Introduction
Depression is a common mood disorder, which is
characterized by mood swings, chaos at work, difficulties in
learning, eating disorders, lack of interest in daily activities
and entertainment, insomnia or excessive sleep, restlessness,
excitement, fatigue, feeling of worthlessness, difficulties in
thinking or concentrating and suicidal thoughts or behaviors. It
has been reported that depression can increase the incidence and
mortality from somatic diseases (1-3).
In 2010, there were ~298 million patients suffering from depression
worldwide, while the annual incidence of depression is estimated to
be ~50%. Therefore, the rising incidence of depression has become a
serious challenge in medical research (4,5).
Currently, first-line antidepressant drugs mainly
include serotonin re-uptake inhibitors and selective
5-HT-norepinephrine re-uptake inhibitors (6). However, the underlying mechanism of
action of vortioxetine differs from other drugs, since it generally
acts in a mixed manner via modulating receptor activity and
inhibiting the re-uptake of neurotransmitters. Vortioxetine exerts
its pharmacological activity in vivo via 5-hydroxytryptamine
type 3 (5-HT3) receptor, 5-HT7 receptor and 5-hydroxytryptamine
(serotonin) receptor 1D (5-HT1D) antagonism, 5-HT1B receptor
partial agonism, 5-HT1A receptor agonism and 5-HT transporter
inhibition (7,8). Interestingly, a previous study
demonstrated that vortioxetine had no effect on norepinephrine and
dopaminergic neurons virtually (9). Additionally, another study showed
that vortioxetine could effectively treat patients with acute-phase
depression, while it could also effectively prevent recurrence
during the maintenance phase (10).
On September 30th, 2013, vortioxetine was approved
by the US Food and Drug Administration (FDA) for the treatment of
adults with depression. The drug is provided in doses of 5, 10, 15
and 20 mg (11). Previous
randomized controlled trials (RCTs) and systematic reviews in
different databases revealed that vortioxetine displayed improved
efficacy and safety compared with the positive drugs paroxetine and
venlafaxine, or placebo (12-14).
However, the results of the aforementioned studies were considered
insufficient, since the different doses of vortioxetine and outcome
measures were limited. The two other meta-analysis about
vortioxetine also provided few outcomes and safety evaluation.
Sufficient outcome evaluation could provide more suggestions to
physicians. With the advancement of clinical trials and the
increased demand for patient medication, it is necessary to
re-evaluate the efficacy and safety of vortioxetine as an
increasingly popular first-line drug for the treatment of
depression in adults. The re-evaluation of its clinical efficacy
and safety has also gained increasing attention from psychiatrists
and clinical pharmacists in several countries. Therefore, in terms
of systematic reviews, collecting more detailed data from clinical
trials on vortioxetine via evaluating more factors associated with
the efficacy and safety of different doses of vortioxetine could
provide the necessary evidence for decision-making on medical
treatments and clinical applications.
Materials and methods
The present study was performed and reported
according to the Cochrane Handbook for Systematic Reviews of
Interventions, which is used for conducting systematic reviews and
meta-analyses for observational studies (15,16),
as well as the Preferred Reporting Items for Systematic reviews and
Meta-Analyses statement (17). To
account for any data that could be missing from the final analysis,
the results were assessed using the Last Observation Carried
Forward method.
Study eligibility criteria
The clinical trials that met the following criteria
were included in the present study meta-analysis: i) Double-blind,
parallel-controlled and randomized clinical trials; ii) Adult
patients suffering from major depressive disorder (MDD), dysthymic
disorder and other psychotic disorders according to the Diagnostic
and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)
(18), DSM-IV Text Revision
(19) and/or ICD-10 Classification
of Mental and Behavioral Disorders (20-22);
iii) Patients treated with 5, 10, 15 or 20 mg/once per day (QD)
vortioxetine and placebo; iv) The efficacy outcome was determined
based on the changes in the total scores of the 24-Items Hamilton
Rating Scale for Depression (HADRS-24) (23), Sheehan Disability Scale (SDS)
(24), Montgomery-Asberg
Depression Rating Scale (MADRS) (25) and Clinical Global Impression
Scale-Improvement (CGI-I) (26),
and changes in HADRS-24 response rate, and MADRS response (≥50%)
and remission (≤10%) rates, from baseline; and v) The safety
outcome was defined as the rate of discontinuation due to adverse
effects (>5%).
The exclusion criteria were as follows: i)
Systematic reviews; ii) Review articles; iii) Case-control studies;
iv) Animal studies; v) Comments; vi) Studies with incomplete data;
vii) Case reports; viii) Studies where inappropriate statistical
methods were used; ix) Duplicate publications; and x) studies that
the diagnostic criteria were not reported.
Data sources and searching
strategy
Literature search was performed on PubMed\Medline,
EBSCO, Embase, Cochrane library, OVID and Web of Science from
database inception to November 2022. There were no limits in terms
of language, race, sex and nationality. Potentially relevant
unpublished data were searched on ClinicalTrials.gov, the FDA web site (Drugs@FDA; https://www.accessdata.fda.gov/), Chinese Clinical
Trial Registry (http://www.chictr.org.cn/), European Union Drug
Regulating Authorities Clinical Trials (https://eudract.ema.europa.eu/index.html), World
Health Organization and International Clinical Trials Registry
Platform (http://www.who.int/ictrp/en/). All studies were
hand-searched for randomized clinical trials that met the inclusion
criteria. The search terms were as follows: ‘vortioxetine’,
‘brintellix’, ‘Lu AA21004’, ‘placebo’, ‘MDD’, ‘dysthymic disorder’,
‘adult patients’, ‘efficacy’, ‘safety ‘, ‘tolerability’, ‘clinical
trial’, ‘randomized controlled trial’, ‘RCT’, ‘double-blind’ and
‘parallel-controlled’. The PubMed search string used was as
follows: ‘(vortioxetine or brintellix or Lu AA21004 or trintellix)
and (placebo) and (MDD or dysthymic disorder) and (adult patients)
and (efficacy) and (safety or tolerability) and (clinical trial or
randomized controlled trial or RCT) and (double-blind) and
(parallel-controlled) and (human or humans)’. In addition, the
Embase search string used was the following:
‘(vortioxetine*.ti or brintellix*.ti or Lu
AA21004*.ti or Trintellix*.ti) and
(MDD*.ti or dysthymic disorder*.ti) and
(adult patients*.ti) and (efficacy*.ti) and
(safety*.ti or tolerability*.ti) and
(clinical trial*.ti or randomized controlled
trial*.ti or RCT*.ti) and
(double-blind*.ti) and
(parallel-controlled*.ti) and (human*.ti or
humans*.ti)’.
Study selection
Each search was performed separately, and each study
was downloaded as a separate file using Endnote X6. To minimize
selection bias, two researchers (SG and XX) independently screened
the titles, abstracts and full texts of each article and data were
extracted based on the pre-defined eligibility criteria. The above
two researchers evaluated the quality of the literature. In case of
disagreement, a third researcher (LF) was involved to reach
consensus.
Data extraction
Two researchers (SG and XX) extracted the study
characteristics, baseline characteristics of patients, including
age, body mass index (BMI), race, HADRS-24 total score, MADRS total
score, CGI-I total score and race, interventions and outcome
measures, including efficacy [HADRS-24 total score change, SDS
total score change, MADRS total score change, CGI-I total score
change, HDRS-24 response rate, MADRS response rate (≥50%) and MADRS
remission rate (≤10%)], and safety (adverse effects) outcomes.
Quality assessment
The quality of literature was evaluated using the
Cochrane Handbook for Systematic Reviews of Interventions (version
5.1.0) for assessing risk of bias in RCTs (27). The evaluation components included
random sequence generation, allocation concealment, blinding,
analysis of incomplete outcome data and intention-to-treat
analysis, while there was no selective reporting or other bias.
Each item was defined as ‘yes’ (low risk of bias), ‘no’ (high risk
of bias) or ‘unclear’.
Statistical analysis
All outcomes were evaluated using Revman 5.3
software (http://www.cochrane.org/). Risk
ratios (RR) with 95% confidence intervals (CIs) were calculated for
dichotomous outcomes, such as response and remission rates.
Continuous outcomes, such as scale score, are expressed as the mean
difference (MD). The I2 statistic was calculated to
estimate heterogeneity using Review Manager. I2≤50% was
considered to indicate that the studies were homogeneous and a
fixed effect model with the Mantel-Haenszel (M-H) method was
performed. Otherwise, the random-effect model (REM) was adopted
(28). Publication bias was
assessed by visually inspecting funnel plots (29).
Results
Literature search and study
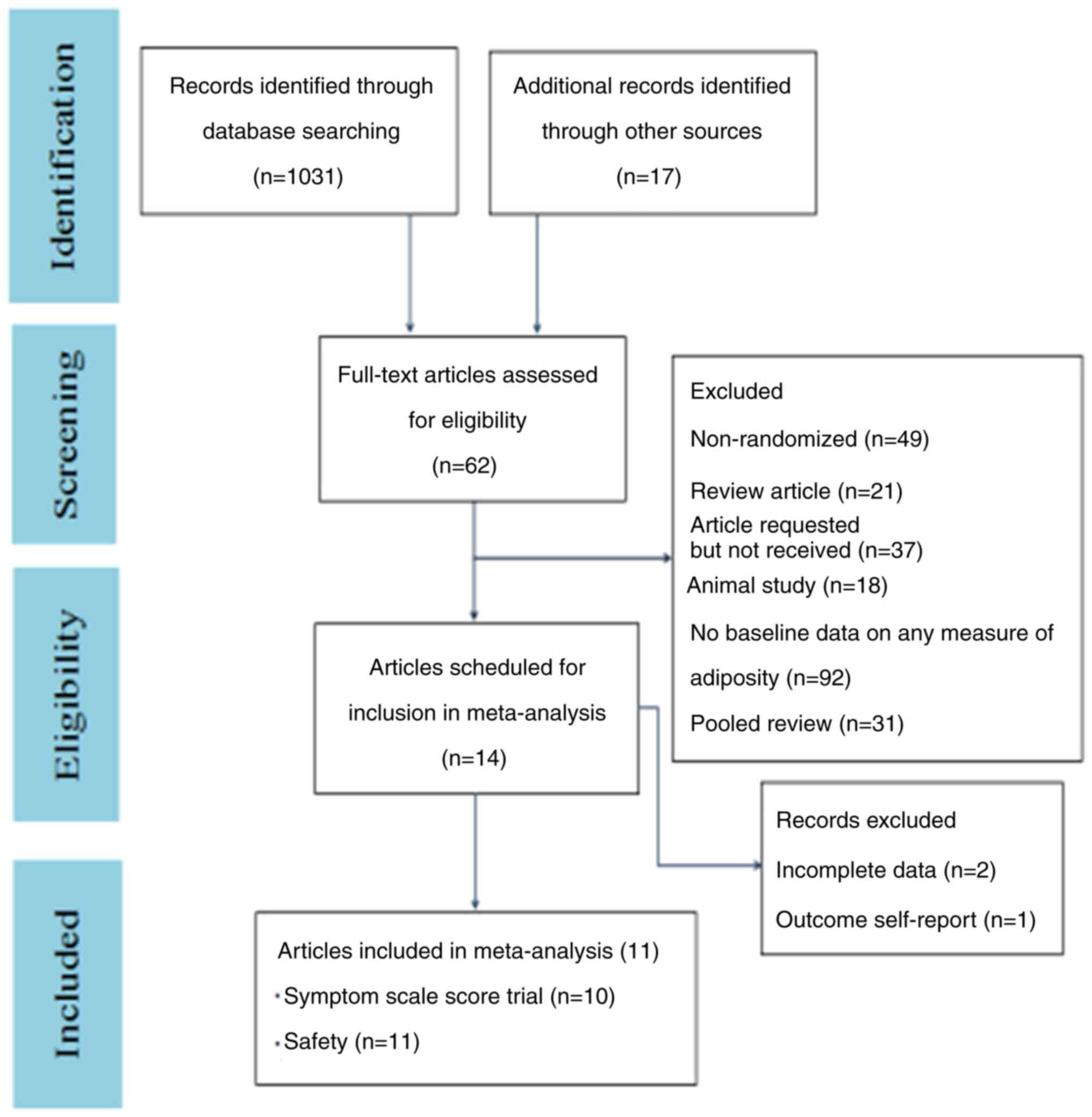
characteristics
After duplicates were removed, a total of 262
studies were screened. Among them, 248 were excluded, since they
did not meet the inclusion criteria based on in vitro
studies, animal studies, trials with healthy volunteers and review
articles. The remaining 14 studies were assessed according to the
pre-determined inclusion criteria. Finally, 11 RCTs were included
in the meta-analysis. The studies evaluated the efficacy and safety
of vortioxetine via randomizing adult patients into different study
arms with different doses of vortioxetine (Fig. 1) (30-39)
with the exception of one study (40). As shown in Table I, in nine studies patients were
treated with vortioxetine for eight weeks (30,31,33-39),
while in the remaining two studies for six weeks (32,40).
The main characteristics of patients included in the 11 studies
(30-40),
such as age, race, BMI, CGI-I and MADRS basic scores were well
described. The demographic or clinical characteristics between
trials were equivalent to the baseline. In the selected studies,
4,098 adult patients with MDD were treated with vortioxetine and
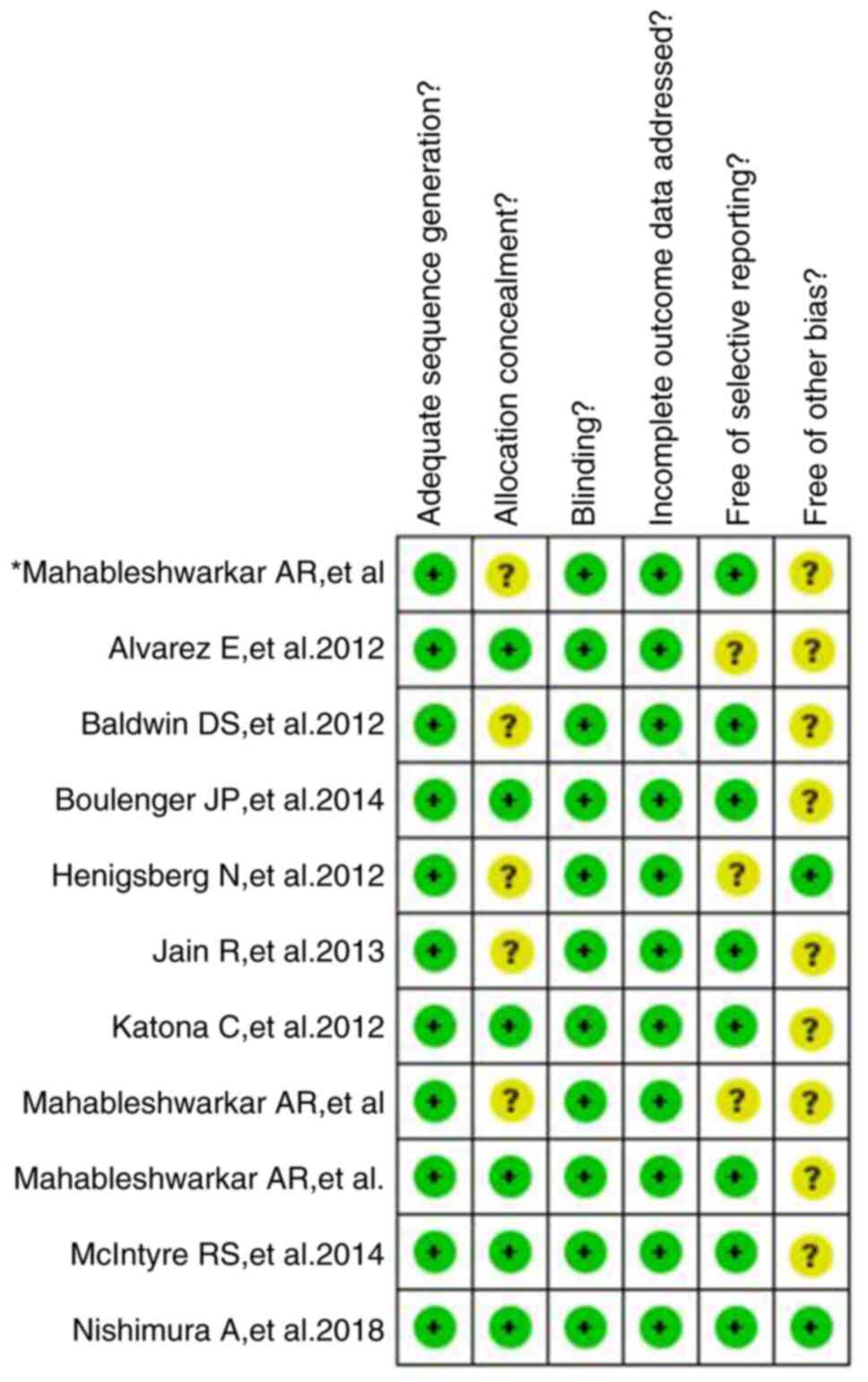
1,852 with placebo. The bias risk assessment demonstrated that
there was a low risk of bias in randomization and blinding.
However, the presence of other biases, such as recruitment bias and
clinical settings-related bias could not be ruled out (Fig. 2).
 | Table IBasic characteristics of literatures
(Baseline/mean ± SD). |
Table I
Basic characteristics of literatures
(Baseline/mean ± SD).
| Study | Interventions | Patients (n) | Age, years | Body mass, index
kg/m2 | CGI-S score | HDRS-24 total
score | MADRS total
score | Treatment duration
(weeks) | Race | Outcome
measure |
|---|
| Henigsberg et
al (30) 2012 | Vorti 5-mg | 140 | 47.3±12.0 | 26.4±5.1 | Not | 32.1±5.04 | 30.6±2.83 | 8 | White, Black, | ①②③④ |
| | Vorti 10-mg | 140 | 46.4±12.3 | 26.2±4.6 | reported | 33.1±4.77 | 31.6±3.83 | | Asian, Other | ⑤⑥⑦⑧ |
| | Placebo | 140 | 46.4±12.3 | 26.4±4.6 | 32.7±4.40 | 30.6±2.89 | | | | |
| Nishimura et
al (31) 2018 | Vorti 5-mg | 144 | 44.2±11.89 | 25.06±5.43 | 4.7±0.65 | Not | 31.6±3.67 | 8 | Asian | ⑤⑥⑦ |
| | Vorti 10-mg | 150 | 45.7±10.9 | 25.93±5.46 | 4.7±0.66 | reported | 31.8±4.02 | | | |
| | Vorti 20-mg | 154 | 44.0±11.79 | 24.82±5.21 | 4.7±0.65 | | 31.7±3.73 | | | |
| | Placebo | 152 | 43.6±11.57 | 24.82±5.13 | | | 31.6±3.56 | | | |
| Jain et al
(32) 2013 | Vorti 5-mg | 300 | 42.5±13.0 | 30.5±8.2 | Not | 32.7±5.4 | 42.5±13.0 | 6 | White, Black, | ①②③④ |
| | Placebo | 300 | 42.4±12.7 | 30.8±7.7 | reported | 32.2±5.5 | 42.4±12.7 | | Asian,
American | ⑤⑥⑧ |
| | | | | | | | | |
Indian/Alaskan, | |
| | | | | | | | | | Pacific
Inlander | |
| McIntyre et
al (33) 2014 | Vorti 10-mg | 195 | 45.4±12.2 | Not | 4.60±0.62 | Not | 45.4±12.2 | 8 | Not reported | ②④⑥⑦ |
| | Vorti 20-mg | 207 | 46.1±11.8 | reported | 4.62±0.58 | reported | 46.1±11.8 | | | |
| | Placebo | 196 | 45.6±12.1 | | 4.55±0.63 | | 45.6±12.1 | | | |
| Mahablesh warkar,
et al (34) 2013 | Vorti 2.5-mg | 153 | 42.6±12.9 | 29.5±7.5 | 4.6±0.62 | 29.8±5.4 | 42.6±12.9 | 8 | White, Black, | ①③④⑤ |
| | Vorti 5-mg | 153 | 43.1±13.9 | 31.4±8.8 | 4.6±0.65 | 29.0±5.6 | 43.1±13.9 | | Asian,
American | ⑥⑧ |
| | Placebo | 153 | 42.6±13.8 | 29.6±7.3 | 4.5±0.62 | 29.5±6.1 | 42.6±13.8 | |
Indian/Alaskan, | |
| | | | | | | | | | Pacific
Inlander | |
| Baldwin et
al (35) 2012 | Voeti 2.5-mg | 155 | 46.0±12.5 | Not | 4.8±0.7 | 29.6±5.8 | 46.0±12.5 | 8 | Asian | ②⑧ |
| | Vorti 5-mg | 157 | 44.7±13.1 | reported | 4.8±0.7 | 31.3±5.8 | 44.7±13.1 | | | |
| | Vorti 10-mg | 151 | 45.2±13.1 | | 4.8±0.7 | 30.4±5.4 | 45.2±13.1 | | | |
| | Placebo | 148 | 43.4±12.5 | | 4.8±0.7 | 29.8±5.1 | 43.4±12.5 | | | |
| Katona et al
(36) 2012 | Vorti 5-mg | 156 | 70.5±4.8 | Not | 4.8±0.7 | 29.2±5.0 | 70.5±4.8 | 8 | Not Reported | ①⑤⑥⑦ |
| | Placebo | 145 | 70.3±4.4 | reported | 4.7±0.7 | 29.4±5.1 | 70.3±4.4 | | | ⑧ |
| Mahablesh warkar
et al (37) 2015 | Vorti 20-mg | 198 | 44.2±12.2 | Not | 4.6±0.6 | Not | 44.2±12.2 | 8 | Black, Asian, | ②④⑧ |
| | Placebo | 194 | 45.0±12.1 | reported | 4.6±0.6 | reported | 45.0±12.1 | | Other | |
| Boulenger et
al (38) 2014 | Vorti 15-mg | 151 | 47.0±14.6 | Not | 4.9±0.6 | Not | 47.0±14.6 | 8 | Not Reported | ⑥⑦⑧ |
| | Vorti 20-mg | 151 | 46.2±13.4 | reported | 4.8±0.7 | reported | 46.2±13.4 | | | |
| | Placebo | 158 | 48.1±13.1 | | 4.9±0.7 | | 48.1±13.1 | | | |
| Mahablesh warkar
et al (39) 2015 | Vorti 15-mg | 147 | 43.1±12.28 | 31.3±7.48 | 4.5±0.55 | Not | 43.1±12.28 | 8 | White, Black, | ⑥⑦⑧ |
| | Vorti 20-mg | 154 | 42.8±12.40 | 30.9±7.63 | 4.5±0.60 | reported | 42.8±12.40 | | Asian, Native | |
| | Placebo | 161 | 42.4±12.55 | 31.1±7.88 | 4.6±0.58 | | 42.4±12.55 | | American/ | |
| | | | | | | | | | Alaskan native | |
| Alvarez et
al (40) 2012 | Vorti 5-mg | 108 | 43.8±11.6 | Not | 5.2±0.7 | 29.9±5.4 | 43.8±11.6 | 6 | Not reported | ⑧ |
| | Vorti 10-mg | 100 | 42.3±13.1 | reported | 5.1±0.7 | 29.3±5.6 | 42.3±13.1 | | | |
| | Placebo | 105 | 42.0±10.9 | | 5.1±0.7 | 29.7±5.0 | 42.0±10.9 | | | |
Efficacy outcomes
To evaluate the efficacy of vortioxetine on MDD, the
changes in the total scores of HADRS-24, MADRS, SDS and CGI-I, and
the HADRS-24 and MADRS response rates (≥50%) and those in MADRS
remission rate (≤10%) were retrieved from the included studies. The
HADRS-24 response rate, MADRS response rate (≥50%) and MADRS
remission rate (≤10%) were considered as the primary efficacy
outcomes. In addition, the HADRS-24, MADRS, SDS and CGI-I total
score changes were considered as the secondary efficacy outcomes.
The effect of vortioxetine (5, 10, 15 and 20 mg) compared with
placebo on the response of patients with MDD was assessed via
measuring the changes in the total scores of HADRS-24, MADRS, SDS
and CGI-I, and in HADRS-24 response rate, MADRS response rate
(≥50%) and MADRS remission rate (≤10%) at 8 or 6 weeks. Patients
were considered to be the measure index responders when reduced
total scores from baseline at the end of the study were obtained.
The present study analysis revealed the following results: i) In
the 5-mg vortioxetine group vs. the placebo group, no statistically
significant difference was observed in terms of HADRS-24, MADRS,
SDS and CGI-I total score change, and HADRS-24 response rate
(P>0.05). However, the MADRS response (≥50%; M-H RR=1.57; 95%
CI=1.23-1.99) and remission (≤10%; M-H RR=1.28; 95% CI=1.10-1.49;
I2=49%) rates were significantly enhanced in the 5-mg
vortioxetine dose group compared with the placebo group
(P<0.05). ii) In the 10-mg vortioxetine group vs. the
placebo group, a statistically significant difference was observed
in the total scores of HADRS-24 [MD=-4.93; 95% CI=-5.12-(-4.76)],
MADRS [MD=-3.65; 95% CI=-5.61-(-1.69)], SDS [MD=-1.54; 95%
CI=-1.76-(-1.32)] and CGI-I [MD=-0.58; 95% CI=-0.64-(-0.52)], and
in HDRS-24 response rate (M-H RR=2.16; 95% CI=1.52-3.05), MADRS
response rate (≥50%; M-H RR=1.44; 95% CI=1.15-1.81;
I2=39%) and MADRS remission rate (≤10%; M-H RR=1.61; 95%
CI=1.38-1.89; I2=34%; P<0.05). iii) The
HADRS-24, MADRS, SDS, CGI-I total scores and HDRS-24 response rate
were not reported for the 15-mg vortioxetine group. However, there
was no statistically significant difference in the MADRS response
(≥50%) and remission rates (≤10%) between the two groups
(P>0.05) (4). Consistently, in
the 20-mg vortioxetine group vs. placebo, the HADRS-24 and SDS
total score changes, and the HADRS-24 response rate were not
reported. A statistically significant difference was obtained
between the above groups in terms of the total scores of MADRS
[MD=-2.30; 95% CI=-2.45-(-2.15)] and CGI-I [MD=-0.58; 95%
CI=-1.13-(-0.02)], MADRS response rate (≥50%; M-H RR=1.55; 95%
CI=1.07-2.23) and MADRS remission rate (≤10%; M-H RR=1.54; 95%
CI=1.17-2.03; all P<0.05). Details of efficacy and heterogeneity
assessment are presented in Table
II and Fig. S1, Fig. S2, Fig. S3 and Fig. S4.
 | Table IIComparison of therapeutic effect
analysis in each trial group. |
Table II
Comparison of therapeutic effect
analysis in each trial group.
| | HADRS-24 total
score change (MD) | MADRS total score
change (MD) | SDS total score
change (MD) | CGI-I total score
change (MD) | HADRS-24 response
rate (RR) | MADRS response rate
(≥50%) (RR) | MADRS remission
rate (≤10%) (RR) |
|---|
| Vortioxetine 5-mg
vs. Placebo | -7.46 (-16.69 to
1.77) Z=1.58; (P=0.11) 4 trials | -2.07 (-4.37 to
0.23) Z=1.76 (P=0.08); 3 trials | -0.27 (0.94 to
0.40) Z=0.79 (P=0.43); 3 trials | -0.22 (0.48 to
0.03) Z=1.71 (P=0.09); 3 trials | 1.34 (1.00 to 1.80)
Z=1.96 (P=0.05); 4 trials | 1.57 (1.23 to 1.99)
Z=3.66 (P<0.05); 3 trials | 1.28 (1.10 to
1.49)a Z=3.13
(P<0.05); 5 trials |
| Vortioxetine 10-mg
vs. Placebo | -4.93 (-5.12 to
-4.74) Z=52.18 (P<0.05); 1 trial | -3.65 (-5.61 to
-1.69) Z=3.65 (P<0.05); 3 trials | -1.54 (-1.76 to
-1.32) Z=13.86 (P<0.05); 1 trial | -0.58 (-0.64 to
-0.52) Z=19.35 (P<0.05); 2 trials | 2.16 (1.52 to 3.05)
Z=4.34 (P<0.05); 1 trial | 1.44 (1.15 to
1.81)a Z=3.13
(P<0.05); 3 trials | 1.61 (1.38 to
1.89)a Z=5.88
(P<0.05); 3 trials |
| Vortioxetine 15-mg
vs. Placebo | Not reported | Not reported | Not reported | Not reported | Not reported | 1.36 (0.75 to 2.47)
Z=1.00 (P=0.32); 2 trials | 1.41 (0.91 to 2.19)
Z=1.53 (P=0.13); 2 trials |
| Vortioxetine 20-mg
vs. Placebo | Not reported | -2.30 (-2.45 to
-2.15) Z=30.37 (P<0.05); 1 trial | Not reported | -0.58 (-1.13 to
-0.02) Z=2.02 (P<0.05); 2 trials | Not reported | 1.55 (1.07 to 2.23)
Z=2.32 (P<0.05); 4 trials | 1.54 (1.17 to 2.03)
Z=3.06 (P<0.05); 4 trials |
Safety outcomes
Subsequently, a parallel, independent meta-analysis
was performed. The meta-analysis of the 11 articles included 16
adverse reactions, including nausea, headache, nasopharyngitis,
dizziness, diarrhea, constipation, dry mouth, insomnia, adverse
events (AEs) leading to discontinuation, serious AEs (SAEs),
fatigue, hyperhidrosis, decreased appetite, somnolence, vomiting
and upper respiratory tract infection, with a total adverse drug
reaction rate of 5%. Therefore, the analysis revealed that compared
with the placebo group: i) A statistically significant difference
was observed in the onset of nausea (response rate, M-H RR=2.48;
95% CI=1.99-3.10; I2=0%) in the 5-mg vortioxetine group.
However, no difference was observed in headache, nasopharyngitis,
dizziness, diarrhea, constipation, dry mouth, insomnia, AEs leading
to discontinuation, SAEs, fatigue, hyperhidrosis, decreased
appetite, somnolence and vomiting, between the two groups
(P>0.05). ii) A statistically significant difference was
obtained in nausea (response rate, M-H RR 3.02; 95% CI, 2.16=4.23;
I2=0%) and hyperhidrosis (response rate, M-H RR=4.65;
95% CI=1.36-15.95; I2=0%) between the 10-mg vortioxetine
group compared with the placebo group. No differences in the
remaining AEs were recorded (P>0.05). iii) In the 15-mg
vortioxetine group, a statistically significant difference was only
obtained in nausea (response rate, M-H RR=3.12; 95% CI=2.18-4.46;
I2=0%), compared with the placebo group, and not for the
other AEs (P>0.05). iv) In the 20-mg vortioxetine group, a
statistically significant difference in nausea (response rate, M-H
RR=3.39; 95% CI=2.54-4.51; I2=0%), insomnia (response
rate, M-H RR=2.25; 95% CI=1.09-4.68; I2=22%) and
vomiting (response rate, M-H RR=13.42; 95% CI=1.78-101.37) was
observed. There were no statistically significant differences in
headache, nasopharyngitis, dizziness, diarrhea, constipation, dry
mouth, AEs leading to discontinuation, SAEs, fatigue, decreased
appetite, vomiting and upper respiratory tract infection between
the two groups (P>0.05). The details of AE assessment are
demonstrated in Table III and
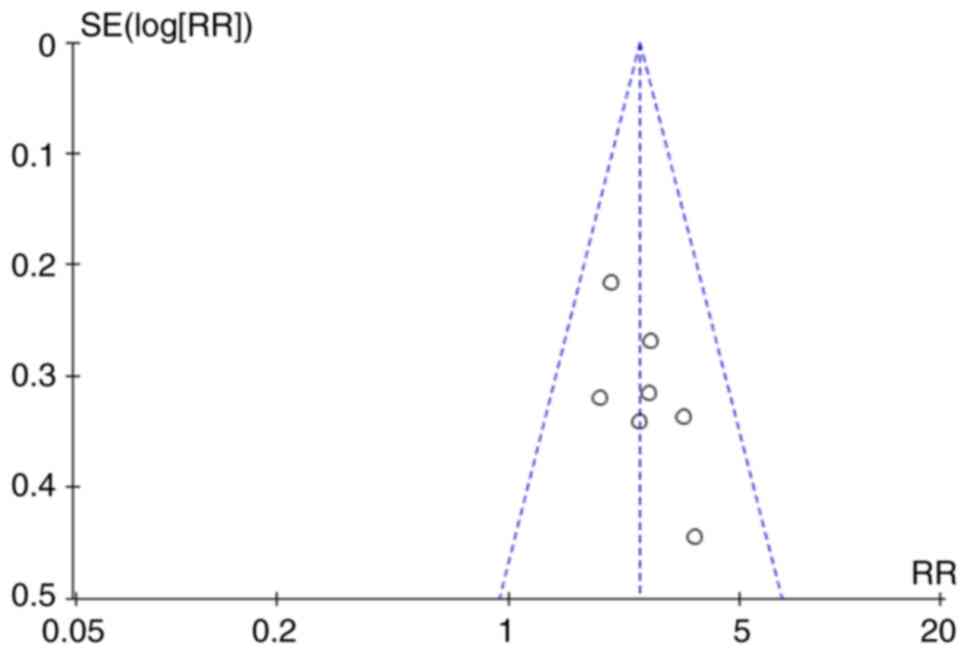
Fig. S5, Fig. S6, Fig. S7 and Fig. S8. Furthermore, the visual
inspection of funnel plots revealed low obvious publication bias
(Fig. 3).
 | Table IIIComparison of safety analysis in each
trial group. |
Table III
Comparison of safety analysis in each
trial group.
| | Vortioxetine
5-mg | Vortioxetine
10-mg | Vortioxetine
15-mg | Vortioxetine
20-mg |
|---|
| Nausea | 2.48% (1.99 to
3.10%)a
I2=0%; 7 trials | 3.02% (2.16 to
4.23%)a
I2=0%; 4 trials | 3.12% (2.18 to
4.46%)a
I2=0%; 2 trials | 3.39% (2.54 to
4.51%)a
I2=0%; 4 trials |
| Headache | 1.01% (0.82 to
1.25%) I2=4%; 6 trials | 0.89% (0.67 to
1.20%) I2=0%; 4 trials | 1.36% (0.89 to
2.08%) I2=0%; 2 trials | 1.19% (0.88 to
1.60%) I2=0%; 4 trials |
|
Nasopharyngitis | 1.24% (0.84 to
1.84%) I2=0%; 4 trials | 0.81% (0.52 to
1.27%) I2=0%; 4 trials | 0.65% (0.24 to
1.74%) 1 trial | 0.95% (0.62 to
1.46%) I2=0%; 3 trials |
| Dizziness | 1.00% (0.72 to
1.40%) I2=0%; 7 trials | 1.20% (0.72 to
2.01%) I2=35%; 4 trials | 1.53% (0.35 to
6.60%) I2=78%; 2 trials | 1.70% (0.82 to
3.52%) I2=52%; 4 trials |
| Diarrhea | 0.95% (0.52 to
1.73%) I2=61%; 5 trials | 0.75% (0.43 to
1.29%) I2=34%; 3 trials | 1.87% (1.04 to
3.38%) I2=33%; 2 trials | 1.13% (0.54 to
2.39%) I2=59%; 4 trials |
| Constipation | 1.00% (0.60 to
1.68%) I2=0%; 5 trials | 1.12% (0.49 to
2.60%) I2=25%; 3 trials | 0.87% (0.35 to
2.13%) 1 trial | 1.73% (0.89 to
3.37%) I2=0%; 2 trials |
| Dry mouth | 1.20% (0.86 to
1.68%) I2=0%; 6 trials | 0.99% (0.55 to
1.80%) I2=31%; 3 trials | 0.97% (0.54 to
1.76%) I2=0%; 2 trials | 1.42% (0.92 to
2.18%) I2=23%; 4 trials |
| Insomnia | 1.46% (0.83 to
2.58%) I2=0%; 4 trials | 1.05% (0.50 to
2.23%) I2=0%; 3 trials | 0.68% (0.23 to
2.02%) 1 trial | 2.25% (1.09 to
4.68%)a
I2=22%; 2 trials |
| AE leading to
discontinuation | 0.35% (0.07 to
1.70%) 1 trial | 2.38% (0.94 to
6.03%) 1 trial | Not reported | 1.72% (0.60 to
4.92%) I2=58%; 2 trials |
| Any SAE | 2.10% (0.19 to
22.88%) 1 trial | 3.06% (0.32% to
29.09%); 1 trial | Not reported | 5.03% (0.60 to
42.57%); 1 trial |
| Fatigue | 1.07% (0.58 to
1.97%) I2=0%; 4 trials | 1.08% (0.44 to
2.66%) I2=0%; 2 trials | 1.57% (0.45 to
5.45%) 1 trial | 1.31% (0.36 to
4.78%) 1 trial |
| Hyperhidrosis | 1.58% (0.75 to
3.31%) I2=0%; 4 trials | 4.65% (1.36 to
15.95%)a
I2=0%; 2 trials | 0.87% (0.27 to
2.80%) 1 trial | 0.08% (0.00 to
1.42%) 1 trial |
| Decreased
appetite | 2.07% (0.73 to
5.92%) I2=0%; 3 trials | 0.47% (0.04 to
5.14%) 1 trial | Not reported | 2.92% (0.31 to
27.86%) 1 trial |
| Somnolence | 1.16% (0.58 to
2.32%) I2=0%; 3 trials | 0.94% (0.28 to
3.19%) 1 trial | Not reported | Not reported |
| Vomiting | 1.28% (0.45 to
3.63%) I2=0%; 2 trials | 1.32% (0.43 to
4.07%) 1 trial | 7.57% (0.94 to
60.80%); 1 trial | 13.42% (1.78 to
101.37%)a 1
trial |
| Upper respiratory
tract infection | Not reported | Not reported | 0.49% (0.17 to
1.38%) 1 trial | 0.75% (0.31 to
1.82%) 1 trial |
Discussion
Summary of findings. It has been reported
that serious mental illness (SMI), such as depression, bipolar
disorder and schizophrenia, not only seriously affects patient's
interpersonal relationships, work and independent living
capabilities, but also is a significant risk factor for increasing
the prevalence and mortality of somatic diseases (41). Previous studies verified that
patients with SMI were at a higher risk of developing acute organ
dysfunction (42) and their life
expectancy was lower by an average of 20 years compared with the
general population (2). Among the
aforementioned conditions, cardiovascular diseases, cancer and
diseases of the endocrine system, such as diabetes and obesity, and
respiratory system have become the main cause of SMI-related death
(43). Numerous studies suggested
that cardiovascular diseases were the most common diseases among
patients with SMI, characterized by relatively high mortality rate
(44,45). Among them, the lifetime prevalence
rate of myocardial infarction, angina pectoris and stroke were ~30%
(46). Cognitive dysfunction is
common in patients with MDD, while it has been reported that
cognitive impairment may persist after the relief of depressive
symptoms (47). Since cognitive
impairment is the most significant residual symptom and can reduce
the quality of life of patients with MDDs, persistent cognitive
impairment may prevent full recovery from depressive episodes.
Several scholars have advocated ‘remission of cognitive function’
as a novel target for the treatment of MDD. Chen et al
(48) confirmed that the changes
in the number of dendritic spines, dendritic spine density,
dendritic length and branching in the hippocampus could promote the
maturation of hippocampal dendritic spines and improve cognitive
function in animals, thus indicating that they could also
potentially affect cognitive function in humans. Another study
suggested that vortioxetine could increase histamine levels in the
cerebral cortex and hippocampus, and it could indirectly improve
cognitive function via regulating serotonin in the vegetarian
system (49).
In the present systematic review, 11 randomized,
double-blind, placebo-controlled trials were included based on the
inclusion and exclusion criteria (30-40).
Subsequently, an evidence-based medical evaluation of the efficacy
and safety of the four vortioxetine preparation doses (5, 10, 15
and 20 mg) was carried out. All 11 studies showed that the
methodological assessments performed were of high quality, with a
low risk of bias, thus verifying that this was a systematic review
with high data credibility. Additionally, all included studies
displayed favorable general-data consistency and baseline balance,
while they were comparable. Furthermore, the heterogeneity of
several indicators in the reference material was assessed by Q
test. In the efficacy assessment, there were varying degrees of
heterogeneity in each group (5, 10, 15 and 20 mg). Due to the
heterogeneity (I2>50%) in the reference material and
since all the clinical research data were valid, the weight ratio
difference analysis of the relevant effect indicators could not
accurately exclude heterogeneous sources. Therefore, to evaluate
the efficacy of each indicator in the four groups (5-, 10-, 15- and
20-mg vortioxetine groups), a meta-analysis using a fixed effect
model was performed for only a few indicators (MADRS remission rate
in the 5- and 10-mg vortioxetine groups and MADRS response rate in
the 10-mg vortioxetine group), while the remaining indicators were
assessed by a REM. For patients with depression, who were initially
treated with 5 mg vortioxetine QD, the results revealed that there
was a significant difference in the MADRS response and remission
rates at the end of the treatment period. In addition, the clinical
symptom scores of patients in the 10- and 20-mg QD vortioxetine
groups were markedly changed with increasing dosage. Compared with
the placebo group, there was a significant difference in symptom
scores and response rates (P<0.05). However, data processing
revealed that the data from five indicators, namely HADRS-24 total
score, MADRS total score, SDS total score, CGI-I total score and
HADRS-24 response rate, were missing for the 15-mg QD vortioxetine
group. Therefore, only the data for two indicators, namely MADRS
response rate and MADRS remission rate, were used for the combined
analysis in this group. There was no statistically significant
difference between the aforementioned two indicators in the 15-mg
QD vortioxetine group compared with the placebo group (P>0.05),
possibly due to the effect of reference source, selection and
publication bias. Therefore, a descriptive analysis was performed,
and a single double-blind RCT showed that patient treatment with 15
mg vortioxetine QD could significantly improve symptom score
(39). The aforementioned results
were consistent with those reported by Meeker et al
(50), which verified that patient
treatment with 5, 10, 15 or 20 mg vortioxetine could significantly
affect the response and remission rates. In the present study, the
results also showed that administration of 5, 10, 15 or 20 mg
vortioxetine could notably alter HADRS-24, MADRS, SDS and CGI-I
total scores, and the HADRS-24 response rate compared with placebo.
However, the results also demonstrated that treatment with 5 mg
vortioxetine had no effect on HADRS-24, MADRS, SDS and CGI-I total
scores, and HADRS-24 response rate, compared with placebo. There
was an effort to use the subgroup analysis due to the
heterogeneity, but the included research in every efficacy outcome
measure is no more than four, therefore the subgroup analysis
cannot solve heterogeneity (I2>50%), Finally, it was
decided not to use the subgroup analysis. Nevertheless, more
high-quality clinical studies are urgently needed to further
investigate and confirm the aforementioned findings.
In terms of safety evaluation, an independent merged
meta-analysis on 16 adverse reactions, namely nausea, headache,
nasopharyngitis, dizziness, diarrhea, constipation, dry mouth,
insomnia, AEs leading to discontinuation, SAEs, fatigue,
hyperhidrosis, decreased appetite, somnolence, vomiting and upper
respiratory tract infection was carried out. The total incidence
rate of AEs in the 11 included studies was >5% (30-40).
The results revealed that nausea, hyperhidrosis, insomnia and
vomiting were more common in all trial vortioxetine groups (5, 10,
15 and 20 mg) compared with placebo. Regarding the association
between AEs and vortioxetine dosage, no absolute linear association
was found in this evaluation system. However, this result could be
greatly affected by bias and the completeness of the data.
Therefore, further high-quality clinical studies are required to
verify the aforementioned results.
Moreover, there have been two systematic reviews
about vortioxetine (51,52); the study conducted by Zhang et
al (51) only the response
rate, remission rate and tolerability were analyzed, which is
insufficient. In the study conducted by Thase et al
(52), the safety outcomes were
not analyzed. In addition, in the present study more and new
outcome measures were analyzed. The efficiency outcome measures not
only contained MADRS response rate, MADRS remission rate, but also
HADRS-24, MADRS, SDS, CGI-I and HADRS-24 response rate. The safety
outcome measure was also substantial. Although the conclusion was
different, Zhang et al (51) considered that vortioxetine is more
advantageous over placebo in treating MDD among adults, while the
present study revealed that there was no statistical difference
between vortioxetine 5-mg and placebo in HADRS-24, MADRS, SDS,
CGI-I total score change and HADRS-24 response rate. Overall, the
present study provided sufficient outcome measure, new conclusions
compared with other meta-analysis of vortioxetine and could offer
significant information to clinicians who prescribe vortioxetine to
patients suffering from SMI.
Limitations
Even though the included studies in the present
meta-analysis were strictly screened according to the inclusion and
exclusion criteria, a rigorous standardized quality evaluation was
conducted for the grouped 11 references. There were nevertheless
certain limitations. The following deficiencies still exist in the
meta-analysis of the system evaluation and effect indicators: i) In
multiple databases, there could be omissions in the collection of
references due to the retrieval strategy applied; ii), Since two
researchers reviewed the references in an independent and parallel
way according to the inclusion and exclusion criteria, the risk of
reference selection bias during the screening of reference material
should not be excluded; iii) Screening results from relevant
clinical trial registration websites were unsatisfactory. For
several grey references and evidence from non-traditional sources,
the query results may not be a strong supplement for the required
entry data due to the effects of confidentiality agreements.
Therefore, a particular degree of publication bias risk could be
included in the analysis. iv) Since the 11 studies included into
the analysis were all in English, the risk of language bias could
not be avoided. However, the aforementioned risks could be present
in any systematic review and meta-analysis. The strict and careful
reference screening and quality assessment, and the appropriate
methodological handling can guarantee scientific, complete and
accurate results with high clinical reference value.
The present systematic review demonstrated that 5-20
mg was significantly effective compared with placebo in the
treatment of MDD. However, treatment of patients with 5 mg
vortioxetine displayed no difference in the HADRS-24, MADRS, SDS
and CGI-I total scores, and HADRS-24 response rate. Interestingly
enough, increased vortioxetine doses were associated with improved
tolerance and high safety. Considering the potential bias and
confounding of the studies included in this meta-analysis, more
well-conducted and large-scale RCTs are needed to confirm the
findings of the present study.
Supplementary Material
Forest plot of comparison of (A)
HDRS-24, (B) MADRS, (C) Sheehan Disability Scale and (D) Clinical
Global Impression Scale-Improvement total score changes, and (E)
HDRS-24 response rate, (F) MADRS response rate (≥50%) and (G) MADRS
remission rate (≤10%) when administered 5 mg vortioxetine vs.
placebo. HDRS-24, 24-Items Hamilton Rating Scale for Depression;
MADRS, Montgomery-Asberg Depression Rating Scale.
Forest plot of comparison of (A)
HDRS-24, (B) MADRS, (C) Sheehan Disability Scale and (D) Clinical
Global Impression Scale-Improvement total score changes, and (E)
HDRS-24 response rate, (F) MADRS response rate (≥50%) and (G) MADRS
remission rate (≤10%) when administered 10 mg vortioxetine vs.
placebo. HDRS-24, 24-Items Hamilton Rating Scale for Depression;
MADRS, Montgomery-Asberg Depression Rating Scale.
Forest plot of comparison of (A) MADRS
response rate (≥50%) and (B) MADRS remission rate (≤10%) when
administered 15 mg vortioxetine vs. placebo. HADRS-24 total score
change, MADRS total score change, Sheehan Disability Scale total
score change, Clinical Global Impression Scale-Improvement total
score change and HDRS-24 response rate not reported. HDRS-24,
24-Items Hamilton Rating Scale for Depression; MADRS,
Montgomery-Asberg Depression Rating Scale.
Forest plot of comparison of (A) MADRS
and (B) Clinical Global Impression Scale-Improvement total score
changes, and (C) MADRS response rate (≥50%) and (D) MADRS remission
rate (≤10%) when administered 20 mg vortioxetine vs. placebo.
HADRS-24 and Sheehan Disability Scale total score change, and
HDRS-24 response rate not reported. HDRS-24, 24-Items Hamilton
Rating Scale for Depression; MADRS, Montgomery-Asberg Depression
Rating Scale.
Forest plot of comparison of (A)
nausea, (B) headache, (C) nasopharyngitis, (D) dizziness, (E)
diarrhea, (F) constipa-tion, (G) dry mouth, (H) insomnia, (I) AE
leading to discontinuation, (J) any serious AE leading to
discontinuation, (K) fatigue, (L) hyperhidrosis, (M) decreased
appetite, (N) somnolence and (O) vomiting when administered 5 mg
vortioxetine vs. placebo. Upper respiratory tract infection was not
reported. AE, adverse event.
Forest plot of comparison of (A)
nausea, (B) headache, (C) nasopharyngitis, (D) dizziness, (E)
diarrhea, (F) constipa-tion, (G) dry mouth, (H) insomnia, (I) AE
leading to discontinuation, (J) any serious AE leading to
discontinuation, (K) fatigue, (L) hyperhidrosis, (M) decreased
appetite, (N) somnolence and (O) vomiting when administered 10 mg
vortioxetine vs. placebo. Upper respiratory tract infection was not
reported. AE, adverse event.
Forest plot of comparison of (A)
nausea, (B) headache, (C) nasopharyngitis, (D) dizziness, (E)
diarrhea, (F) constipa-tion, (G) dry mouth, (H) insomnia, (I)
fatigue, (J) hyperhidrosis, (K) vomiting and (L) upper respiratory
tract infection when administered 15 mg vortioxetine vs. placebo.
AEs, serious AEs and decreased appetite were not reported. AE,
adverse event.
Forest plot of comparison of (A)
nausea, (B) headache, (C) nasopharyngitis, (D) dizziness, (E)
diarrhea, (F) constipa-tion, (G) dry mouth, (H) insomnia, (I) AE
leading to discontinuation, (J) any serious AE leading to
discontinuation, (K) fatigue, (L) hyperhidrosis, (M) decreased
appetite, (N) vomiting and (O) upper respiratory tract infection
when administered 20 mg vortioxetine vs. placebo. Somnolence was
not reported. AE, adverse event.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Plan Fund from Yaan Science and Technology Bureau (grant
no. 22KJJH0039).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SG, LF, XX and DZ designed the study, collected the
data, undertook the statistical analyses, drafted the manuscript
and contributed equally to this work. SG and XX participated in the
review of the study, performed the statistical analyses, helped to
interpret data and drafted the manuscript. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirschfeld RM: Differential diagnosis of
bipolar disorder and major depressive disorder. J Affect Disord.
169 (Suppl 1):S12–S16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
DE Hert M, Correll CU, Bobes J,
Cetkovich-Bakmas M, Cohen D, Asai I, Detraux J, Gautam S, Möller
HJ, Ndetei DM, et al: Physical illness in patients with severe
mental Disorders. I. Prevalence, impact of medications and
disparities in health care. World Psychiatry. 10:52–77.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grigoriadis S, VonderPorten EH,
Mamisashvili L, Eady A, Tomlinson G, Dennis CL, Koren G, Steiner M,
Mousmanis P, Cheung A, et al: The effect of prenatal antidepressant
exposure on neonatal adaptation: A systematic review and
meta-analysis. J Clin Psychiatry. 74:e309–e320. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hair P, Cameron F and Garnock-Jones KP:
Levomilnacipran extended-release: First global approve. Drugs.
73:1639–1645. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sartorius N: Comorbidity of mental and
physical disorders: A main challenge to medicine in the 21st
century. Psychiatr Danub. 25 (Suppl 1):S4–S5. 2013.PubMed/NCBI
|
|
6
|
He S, Yang XM and Li HF: Levomilnacipran:
A new drug for treatment of major depressive disorder. Chin J New
Drugs Clin Rem. 34:418–422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bang-Andersen B, Ruhland T, Jørgensen M,
Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S,
Mørk A and Stensbøl TB: Discovery of
1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): A
novel multimodal compound for the treatment of major depressive
disorder. J Med Chem. 54:3206–3221. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pehrson AL, Cremers T, Bétry C, van der
Hart MG, Jørgensen L, Madsen M, Haddjeri N, Ebert B and Sanchez C:
Lu AA21004, a novel multimodal antidepressant, produces regionally
selective increases of multiple neurotransmitters a rat
microdialysis and electrophysiology study. Eur
Neuropsychopharmacol. 23:133–145. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gibb A and Deeks ED: Vortioxetine: First
global approval. Drugs. 74:135–145. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Boulenger JP, Loft H and Florea I: A
randomized clinical study of Lu AA21004 in theprevention of relapse
in patients with major depressive disorder. J Psychopharmacol.
26:1408–1416. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
FDA. Vortioxetine(EB/OL). http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204447s000lbl.pdf,
2013-09-30.
|
|
12
|
Li G, Wang X and Ma D: Vortioxetine versus
duloxetine in the treatment of patients with major depressive
disorder: A Meta-analysis of randomized controlled trials. Clin
Drug Investig. 36:509–517. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pae CU, Wang SM, Han C, Lee SJ, Patkar AA,
Masand PS and Serretti A: Vortioxetine: A meta-analysis of 12
short-term, randomized, placebo-controlled clinical trials for the
treatment of major depressive disorder. J Psychiatry Neurosci.
40:174–186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baldwin DS, Chrones L, Florea I, Nielsen
R, Nomikos GG, Palo W and Reines E: The safety and tolerability of
vortioxetine: Analysis of data from randomized
Placebo-controlledtrials and open-label extension studies. J
Psychopharmacol. 30:242–252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Page MJ and Moher D: Evaluations of the
uptake and impact of the preferred reporting items for systematic
reviews and Meta-analysis (PRISMA) statement and extensions: A
scoping review. Syst Rev. 6(263)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sandini M, Mattavelli I, Nespoli L, Uggeri
F and Gianotti L: Systematic review and meta-analysis of sutures
coated with triclosan for the prevention of surgical site infection
after elective colorectal surgery according to the PRISMA
statement. Medicine (Baltimore). 95(e4057)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Panic N, Leoncini E, de Belvis G,
Ricciardi W and Boccia S: Evaluation of the endorsement of the
preferred reporting items for systematic reviews and meta-analysis
(PRISMA) statement on the quality of published systematic review
and meta-analyses. PLoS One. 8(e83138)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Durak S, Ercan ES, Ardic UA, Yuce D, Ercan
E and Ipci M: Effect of methylphenidate on neurocognitive test
battery: An evaluation according to the diagnostic and statistical
manual of mental disorders, fourth edition, subtypes. J Clin
Psychopharmacol. 34:467–474. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shabsigh R and Rowland D: The Diagnostic
and statistical manual of mental distal disorders, Fouth edition,
text revision as an appropriate diagnostic for premature
ejaculation. J Sex Med. 4:1468–1478. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Faiad Y, Khoury B, Daouk S, Maj M, Keeley
J, Gureje O and Reed G: Frequency of use of the international
classification of diseases ICD-10 diagnostic categories for mental
and behavioural disorders across world regions. Epidemiol Psychiatr
Sci. 11:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Janca A, Ustün TB, Early TS and Sartorius
N: The ICD-10 symptom checklist: A companion to the ICD-10
classification of mental and behavioural disorders. Soc Psychiatry
Psychiatr Epidemiol. 28:239–242. 1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
International Advisory Group for the
Revision of ICD-10 Mental and Behavioural Disorders. A conceptual
framework for the revision of the ICD-10 classification of mental
and behavioural disorders. World Psychiatry. 10:86–92.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khan A, Lewis C and Lindenmayer JP: Use of
non-parametric item response theory to develop a shortened version
of the Positive and Negative Syndrome Scale (PANSS). BMC
Psychiatry. 11(178)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Garcia-Caballero A, Torrens-Lluch M,
Ramírez-Gendrau I, Garrido G, Vallès V and Aragay N: The efficacy
of motivational intervention and cognitive-behavioral therapy for
pathological gambling. Adicciones. 30:219–224. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
25
|
Health Quality Ontario. Psychotherapy for
Major depressive disorder and generalized anxiety disorder: A
health technology assessment. Ont Health Technol Assess Ser.
17:1–167. 2017.PubMed/NCBI
|
|
26
|
Hedges DW, Brown BL and Shwalb DA: A
direct comparison of effect sizes from the clinical global
impression-improvement scale to effect sizes from other rating
scales in controlled trials of adult social anxiety disorder. Hum
Psychopharmacol. 24:35–40. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Higgins JPT and Green S, (eds.): Cochrane
Handbook for Systematic Reviews of Interventions Version 5.1.0. The
Cochrane Collaboration, Available from: http://handbook.cochrane.org, (Updated March
2011).
|
|
28
|
Berhan A and Barker A: Vortioxetine in the
treatment of adult patients with major depressive disorder: A
meta-analysis of randomized double-blind controlled trials. BMC
Psychiatry. 14:276–283. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Braga VL, Rocha LPDS, Bernardo DD, Cruz CO
and Riera R: What do Cochrane systematic reviews say about
probiotics as preventive interventions? Sao Paulo Med J.
135:578–586. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Henigsberg N, Mahableshwarkar AR, Jacobsen
P, Chen Y and Thase ME: A randomized, double-blind,
placebo-controlled 8-week trial of the efficacy and tolerability of
multiple doses of Lu AA21004 in adults with major depressive
disorder. J Clin Psychiatry. 73:953–959. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nishimura A, Aritomi Y, Sasai K, Kitagawa
T and Mahableshwarkar AR: Randomized, double-blind,
placebo-controlled 8-week trial of the efficacy, safety, and
tolerability of 5, 10, and 20 mg/day vortioxetine in adults with
major depressive disorder. Psychiatry Clin Neurosci. 72:64–72.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jain R, Mahableshwarkar AR, Jacobsen PL,
Chen Y and Thase ME: A randomized, double-blind, placebo-controlled
6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in
adults with major depressive disorder. Int J Neuropsychopharmacol.
16:313–321. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
McIntyre RS, Lophaven S and Olsen CK: A
randomized, double-blind, placebo-controlled study of vortioxetine
on cognitive function in depressed adults. Int J
Neuropsychopharmacol. 17:1557–1567. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mahableshwarkar AR, Jacobsen PL and Chen
Y: A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine
(Lu AA21004) versus placebo for 8 weeks in adults with major
depressive disorder. Curr Med Res Opin. 29:217–226. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Baldwin DS, Loft H and Dragheim M: A
randomised, double-blind, placebo controlled duloxetine-referenced,
fixed-dose study of three dosages of Lu AA21004 in acute treatment
of major depressive disorder (MDD). Eur Neuropsychopharmacol.
22:482–491. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Katona C, Hansen T and Olsen CK: A
randomized, double-blind, placebo-controlled,
duloxetine-referenced, fixed-dose study comparing the efficacy and
safety of Lu AA21004 in elderly patients with major depressive
disorder. Int Clin Psychopharmacol. 27:215–223. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mahableshwarkar AR, Zajecka J, Jacobson W,
Chen Y and Keefe RS: A randomized, placebo-controlled,
active-reference, double-blind, flexible-dose study of efficacy of
vortioxetine on cognitive function in major depressive disorder.
Neuropsychopharmacology. 40:2025–2037. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Boulenger JP, Loft H and Olsen CK:
Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day:
A randomized, double-blind, placebo-controlled,
duloxetine-referenced study in the acute treatment of adult
patients with major depressive disorder. Int Clin Psychopharmacol.
29:138–149. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mahableshwarkar AR, Jacobsen PL, Chen Y,
Serenko M and Trivedi MH: A randomized, double-blind,
duloxetine-referenced study comparing efficacy and tolerability of
2 fixed doses of vortioxetine in the acute treatment of adults with
MDD. Psychopharmacology (Berl). 232:2061–2070. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Alvarez E, Perez V, Dragheim M, Loft H and
Artigas F: A double-blind, randomized, placebo-controlled, active
reference study of Lu AA21004 in patients with major depressive
disorder. Int J Neuropsychopharmacol. 15:589–600. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ma XR, Hou CL and Xia FJ: Present
situation and challenges of somatic syndromes in patients with
serious mental illness. Chin J Behav Med Brain Sci. 23:181–183.
2014.
|
|
42
|
Shen HN, Lu CL and Yang HH: Increased
risks of acute organ dysfunction and mortality in intensive care
unit patients with schizophrenia: A nationwide population-based
study. Psychosom Med. 73:620–626. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lawrence D and Kisely D: Inequalities in
healthcare provision for people with severe mental iuness. J
Psychopharmacol. 24 (4 Suppl):S6l–S68. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ritchie S and Muldoon L: Cardiovascular
preventive care for patients with serious mental illness. Can Fam
Physician. 63:e483–e487. 2017.PubMed/NCBI
|
|
45
|
Kilbourne AM, Welsh D, McCarthy JF, Post
EP and Blow FC: Quality of care for cardiovascular disease-related
conditions in patients with and without mental disorders. J Gen
Intern Med. 23:1628–1633. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhao G, Ford ES, Dhingra S, Li C, Strine
TW and Mokdad AH: Depression and anxiety among US adults:
Associations with body mass index. Int J Obes (Lond). 33:257–266.
2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Waller JA, Chen F and Sanchez C:
Vortioxetine promotes maturation of dendritic spines in vitro: A
comparative study in hippocampal cultures. Neuropharmacology.
103:143–154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen F, du Jardin KG, Waller JA, Sanchez
C, Nyengaard JR and Wegener G: Vortioxetine promotes early changes
in dendritic morphology compared to fluoxetine in rat hippocampus.
Eur Neuropsychopharmacol. 26:234–245. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Smagin GN, Song D, Budac DP, Waller JA, Li
Y, Pehrson AL and Sánchez C: Histamine may contribute
Tovortioxetine's procognitive effects; possibly through an
orexigenic mechanism. Prog Neuropsychopharmacol Biol Psychiatry.
68:25–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Meeker AS, Herink MC, Haxby DG and Hartung
DM: The safety and efficacy of vortioxetine for acute treatment of
major depressive disorder: A systematic review and meta-analysis.
Syst Rev. 4(21)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang X, Cai Y, Hu X, Lu CY, Nie X and Shi
L: Systematic review and meta-analysis of vortioxetine for the
treatment of major depressive disorder in adults. Front Psychiatry.
13(922648)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Thase ME, Mahableshwarkar AR, Dragheim M,
Loft H and Vieta E: A meta-analysis of randomized,
placebo-controlled trials of vortioxetine for the treatment of
major depressive disorder in adults. Eur Neuropsychopharmacol.
26:979–993. 2016.PubMed/NCBI View Article : Google Scholar
|