526-116,433) copies/µl gDNA in CAR-T cells.
Discussion
CARs typically consist of high-affinity
antigen-binding sites and T cell activation signal transduction
domains on single-chain fragment variants (scFv) of monoclonal
antibodies that are not limited by major histocompatibility
complexes. So far, the first-generation CARs combine scFV domain
and CD4 (extracellular domain) with CD3ζ (intracellular domain),
but the stability and clinical efficacy are poor. The
second-generation CARs add additional co-stimulatory signal domains
on the intracellular domain, such as CD28 or 4-1BB, which enhance
the in vivo amplification and durability of efficacy. Third-
and fourth-generation CARs exhibit different characteristics in
combination with other co-stimulatory domains (15). After CAR-T cells bind to target
antigens in vivo, activation and proliferation occur,
followed by cytotoxicity, which ultimately kills target cells
(16).
A number of clinical studies have shown that CAR-T
cell immunotherapy plays an important role in the treatment of
hematological malignancies. In the peripheral blood of patients
with B-cell acute lymphoblastic leukemia, diffuse large B-cell
lymphoma and follicular lymphoma, it lasted for at least 5-6 months
(5,17), and 30-40% of patients achieved
remission and maintained for 12 months (18,19).
In the research area of MM, CAR-T therapeutics are rapidly
evolving, with 50 clinical trials involving all phases and
different CAR-T designs or targets, among which the efficacy of
anti-BCMA CAR-T is more prominent. BCMA is not expressed by
hematopoietic stem cells or non-hematological cells, but is highly
expressed in all MM cells and malignant PCs. Its expression levels
increased sequentially from monoclonal immunoglobulinemia to
smoking myeloma to MM (20). BCMA
is shed from the PC surface by secretase-mediated division to form
a soluble form (21), which can be
detected in peripheral blood. Therefore, BCMA is an ideal target
for CAR-T cells against myeloma (22). Although CAR-T cell therapy has
shown good efficacy so far, there are still some specific problems
to be solved, such as the efficacy of RRMM after multi-line
therapy, treatment response time, efficacy maintenance time,
adverse events and related treatment and treatment effect of
extramedullary diseases. Therefore, the present study was designed
to study the efficacy and adverse effects of second-generation
CAR-T in the treatment of RRMM.
The present study collected 10 patients with RRMM
who had received multiple lines of treatment but had poor response
to proteasome inhibitors or immunomodulators. All patients had
advanced clinical stages and a high level of disease control
difficulty, leading to poor prognosis. The present study observed
the disease remission and adverse reactions after anti-BCMA CAR-T
cell therapy, suggesting that RRMM patients are generally sensitive
to BCMA CAR-T cell therapy and achieve a high response rate. Among
the 10 patients, one patient succumbed due to the rapid progress of
the disease, so the efficacy could not be evaluated. The ORR of the
remaining nine patients was 88.9%, and 8 (88.9%) patients achieved
PR or above, of which seven (77.8%) patients achieved CR with
negative MRD, one (11.1%) patient had PR. The disease maintenance
time of eight patients with PR or above exceeded 6 months, and
three of them maintained the disease remission state for >1
year. The condition of one (11.1%) patient remained SD. Among the
three patients with extramedullary invasion, two extramedullary
lesions disappeared and one was SD. The results of the present
study suggested that RRMM patients were sensitive to BCMA CAR-T
cell therapy and achieved a high response rate. In addition, only
one patient with SD progressed 337 days after CAR-T infusion and
the disease of the remaining patients remained in the current state
after the median follow-up time of 337 (253-504) days. It is
suggested that CAR-T cell immunotherapy not only has good efficacy
but also has a long duration of response, as well as showing good
therapeutic effect on extramedullary myeloma involvement. These
results suggested that CAR-T cell therapy holds great promise for
the treatment of RRMM. In the initial stage of this project, two
patients with CAR-T cell infusion concentration of
0.5x106/kg were set up and the treatment process was
smooth without obvious adverse reactions, so 2.0x106/kg
and 4.0x106/kg concentration groups were set up in the
dose escalation stage. Of the 10 patients, nine (90%) had grade II
or lower CRS after infusion, which improved with NSAIDs or one time
tocilizumab treatment. Among the patients, five (50%) had grade III
pulmonary infection, which improved after anti-infection treatment.
A total of two (20%) patients had elevated glutamic oxaloacetic
transaminase, which improved within 14 days after liver protection
treatment. In addition, 10% of patients had bone marrow failure,
bacteremia and grade III pulmonary infection at the same time. The
large number of patients with hematological adverse reactions may
be related to the fact that most patients had concomitant
disease-related cytopenia prior to treatment, and cytopenia may
also be associated with pretreatment chemotherapy and most patients
recovered significantly within 1 month of treatment. Abnormal liver
function has also appeared in other studies of CAR-T cell therapy
for MM, which may be part of systemic inflammatory syndrome
(11).
Currently, immunotherapy drugs that have been proved
to significantly improve the overall survival of patients with RRMM
include targeted monoclonal antibody Daratumumab, in addition to
CAR-T cells. In studies targeting RRMM, patients who received
Daratumumab combined with chemotherapy had an ORR of 92.9% and a CR
of 56.6% (23,24). Although Daratumumab has also shown
desirable efficacy, patients who relapse after receiving third-line
or above treatment and those who are resistant to proteasome
inhibitors and immunomodulators do not receive significant benefits
(25) and the repeated appearance
of adverse reactions and the need for frequent hospitalization have
caused difficulties for patients. In a recent study of 16 cases of
refractory myeloma treated with anti-BCMA CAR-T cells, the ORR was
81%, among them, 63% achieved CR or VGPR (26). In a clinical study of bb2121 CAR-T
in the treatment of RRMM, the ORR was 85%, CR was 45% and the
median disease-free progression time was 10.9 months (27). In a domestic study on the treatment
of RRMM with BCMA CAR-T cells with double epitopes, the ORR was 88%
and the CR was 68% (28). In the
present study, the ORR and CR were 88.9 and 77.8% respectively,
which was basically consistent with other reported results. One
patient succumbed on day 24 following CAR-T cell infusion due to
rapid progression. The median follow-up time, median onset time and
median PFS of nine patients were 337 (253-504) days, 43 (22-169)
days and 337 (253-504) days respectively. The disease maintenance
time exceeded 6 months, and 33.3% patients maintained the disease
remission state for more than a year.
The PFS in this study was affected due to the short
observation time of some cases. Nevertheless, the disease
maintenance time of patients in the present study was longer than
that of other domestic studies and the treatment effect was
improved. CAR-T cells with a 4-1BB:CD3ζ co-stimulated domain had
significantly longer duration and improved safety than those with
CD28 co-stimulated domain (29).
Studies have shown that the peak blood CAR-T cell copy number is
not related to the dose of CAR-T cell infusion (26), but is related to the anti-MM
response (28), which is basically
consistent with the results of the present study. All patients
achieved the best treatment effect within 30 (7-30) days after the
copy number of CAR-T cells reached 1x105 copies/µl gDNA.
The number was reduced to 84 copies/µl gDNA in one patient 30 days
before relapse. A high level of expanded CAR-T copy number
indicates good efficacy, while a sudden decline to low copy number
predicts disease recurrence.
Although these results were preliminary and the
sample size was small, the clinical research results of CAR-T cells
showed encouraging efficacy and disease remission rate compared
with other salvage treatments for RRMM. In addition, although some
adverse reactions occurred, the adverse reactions were controllable
and, after symptomatic supportive therapy and anti-IL6 antibody
therapy, the symptoms were alleviated and there were no
uncontrollable and long-term adverse reactions. This caused less
harm to patients, so the safety of CAR-T therapy was high. CAR-T
cell levels have been shown to correlate with IL6 after CAR-T cell
therapy, and high levels of IL6 drive CRS and are also associated
with high survival (30).
Due to the persistence of antibody-producing cells
after CAR-T cell therapy, the treatment cannot completely cure the
disease, and there may still be disease recurrence after a certain
period of time (31,32). Therefore, the treatment exploration
for RRMM continues, including bispecific CAR therapy, combination
CAR therapy and CAR combination therapy and it is hoped that CAR-T
cell therapy can once again bring medical miracles in the
future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and XZ were responsible for the study conception
and design. Data collection, analysis and interpretation were
performed by JQ, XZ and GS. CO and HL performed experiments. XZ
drafted the manuscript. XS and JQ reviewed the manuscript. XS and
XZ confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Handan Central Hospital (Handan, China; approval no.
007) and written informed consent was obtained from each patient.
The study was performed in accordance with the principles of the
Declaration of Helsinki and the International Conference on
Harmonisation Guidelines for Good Clinical Practice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cowan AJ, Allen C, Barac A, Basaleem H,
Bensenor I, Curado MP, Foreman K, Gupta R, Harvey J, Hosgood HD, et
al: Global burden of multiple myeloma: A systematic analysis for
the global burden of disease study 2016. JAMA Oncol. 4:1221–1227.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van Beurden-Tan CHY, Franken MG,
Blommestein HM, Uyl-de Groot CA and Sonneveld P: Systematic
literature review and network meta-analysis of treatment outcomes
in relapsed and/or refractory multiple myeloma. J Clin Oncol.
35:1312–1319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Furukawa Y and Kikuchi J: Molecular basis
of clonal evolution in multiple myeloma. Int J Hematol.
111:496–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D,
Han X, Liu Y, Zhang W, Wang C, et al: Correction to: Bispecific
CAR-T cells targeting both CD19 and CD22 for therapy of adults with
relapsed or refractory B cell acute lymphoblastic leukemia. J
Hematol Oncol. 13(53)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Finney OC, Brakke HM, Rawlings-Rhea S,
Hicks R, Doolittle D, Lopez M, Futrell RB, Orentas RJ, Li D,
Gardner RA and Jensen MC: CD19 CAR T cell product and disease
attributes predict leukemia remission durability. J Clin Invest.
129:2123–2132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abramson JS: Anti-CD19 CAR T-cell therapy
for B-cell non-hodgkin lymphoma. Transfus Med Rev. 34:29–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van de Donk NWCJ, Usmani SZ and Yong K:
CAR T-cell therapy for multiple myeloma: State of the art and
prospects. Lancet Haematol. 8:e446–e461. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cho SF, Anderson KC and Tai YT: Targeting
B cell maturation antigen (BCMA) in multiple myeloma: Potential
uses of BCMA-Based immunotherapy. Front Immunol.
9(1821)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Friedman KM, Garrett TE, Evans JW, Horton
HM, Latimer HJ, Seidel SL, Horvath CJ and Morgan RA: Effective
targeting of multiple B-Cell maturation antigen-expressing
hematological malignances by Anti-B-Cell maturation antigen
chimeric antigen receptor T cells. Hum Gene Ther. 29:585–601.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin
D, Guo T, Kou H, Liu L, Tang L, et al: A bispecific CAR-T cell
therapy targeting BCMA and CD38 in relapsed or refractory multiple
myeloma. J Hematol Oncol. 14(161)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bao L, Bo XC, Cao HW, Qian C, Wang Z and
Li B: Engineered T cells and their therapeutic applications in
autoimmune diseases. Zool Res. 43:150–165. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
June CH and Sadelain M: Chimeric antigen
receptor therapy. N Engl J Med. 379:64–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brudno JN, Lam N, Vanasse D, Shen YW, Rose
JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, et al: Safety
and feasibility of anti-CD19 CAR T cells with fully human binding
domains in patients with B-cell lymphoma. Nat Med. 26:270–280.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Majzner RG and Mackall CL: Clinical
lessons learned from the first leg of the CAR T cell journey. Nat
Med. 25:1341–1355. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kersten MJ, Spanjaart AM and Thieblemont
C: CD19-directed CAR T-cell therapy in B-cell NHL. Curr Opin Oncol.
32:408–417. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mikkilineni L and Kochenderfer JN:
Chimeric antigen receptor T-cell therapies for multiple myeloma.
Blood. 130:2594–2602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tai YT and Anderson KC: B cell maturation
antigen (BCMA)-based immunotherapy for multiple myeloma. Expert
Opin Biol Ther. 19:1143–1156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Berdeja JG, Madduri D, Usmani SZ,
Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M,
Lesokhin A, et al: Ciltacabtagene autoleucel, a B-cell maturation
antigen-directed chimeric antigen receptor T-cell therapy in
patients with relapsed or refractory multiple myeloma
(CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 398:314–324.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bahlis NJ, Dimopoulos MA, White DJ,
Benboubker L, Cook G, Leiba M, Ho PJ, Kim K, Takezako N, Moreau P,
et al: Daratumumab plus lenalidomide and dexamethasone in
relapsed/refractory multiple myeloma: Extended follow-up of POLLUX,
a randomized, open-label, phase 3 study. Leukemia. 34:1875–1884.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Usmani SZ, Nahi H, Legiec W, Grosicki S,
Vorobyev V, Spicka I, Hungria V, Korenkova S, Bahlis NJ, Flogegard
M, et al: Final analysis of the phase III non-inferiority COLUMBA
study of subcutaneous versus intravenous daratumumab in patients
with relapsed or refractory multiple myeloma. Haematologica.
107:2408–2417. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stork M, Spicka I, Radocha J, Minarik J,
Jelinek T, Jungova A, Pavlicek P, Pospisilova L, Sedlak F, Straub
J, et al: Daratumumab with lenalidomide and dexamethasone in
relapsed or refractory multiple myeloma patients-real world
evidence analysis. Ann Hematol. 102:1501–1511. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brudno JN, Maric I, Hartman SD, Rose JJ,
Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et
al: T cells genetically modified to express an Anti-B-Cell
maturation antigen chimeric antigen receptor cause remissions of
poor-prognosis relapsed multiple myeloma. J Clin Oncol.
36:2267–2280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-Cell Therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao WH, Liu J, Wang BY, Chen YX, Cao XM,
Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, et al: A phase 1,
open-label study of LCAR-B38M, a chimeric antigen receptor T cell
therapy directed against B cell maturation antigen, in patients
with relapsed or refractory multiple myeloma. J Hematol Oncol.
11(141)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Maus MV and June CH: Making better
chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer
Res. 22:1875–1884. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Enblad G, Karlsson H, Gammelgard G, Wenthe
J, Lövgren T, Amini RM, Wikstrom KI, Essand M, Savoldo B, Hallböök
H, et al: A Phase I/IIa trial using CD19-Targeted Third-Generation
CAR T cells for lymphoma and leukemia. Clin Cancer Res.
24:6185–6194. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schultz L and Mackall C: Driving CAR T
cell translation forward. Sci Transl Med.
11(eaaw2127)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kansal R, Richardson N, Neeli I, Khawaja
S, Chamberlain D, Ghani M, Ghani QU, Balazs L, Beranova-Giorgianni
S, Giorgianni F, et al: Sustained B cell depletion by CD19-targeted
CAR T cells is a highly effective treatment for murine lupus. Sci
Transl Med. 11(eaav1648)2019.PubMed/NCBI View Article : Google Scholar
|