Introduction
Acute lung injury (ALI) is a hyper-inflammatory
syndrome characterized by progressive dyspnea and refractory
hypoxemia. It is caused by various pathogenic factors, both
internal and external to the lung (1). These factors contribute to the
dysregulation of lung function, leading to the development of ALI.
Moreover, when the condition of patients with ALI deteriorates and
the oxygenation index decreases to PaCO2/FiO2
≤200 mmHg (1 mmHg=0.133 kPa), severe acute respiratory distress
syndrome (ARDS) develops (2). ALI
and ARDS are the leading causes of acute respiratory failure, with
an incidence of 789 per 100,000 people per year in the United
States, according to a report in 2005(3). Globally, the mortality rate for ALI
and ARDS is 30-40% (4). While the
cascade of inflammation is considered the typical pathogenesis of
ALI/ARDS, there are still several controversies surrounding this
mechanism (5). Based on the
pathophysiological changes and clinical manifestations of ALI/ARDS,
its treatment measures can be divided into mechanical ventilation
support and drug therapy (6-8).
Mechanical ventilation adopts a protective lung ventilation
strategy, which is considered the cornerstone of the treatment of
ALI/ARDS (9). Drug therapy
primarily includes anti-inflammatory agents, antioxidants,
vasodilators, and lung surfactants (10). Unfortunately, despite significant
progress in treatment methods for ALI/ARDS, the clinical mortality
rate remains high (11).
Therefore, there is an urgent to identify safe and effective
treatment methods. In recent years, with the deepening research on
the prevention and treatment of ALI/ARDS using traditional Chinese
medicines (TCMs), numerous basic studies have demonstrated the
beneficial effects of TCM on ALI/ARDS (12). CFYH is a TCM formula that was
developed for ALI, which consists of the following ingredients:
Poria Cocos (Schw.) Wolf. (Fu ling),
Zingiber Officinale Roscoe (Sheng jiang), Atractylodes
Macrocephala Koidz. (Bai zhu), Typhonii Rhizoma (Fu zi),
Hedysarum Multijugum Maxim. (Huang qi), Angelicae Sinensis
Radix (Dang gui), Radix Paeoniae Rubra (Chi shao),
Pheretima Aspergillum (Di long), Chuanxiong Rhizoma
(Chuan xiong), Carthami Flos (Hong hua), and Persicae
Semen (Tao ren). CFYH is designed to remove water from the
lungs, and relieve coughs and asthma. Our previous clinical studies
have confirmed that CFYH is effective for the treatment of
ALI/ARDS, and it has been shown to reduce the risk of progression
to severe disease (13,14). In the present study, the underlying
mechanisms of action of CFYH in ALI/ARDS were assessed to provide
evidence-based support for its integration into clinical practice,
increasing treatment options and improving patient outcomes.
Materials and methods
Reagents
LPS (cat. no. 0000081275; purity>99% by HPLC) was
purchased from MilliporeSigma. Dexamethasone acetate tablets (Dex;
cat. no. H51022823; 0.75 mg/tablet) were obtained from Chengdu No.
1 Pharmaceutical Co. Ltd. BCA Protein Concentration Determination
Kit (cat. no. MA0082), Rapid Gel Kit (cat. no. MA0159), and ECL
chemiluminescence kit (cat. no. MA0186) were purchased from Dalian
Meilun Biotechnology Co., Ltd. The cDNA First Strand Synthesis Kit
(cat. no. FP205) and 2x SYBR Green PCR MasterMix (cat. no. KR118)
were purchased from Tiangen Biochemical Technology Co., Ltd. RNA
Extraction Kit (cat. no. R1200) was purchased from Beijing Solarbio
Technology Co., Ltd. ELISA kits for IL-6 (cat. no. 22A109), TNF-a
(cat. no. 22A116), and IL-1β (cat. no. ITWE8PMAF6) were purchased
from Excell Biochemical Technology Co., Ltd. Antibodies against
NF-κB p65 (cat. no. 380172), HMGB1 (cat. no. R22773), RAGE (cat.
no. 381618), HIF-1α (cat. no. 340462), AMPK α1 (cat. no. 380431),
phospho-AMPK α1 (Thr183)/AMPK α2 (Thr172) Rabbit pAb (cat. no.
383462), mTOR/phospho-mTOR (Ser2481) Rabbit pAb (cat. no. 381548),
LC3A/B (cat. no. 306019), Bax (cat. no. 380709) and SQSTM1/p62
Rabbit mAb (cat. no. R27312) were purchased from Chengdu Zhengneng
Biotechnology Co., Ltd. Antibodies against TLR4 (cat. no. AF7017),
NLRP3 (cat. no. DF7438), GSDMD-NT (cat. no. AF4012), and caspase-3
were obtained from Affinity Biologicals Co., Ltd. Reactive oxygen
Species testing kit (cat. no. S0033S) was purchased from Shanghai
Biyuntian Biotechnology Co., Ltd. LDH (cat. no. 20210803) was
obtained from Nanjing Jiancheng Biological Co., Ltd. Antibodies
against Beclin-1 (cat. no. 11306-1-AP), Bcl2 (cat. no. 26593-1-AP),
Caspase-1/P20/P10 Polyclonal Antibody Bcl2 Polyclonal Antibody
(cat. no. 26593-1-AP), Caspase-1/P20/P10 Polyclonal Antibody (cat.
no. 22915-1-AP) were obtained from Wuhan Sanying Biotechnology Co.,
Ltd. Rat IL-18 (cat. no. E-EL-R0567c) was purchased from Wuhan
Elite Biotechnology Co. Ltd.
Preparation of CFYH
The drug composition of CFYH is shown in Table I. The Chinese herbal medicines that
constitute CFYH were provided by the Sichuan New Green
Pharmaceutical Technology Development Co., Ltd. All medicinal
materials were extracted twice with boiling water, combined with
the filtrate, and concentrated to form a decoction (2.0 g/ml). The
middle dose was calculated according to the equivalent dose in
rats, which was ~6.3x that of a 70 kg adult (15). Therefore, the dose of CFYH in rats
was 41.13 g/kg/day. The lowest dose of CFYH was 20.565 g/kg/day,
and the highest dose was 82.26 g/kg/day. Based on the required
concentration of CFYH, pure water was added and stored at room
temperature until further use.
 | Table IIngredients list of
Chuanfangyihao. |
Table I
Ingredients list of
Chuanfangyihao.
| Medicine | Latin name | Weight, g | Place of production
in China | Batch no. |
|---|
| Fu ling | Poria Cocos
(Schw.) Wolf. | 9 | Yunnan | 21060033 |
| Sheng jiang | Zingiber
Officinale Roscoe | 9 | Sichuan | 20060086 |
| Bai zhu | Atractylodes
Macrocephala Koidz. | 6 | Hebei | 21030002 |
| Fu zi | Typhonii
Rhizoma | 9 | Sichuan | 20120037 |
| Huang qi | Hedysarum
Multijugum Maxim. | 40 | Gansu | 21030101 |
| Dang gui | Angelicae
Sinensis Radix | 10 | Gansu | 21020050 |
| Chi shao | Radix Paeoniae
Rubra | 20 | Sichuan | 20100082 |
| Di long | Pheretima
aspergillum | 10 | Guangxi | 21030065 |
| Chuan xiong | Chuanxiong
Rhizoma | 20 | Sichuan | 21050011 |
| Hong hua | Carthami
Flos | 15 | Xinjiang | 21020096 |
| Tao ren | Persicae
Semen | 15 | Gansu | 20040082 |
Animals and experimental design
The experimental procedure was approved by the
Medical Ethics Committee of the Sichuan Academy of TCM [grant no.
SYLL (2022)-039] (16). We
strictly followed the replacement, reduction, and refinement
principle, and adhered to the National Institutes of Health
Guidelines for the Care and Use of Laboratory Animals and ARRIVE
Animal Research guidelines (17).
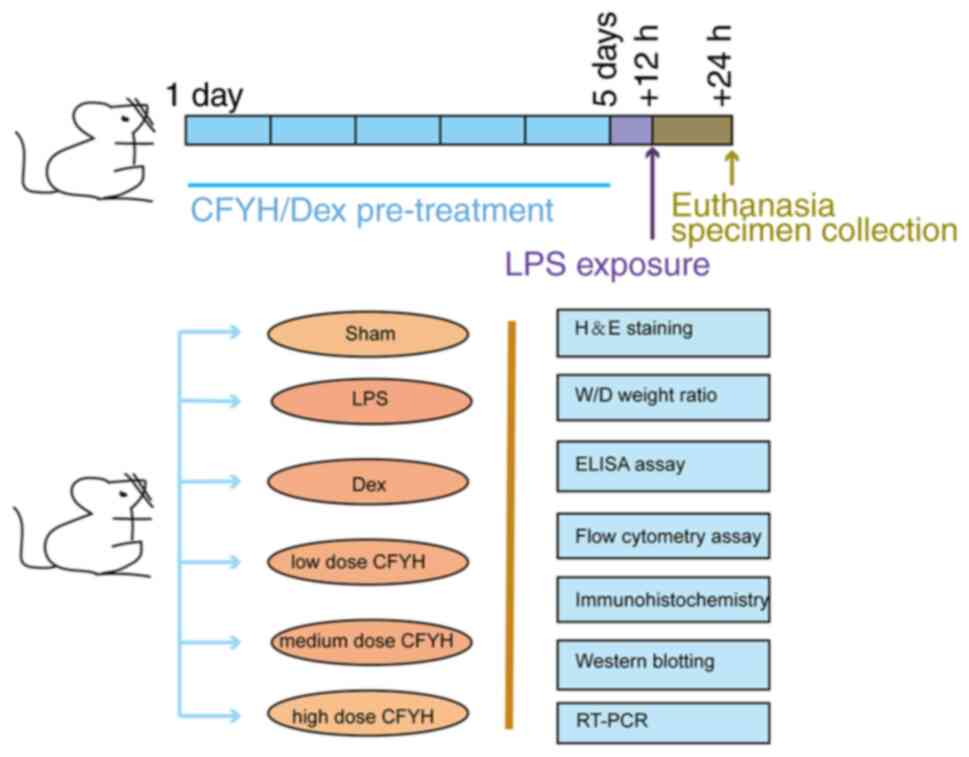
The experiments were performed as shown in Fig. 1. A total of 36 male Sprague-Dawley
rats (10-week-old, weighing 180-220 g) were provided by the
Experimental Animal Center of Sichuan University [license no. SCXK
(Sichuan) 2018-026, certificate no. 00116050]. They were housed in
specific-pathogen-free environments at the Sichuan Academy of TCM
(12 h dark/light cycle; humidity, 55±5%; temperature, 25±1˚C), and
provided with ad libitum access to a standard diet and
water.
The rats were adaptively fed for 1 week and then
divided into groups as follows (n=6/group): i) sham group, ii) LPS
group (10 mg/kg), iii) Dex (DEX) group (0.27 mg/kg/day) (18), iv) CFYH low dose group (20.565
g/kg/day), v) CFYH medium dose group (41.13 g/kg/day), and a vi)
CFYH high dose group (82.26 g/kg/day). The sham and LPS groups
received oral gavage of saline (2 ml), whereas the other groups
received the corresponding drugs twice daily for 5 days. A total of
12 h after the final administration, rats in the sham group were
intratracheally administered normal saline (10 mg/kg), whereas
those in the other groups were intratracheally administered LPS (10
mg/kg). The dosage and usage of LPS were determined based on
previous literature (19,20). The preparation method was as
follows: 10 mg LPS was dissolved in 10 ml normal saline and stored
away from light. After a 24-h period following the establishment of
the LPS model, all rats were sacrificed by injecting 3%
pentobarbital sodium.
Lung wet/dry (W/D) weight ratio
After sacrificing the rats, a portion of the right
lung tissue was removed and weighed to obtain the wet weight (W),
and then it was dried in an 80˚C incubator for 48 h to obtain the
dry weight (D).
Histological analysis
Another region of the lung tissue was soaked in 4%
formaldehyde for 24 h at room temperature, embedded in paraffin,
cut into 3 µm sections, and stained with hematoxylin-eosin
(H&E) at room temperature for 5 min. Finally, the samples were
observed at 20x magnification using a light microscope and scored.
The scoring criteria were as follows: i) alveolar congestion; ii)
hemorrhage; iii) infiltration or aggregation of neutrophils in
airspace or vessel wall; and iv) thickness of alveolar wall/hyaline
membrane formation (21). Each
item was scored on a five-point scale as follows: 0, minimal
damage; 1, mild damage; 2, moderate damage; 3, severe damage; and
4, maximal damage.
ROS content of lung tissue
The lung tissues were flushed with PBS, and 1 ml
collagenase was added to dissociate the tissues, which were then
incubated at 37˚C for 10 min. Digestion was terminated with 3% FBS
and filtered with a 40-µm cell screen. Then, 10 ml PBS was added,
and the mixture was centrifuged at 186 x g for 5 min at room
temperature to remove the supernatant. 2',7'-dichlorofluorescein
diacetate (DCFH-DA) was diluted with a serum-free medium at a ratio
of 1:1,000, resulting in a final concentration of 10 µmol/l. After
adding 500 µl diluted DCFH-DA, the samples were incubated in a cell
culture box at 37˚C for 20 min. The cells were rinsed three times
with serum-free cell culture medium. Finally, the cells were
resuspended in PBS, and FITC fluorescence was detected using a
CytoFLEX flow cytometer (Beckman Coulter, Inc.) with CytExpert
software (version, 2.3.0.84; Beckman Coulter, Inc.).
ELISA
Lung tissues (100 mg) were rinsed with PBS and dried
on filter paper. The lung tissue was homogenized, and the
homogenate was centrifuged at 5,000 x g for 10 min at 4˚C to obtain
the supernatant for analysis. TNF-α, IL-6, IL-18, IL-1β, and LDH
levels were determined in the lung tissue homogenates using
specific ELISA kits.
Immunohistochemistry assay
The paraffin-embedded lung sections were dewaxed
with xylene and rehydrated using an ethanol gradient. The slices
were completely immersed in citrate buffer and heated for 20 min at
100˚C. After cooling naturally, the samples were washed with PBS
three times. The sections were incubated in 3%
H2O2 for 20 min and washed with PBS. Then,
the sections were incubated with the primary antibody for 2 h at
37˚C and washed with PBS three times. The secondary antibody was
added, and the sections were incubated for 15 min and washed with
PBS three times. DAB was added to the sections for color
development, hematoxylin was used for re-dyeing at room
temperature, and neutral glue was used to seal the sections and
observed under a microscope.
Western blot analysis
Lung tissues were fully homogenized to extract
proteins, and the protein concentration was determined using a BCA
kit. Equal quantities of protein were separated by 10% SDS-PAGE and
transferred onto PVDF membranes. The membranes were blocked with 5%
skim milk for 1 h at room temperature, then washed three times and
incubated with primary antibodies overnight at 4˚C. The following
day, the membranes were washed three times with PBS for 10 min each
and then incubated with a secondary antibody for 2 h at room
temperature. Finally, the membrane was washed three times for 10
min. Protein bands were detected using an enhanced
chemiluminescence detection kit. ImageJ (version, 1.51j8; National
Institutes of Health) was used for the densitometry analysis.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from rat lung tissues using
a total RNA extraction kit (Beijing Solarbio Technology Co., Ltd.)
according to the manufacturer's instructions. The RNA was reverse
transcribed into cDNA using the FastKing cDNA first-strand
synthesis kit produced by Tiangen Biochemical Technology Co., Ltd.
according to the manufacturer's protocol. qPCR was then used to
determine the relative expression of HMGB1, RAGE, AMPK, mTOR, and
HIF-1α. Primer sequences are listed in Table II. Thermocycling conditions were
as follows: 95˚C for 15 min; then 40 cycles of 95˚C for 10 sec,
55˚C for 20 sec and 72˚C for 30 sec. Compared with housekeeping
gene (β-actin), the relative mRNA expression was calculated using
the 2-ΔΔCq method and expressed as the change compared
with the control group (22).
 | Table IISequences of the primers. |
Table II
Sequences of the primers.
| Gene | Forward primer,
5'-3' | Reverse primer,
5'-3' |
|---|
| HMGB1 |
GCGCGCGCCAGGAAAAT |
GCCTTTGATTTTTGGGCGGT |
| RAGE |
GGGTCACAGAAACCGGTGAT |
ATCATGTGGGCTCTGGTTGG |
| AMPK |
CAAACACCAAGGCGTACG |
TGCTCTACACACTTCTGCCAT |
| mTOR |
CGTCACAATGCAGCCAACAA |
AACAAACTCGTGCCCATTGC |
| HIF-1α |
TCCTGCACTGAATCAAGAGGTTGC |
ACTGGGACTGTTAGGCTCAGGTG |
| β-actin |
CGTTGATATCCGTAAAGACC |
TACATAACAGTCCGCCTAGAAG |
Statistical analysis
SPSS version 26.0 (IBM Corp.) and GraphPad Prism
version 8.0 (GraphPad Software, Inc.) were used for data processing
and developing the figures. Data are presented as the mean ± SD
(23). When multiple groups were
compared, ANOVA was used for parametric data and Kruskal-Wallis was
used for multiple groups that were non-parametric. Pair-based
comparison of homogeneous variances were evaluated using Tukey's
test, heterogeneous variances were determined using Dunnett's T3
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Protective effect of CFYH on ALI
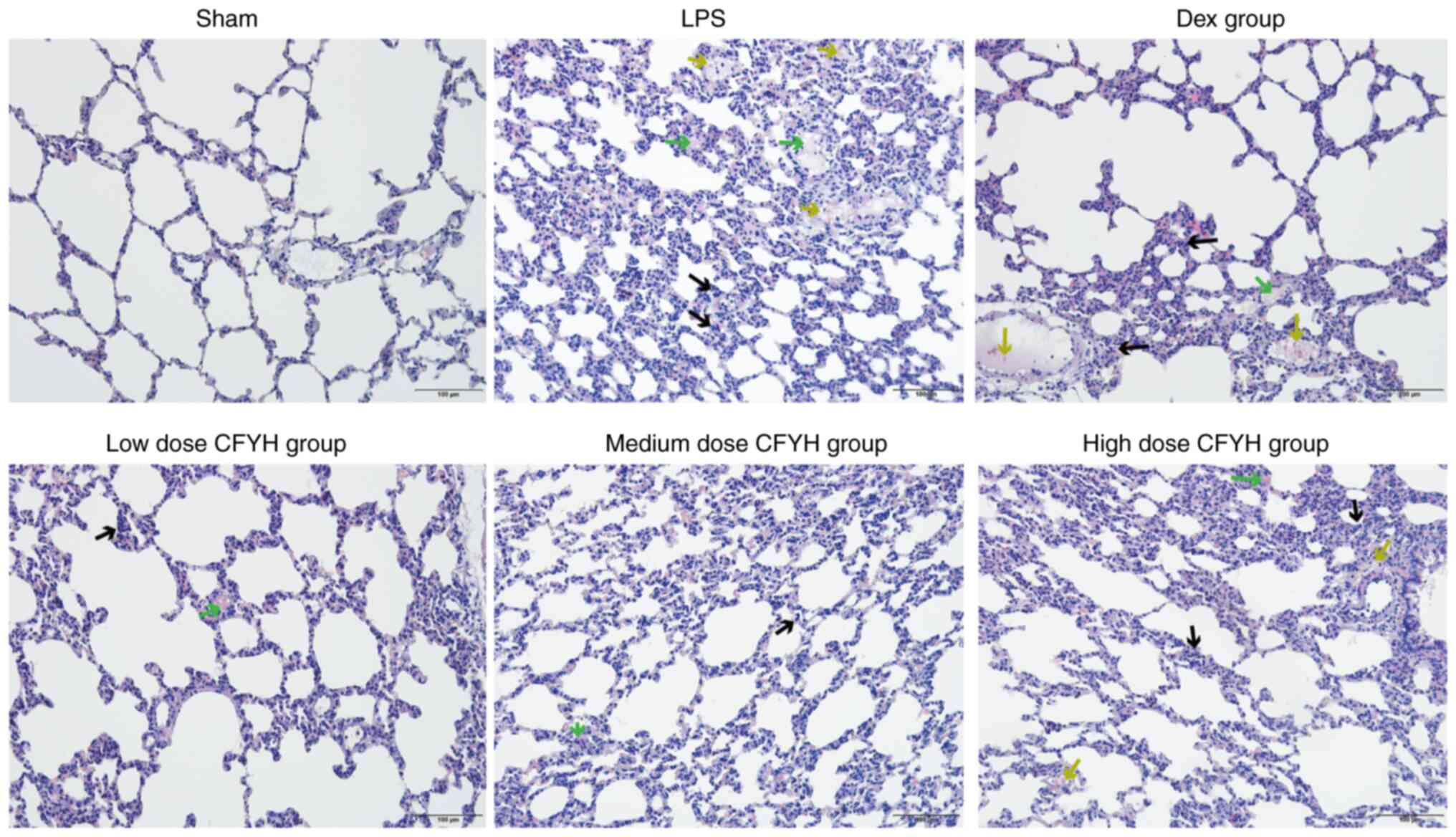
Effect of CFYH on lung histopathology. The
H&E staining results (Fig. 2)
showed that the lung tissue structure of rats in the sham group was
normal, while that of rats in the LPS group showed thickening of
the alveolar wall and widening of the alveolar septum, along with a
large number of red blood cells and inflammatory cells in the
alveolar cavity, and the lung tissue damage score was significantly
increased. In the CFYH and Dex groups, the infiltration of red
blood cells and inflammatory cells into the lung tissue decreased,
and the thickness of the alveolar wall decreased. The decrease in
lung tissue inflammation was most marked in the low-dose CFYH
group, and the lung tissue injury score was significantly lower
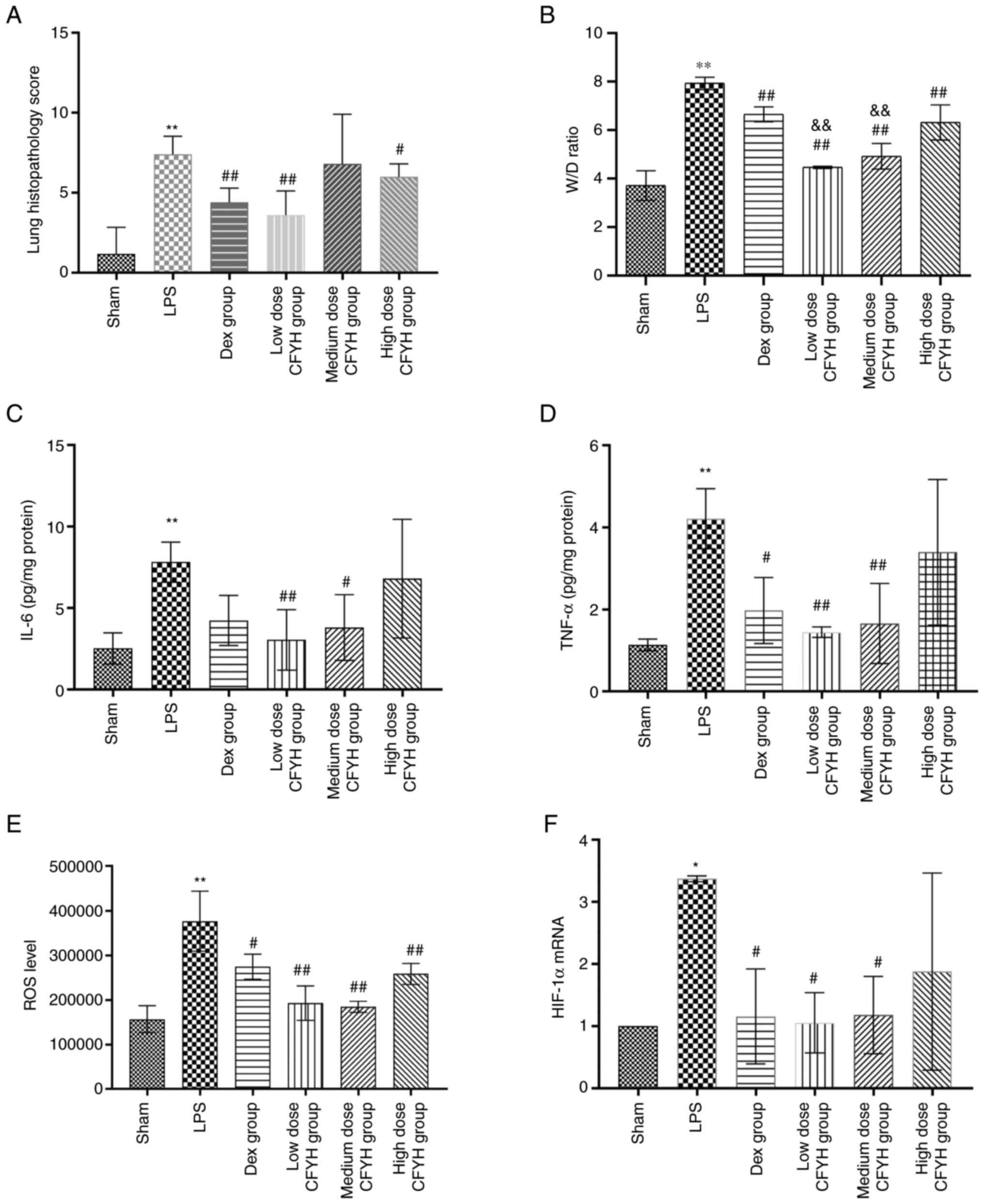
(Fig. 3A). The W/D weight ratio
can be used to reflect pulmonary edema. The W/D weight ratio in the
LPS group was substantially higher than that in the sham group. As
shown in Fig. 3B, the W/D weight
ratio decreased after pretreatment with CFYH and Dex, especially at
low and medium doses of CFYH.
CFYH downregulates the expression of IL-6 and
TNF-α in lung tissues. ALI is characterized by the presence of
inflammatory cytokines. ELISA results showed that LPS exposure
resulted in increased expression of IL-6 and TNF-α compared to that
in the sham group. However, IL-6 was significantly reduced in the
low and medium-dose CFYH group, and TNF-α was notably decreased in
the low and medium-dose CFYH and Dex groups (Fig. 3C and D).
Effects of CFYH on oxidative metabolism in rats
with LPS-induced ALI. ROS content was analyzed by flow
cytometry, and the results revealed that ROS levels increased in
the LPS group, whereas they were downregulated in the CFYH and Dex
groups (Fig. 3E).
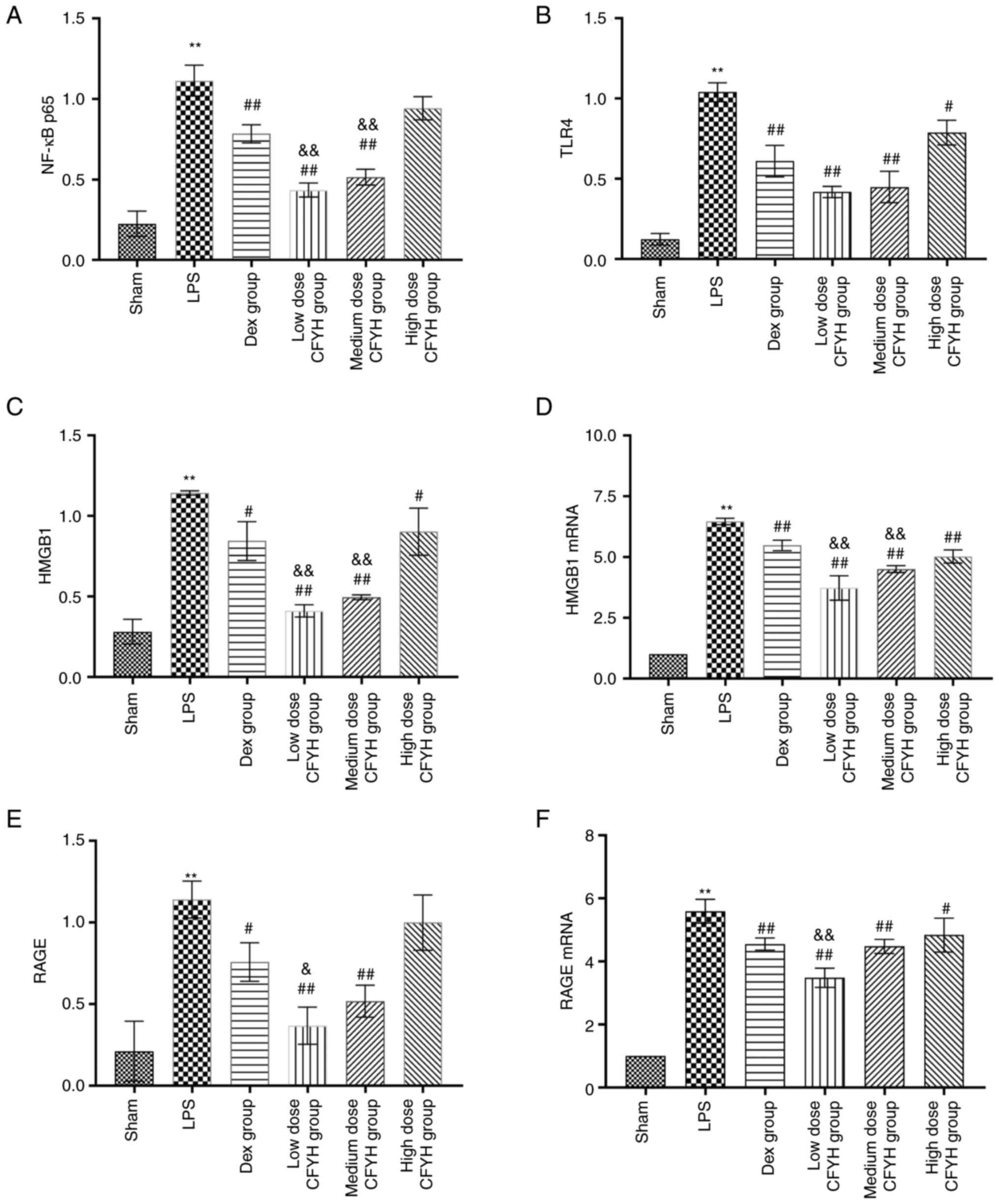
Effects of CFYH on HMGB1/RAGE
signaling
Western blotting was used to detect the expression
of NF-κB P65, TLR4, HMGB1, and RAGE protein expression levels. As
shown in Figs. 4A-E and 5, LPS stimulation enhanced the levels of
NF-κB P65, TLR4, HMGB1, and RAGE. In contrast, CFYH and Dex
pretreatment markedly reversed this effect. The expression of NF-κB
P65 was notably reduced in the low and medium-dose CFYH groups and
Dex group. TLR4 expression also decreased in the CFYH and Dex
groups. Simultaneously, HMGB1 expression in the low and medium-dose
CFYH groups was markedly reduced, and the results of RT-qPCR were
consistent with these results. The expression levels of RAGE in the
low and medium-dose CFYH groups and Dex group were markedly
decreased. The RT-qPCR results revealed that RAGE mRNA levels
decreased after CFYH and Dex pretreatment. Compared with the Dex
group, the low-dose CFYH group was more noticeably reduced
(Fig. 4D-F).
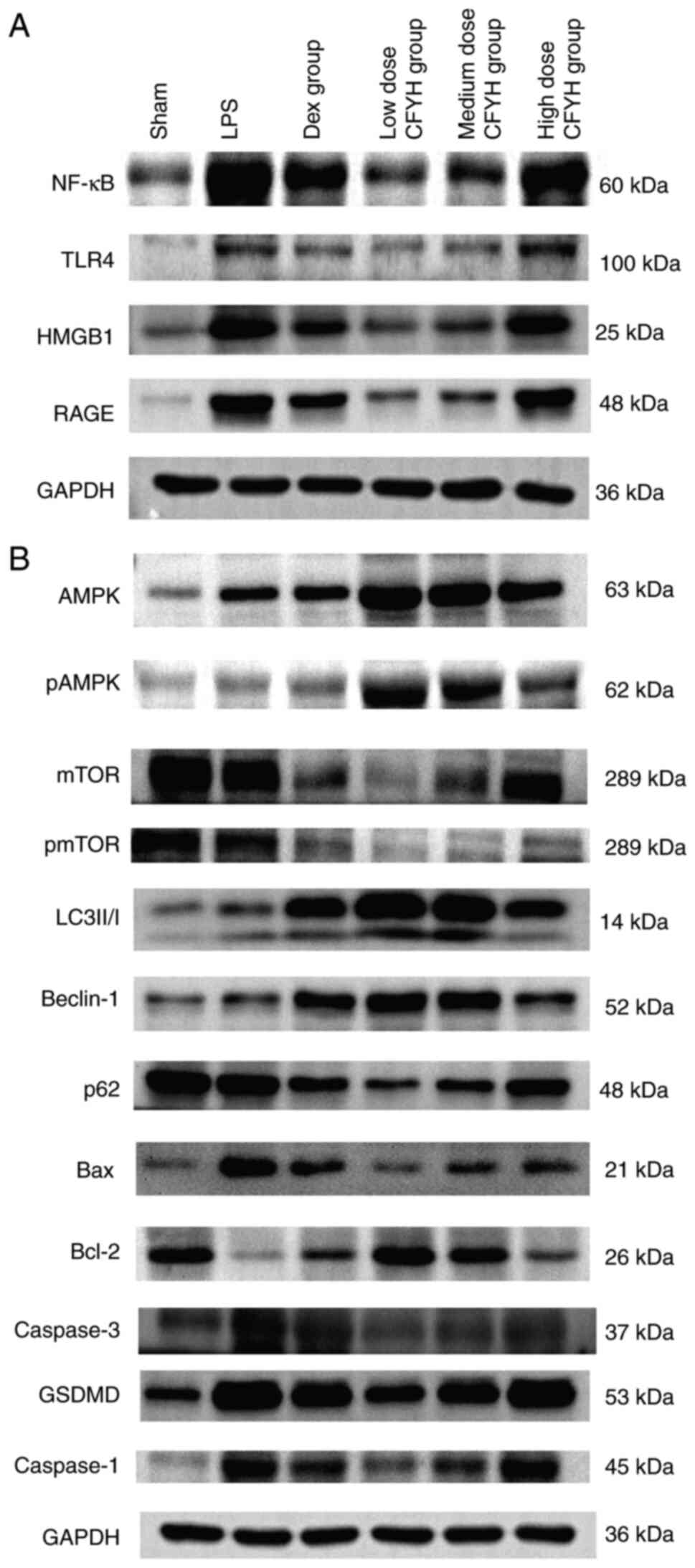 | Figure 5Western blotting analysis. (A)
Western blotting analysis of NF-κB P65, TLR4, HMGB1 and RAGE
expression levels. (B) Western blotting analysis of p-AMPK/AMPK,
p-mTOR/mTOR, LC3-II/I, Beclin-1, P62, Bax, Caspase-3, Bcl-2,
GSDMD-NT, Caspase-1, and NLRP3 expression levels. p,
phosphorylated; LC3, microtubule-associated protein 1 light chain
3; HMGB1, high mobility group box 1; RAGE, receptor for advanced
glycation end products; NLRP3, NOD-like receptor thermal protein
domain associated protein 3; GSDMD-NT, gasdermin D N-terminal. |
The effect of CFYH on HIF-1α was analyzed by PCR;
LPS resulted in HIF-1α upregulation, but pretreatment with CFYH
inhibited these effects, especially in the low and medium-dose
groups (Fig. 3F).
Effects of CFYH on the death pattern
of lung cells in ALI rats
CFYH promotes autophagy in LPS-induced ALI.
Western blotting was performed to detect AMPK, mTOR, phospho-AMPK,
and phospho-mTOR (Fig. 5B). The
phospho-AMPK/AMPK ratio was noticeably higher in the low dose CFYH
group than in the LPS group. The phospho-mTOR/mTOR expression ratio
was lower in the LPS group than in the sham group. Following
pretreatment with CFYH and Dex, the phospho-mTOR/mTOR ratio
decreased. The PCR results for AMPK and mTOR mRNA were consistent
with those of western blotting (Fig.
6A-D).
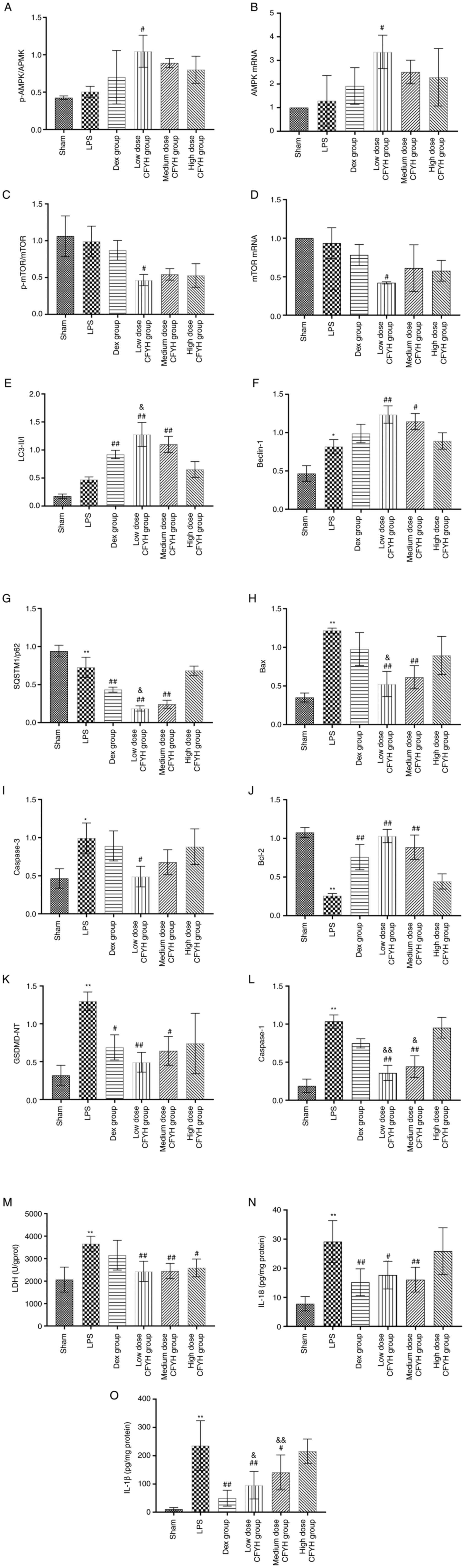 | Figure 6(A) The levels of p-AMPK/AMPK were
determined by western blotting; (B) The mRNA expression levels of
AMPK were determined by PCR. (C) The levels of p-mTOR/mTOR were
determined by western blotting. (D) The mRNA expression levels of
mTOR were determined by PCR. The levels of (E) LC3-II/I, (F)
Beclin-1, (G) P62, (H) Bax, (I) Caspase-3, (J) Bcl-2, (K) GSDMD-NT
and (L) Caspase-1 were determined by western blotting. The levels
of (M) LDH, (N) IL-18 and (O) IL-1β were determined by ELISA.
*P<0.05, **P<0.01 vs. sham group;
#P<0.05, ##P<0.01 vs. LPS group;
&P<0.05, &&P<0.01 vs. Dex
group. P, phosphorylated; LC3, microtubule-associated protein 1
light chain 3; HMGB1, high mobility group box 1; RAGE, receptor for
advanced glycation end products; NLRP3, NOD-like receptor thermal
protein domain associated protein 3; GSDMD-NT, gasdermin D
N-terminal. |
The expression levels of LC3-II/I, Beclin-1, and P62
were detected using western blotting (Fig. 5B). The results showed that the
LC3-II/I ratios of the CFYH and Dex groups were markedly higher
than those of the LPS group, and the CFYH low dose group was higher
than in the Dex group. Similarly, Beclin-1 protein expression
levels were significantly higher in the low and medium-dose CFYH
groups than in the LPS group. The expression trends of P62 at the
protein level in the LPS group were reversed by CFYH and Dex,
particularly in the low-dose CFYH group (Fig. 6E-G). Immunohistochemical analysis
showed that the average optical density of Beclin-1 was higher in
the low-dose CFYH group than in the LPS group (Fig. 7A and C). These results indicated that CFYH
enhanced autophagy in LPS-induced lung injury.
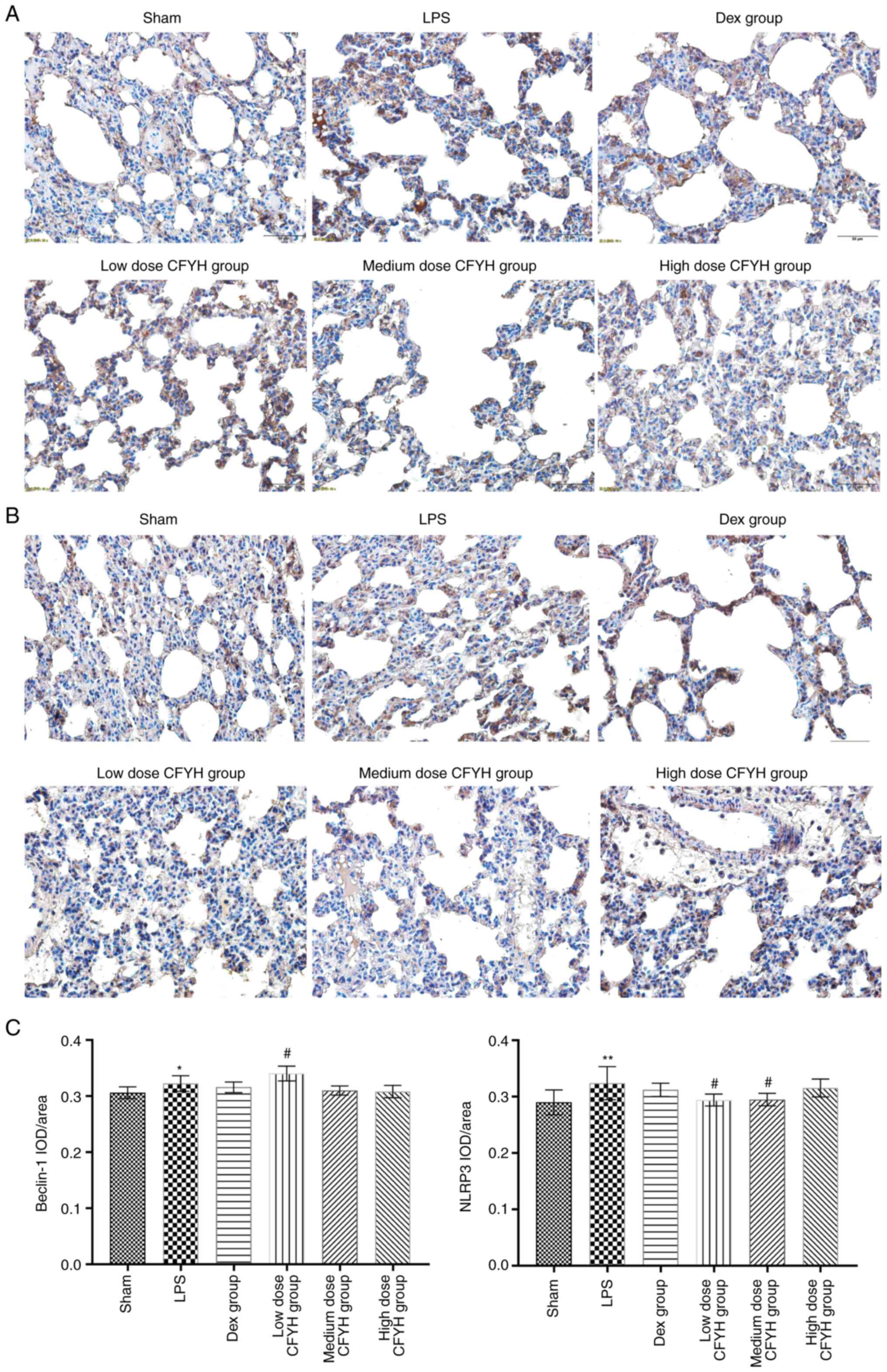 | Figure 7Results of immunohistochemistry
analysis. (A) Immunohistochemistry analysis of Beclin-1 in the
Sham, LPS, Dex, and low, medium, and high-dose CFYH groups. (B)
Immunohistochemistry analysis of NLRP-3 in the Sham, LPS, Dex, and
low, medium, and high-dose CFYH groups. (C) The levels of Beclin-1
and NLRP3 were detected by immunohistochemical analysis.
*P<0.05, **P<0.01 vs. sham group;
#P<0.05, ###P<0.001 vs. LPS group.
CFYH, Chuanfangyihao; Dex, Dexamethasone group; LPS,
lipopolysaccharide group. |
CFYH inhibits apoptosis in the LPS-induced ALI
rat model. To elucidate the relationship between LPS exposure
and apoptosis, the expression of apoptosis-related proteins was
determined. Compared to the LPS group, the expression levels of Bax
protein in the low and medium-dose CFYH groups were markedly
reduced. LPS exposure led to a notable increase in the expression
of Caspase-3 at the protein level, and this increased expression of
caspase-3 was inhibited by pretreatment with CFYH and Dex,
especially by the low-dose CFYH group. The expression levels of
Bcl-2 protein in the low and medium-dose CFYH and Dex groups were
notably higher than those in the LPS group (Figs. 5B and 6H-J). These results suggested that CFYH
suppressed apoptosis in LPS-triggered lung injury.
CFYH attenuates pyroptosis of LPS-induced
ALI. Pyroptosis mediated LPS-induced pulmonary dysfunction. The
protein expression of GSDMD-NT and Caspase-1 was examined by
western blotting (Fig. 5B). These
results suggest that the protein expression levels of GSDMD-NT and
Caspase-1 in the lung tissues of the LPS group were markedly
increased. The expression levels of GSDMD-NT were markedly reduced
in the low and medium-dose CFYH and Dex groups. Additionally, the
expression levels of Caspase-1 protein in the low and medium-dose
CFYH groups were significantly downregulated (Fig. 6K and L). Immunohistochemical analysis was
performed to detect NLRP3 expression. The mean optical density of
NLRP3 was lower in the low and medium-dose CFYH groups than in the
LPS group (Fig. 7B and C).
When a cell undergoes pyroptosis, the cell membrane
is ruptured, and LDH is released extracellularly. ELISA was used to
detect LDH levels, and the results showed that the LDH content in
the CFYH group was notably lower than that in the LPS group
(Fig. 6M). Furthermore, the low
and medium-dose CFYH groups and Dex group reversed the effects of
the LPS group on IL-18 and IL-1β (Fig.
6N and O). These results
indicate that CFYH alleviated pyroptosis.
Taken together, these results suggest that CFYH
attenuated LPS-induced ALI in rats by regulating autophagy and
repressing apoptosis and pyroptosis.
Discussion
In the present study, the potential pharmacological
mechanism of CFYH in the management of ALI was investigated. The
results revealed that CFYH effectively attenuated histopathological
damage in a rat model of ALI induced using LPS while inhibiting
apoptosis, pyroptosis, and inflammation.
ALI/ARDS is one of the leading causes of death in
severe clinical cases of lung disease, characterized by its rapid
onset and high fatality rate (24). Its impact is extensive and
far-reaching (25), imposing a
substantial economic burden on individuals and society. Moreover,
it severely impairs the quality of life of patients, with lasting
effects persisting for up to 5 years after recovery (26). Unfortunately, there is currently no
specific treatment available for this disease in the clinical
practice (27). Therefore,
effective prevention of ALI in its early stages and halting its
progression to ARDS remain critical challenges that demand urgent
attention (28).
In recent years, the application of TCM preparations
and extracts for the prevention and treatment of ALI has garnered
increasing attention from scholars (29). However, the chemical composition of
TCM is complex, and not all compounds have pharmacological
activity. This complexity poses a challenge to comprehensively
studying the potential mechanism of Chinese medicinal formulations
(30). The pathogenesis of ALI is
intricate, and a single drug cannot achieve the expected
therapeutic effect. TCM formulations can exert therapeutic effects
through the synergistic actions of various pharmacologically active
compounds (31). Through
experimental verification, it was systematically elucidated the
possible pharmacological mechanism of CFYH against ALI in the
present study.
CFYH is derived from the classical, TCM
prescriptions, Zhenwu concoction, and Buyanghuanwu concoction.
Studies have demonstrated that Zhenwu concoction protects
mitochondrial function and attenuates apoptosis (32,33).
It has also shown potential in alleviating symptoms and promoting
heart pulmonary function in patients with acute-stage chronic
pulmonary heart disease (34).
Buyanghuanwu concoction diminished the generation of Keap1,
upregulated Nrf2 expression, and promoted the expression of HO-1 in
a bleomycin-induced model (35).
CFYH may be a promising method for managing lung disorders in
clinical practice due it favorable efficacy (13,14),
and the favorable safety profile of CHYF is also a major advantage.
The combination of each Chinese medicine is performed in line with
the composition norms of Chinese medicine formulas (36), and the dosages of the medicines in
CFYH are in agreement with the standards of the safe use of TCM
(37). Thus far, no safety risks
have been found in patients taking CFYH. However, Chinese medicines
often have a poor taste, and certain people who are not accustomed
to the taste of Chinese medicines may find CFYH difficult to
swallow.
The results of the present study provide a basis for
experiments to verify the role of CFYH in LPS-induced ALI. There
are several pathogenic factors that can lead to the development of
ALI/ARDS, among which gram-negative bacterial infection-induced ALI
accounts for a marked proportion (38). LPS, a glycolipid complex, is
present in the cell wall of gram-negative bacteria. LPS activates
mononuclear macrophages and induces the synthesis and release of
pro-inflammatory cytokines, chemokines, growth factors, and a
variety of other factors (39).
The major toxicity centers and bioactive parts of LPS are highly
conserved and non-species specific, thus the toxic effects of LPS
produced by different strains are similar (40). Consequently, LPS is often used to
construct animal models of ALI, as the inflammatory injury induced
by LPS in the body is similar to that of a real infection caused by
gram-negative bacteria (41). In
the LPS-induced ALI rats, massive inflammatory cell infiltration,
alveolar space, interstitial edema, red blood cell leakage,
alveolar wall congestion, and alveolar wall thickening were
observed, indicating the successful establishment of the model.
Moreover, the CFYH group alleviated the histopathological damage of
the rat lung tissues to varying degrees.
Excessive production of inflammatory cytokines and
oxygen free radicals are two major contributors to LPS-induced ALI.
Several inflammatory mediators accumulate in the lungs after LPS
stimulation (42). As a common
DAMP, HMGB1 can amplify the inflammatory cascade, and it is also an
important late inflammatory factor in the inflammatory response of
ALI/ARDS, and its synthesis and release directly affect the
occurrence and development of ALI/ARDS (43,44).
Extracellular HMGB1 binds to receptors such as RAGE and TLR4 on the
cell surface, activating several downstream inflammatory signaling
pathways and inducing inflammatory mediators such as NF-κB, IL-6,
and TNF-α, thus enhancing the inflammatory response in lung
tissues. This interaction between HMGB1 and inflammatory cytokines
creates a vicious cycle, aggravating the degree of respiratory
dysfunction and lung injury.
LPS-induced inflammation is often accompanied by
hypoxia (45). A continuous supply
of ATP is key to maintaining physiological activities. ATP
synthesis is primarily performed by mitochondria (46), which produce large quantities of
ROS during hypoxia. ROS damage of pulmonary microvascular
endothelial cells and epithelial cells, increases pulmonary
vascular permeability, and leads to the formation of pulmonary
edema (47-49).
Both hypoxia and inflammation can stimulate the upregulation of
HIF-1α (50,51). HIF-1α is also an important
inflammatory regulator that promotes the release of various
pro-inflammatory factors, such as TNF-α, IL-1β, and IL-6, which
further aggravates the pulmonary inflammatory response through
their interactions (52,53). These results show that HMGB1 and
RAGE at the protein and mRNA level, IL-6, TNF-α, NF-κB P65, TLR4,
and ROS levels were significantly increased following LPS
induction. Interestingly, these effects were reversed by CFYH
treatment. These results suggest that CFYH protects against ALI
through its anti-inflammatory and antioxidant effects.
Programmed cell death is often associated with
inflammation, and autophagy, apoptosis, and pyroptosis are involved
in the occurrence and development of LPS-related ALI (38,54-59,60).
The entire autophagy process is regulated by >30
autophagy-related genes (Atgs) (61). AMPK acts as an energy metabolism
switch and activates autophagy by inhibiting mTOR phosphorylation
(62,63). Microtubule-associated protein 1
light chain 3 (LC3) is an autophagy marker that is responsible for
regulating the elongation and extension of autophagosome membranes,
and the ratio of LC3-II/I can be used to estimate the levels of
autophagy (64-66).
The Atg Beclin-1 interferes with the formation of autophagosomes at
different stages, and the p62 protein is primarily responsible for
the degradation of autophagy substrates (67,68).
When autophagy occurs, LC3 is transformed from type I to type II,
beclin-1 expression level is increased, and P62 is gradually
degraded (69). The results of the
present study showed that AMPK, LC3-II/I, and Beclin-1 in lung
tissues of rats in the LPS group were increased and significantly
decreased after CFYH intervention. The expression levels of P62 and
mTOR in the LPS group decreased, and CFYH had the opposite effect.
These results suggest that CFYH enhances autophagy.
Bcl-2 and the Caspase families of proteins play
important roles in the regulation of the apoptosis pathway
(70). Bax and Bcl-2 are a pair of
positive and negative regulators, with Bax promoting apoptosis and
Bcl-2 preventing apoptosis (71).
Caspase-3 is located downstream of different apoptosis pathways and
is a key executioner of apoptosis, and its cleavage indicates a
commitment to apoptosis (72). The
experimental results of the present study showed that the
expression levels of Bax and Caspase-3 were significantly increased
in the LPS group, which could not be reversed by Dex, but was
reversed by CFYH. After CFYH intervention, Bcl-2 protein levels
were significantly higher than those in the LPS group. These
results indicate that CFYH inhibits apoptosis.
Pyroptosis, also known as cell inflammatory
necrosis, is induced by the inflammasome and initiates through
gasdermin-D activation by the inflammatory caspase family, which
causes cell membrane perforation, cell lysis, and the release of
cellular contents, such as activated IL-1β and IL-18. IL-1β and
IL-18 inflammatory factors produce pro-inflammatory signals, which
amplify the inflammatory response and lead to ‘pathological
suicide’ of the cell (73). The
occurrence, development, and severity of ALI depend on this process
(74-78).
The results of the present study showed that the levels of key
pyroptosis proteins in the LPS group were significantly higher than
those in the sham group. After CFYH intervention, the expression of
all the measured proteins was reduced to varying degrees. Although
none of the indicators showed obvious dose dependence in the low,
medium, and high-dose CFYH groups, as a whole, CFYH blocked
pyroptosis in rats with ALI.
The functional relationship between autophagy,
apoptosis, and pyroptosis is complicated and subtle (79,80).
Studies have shown that when cells are subjected to a level of low
environmental pressures (slight or early inflammation), they
initiate autophagic mechanisms to overcome the pressure, and
autophagy increases sharply. This increase in autophagy inhibits
the apoptosis and pyroptosis of cells. Conversely, under severe
inflammatory conditions, the body initiates the mechanism of
apoptosis and pyroptosis, leading to further damage (81-86).
The AMPK/mTOR signaling pathway, as an important signaling pathway
regulating the autophagy-apoptosis-pyroptosis balance, has been
shown to play a key role in the improvement of ALI.
The design of the present study was based on the
concept of prevention (87,88),
and the purpose was to observe whether Chinese medicines had a
therapeutic effect for the treatment of ALI, after reaching its
requisite blood concentration in animals. Future studies will focus
on the idea of developing and administering TCM after therapeutic
modeling.
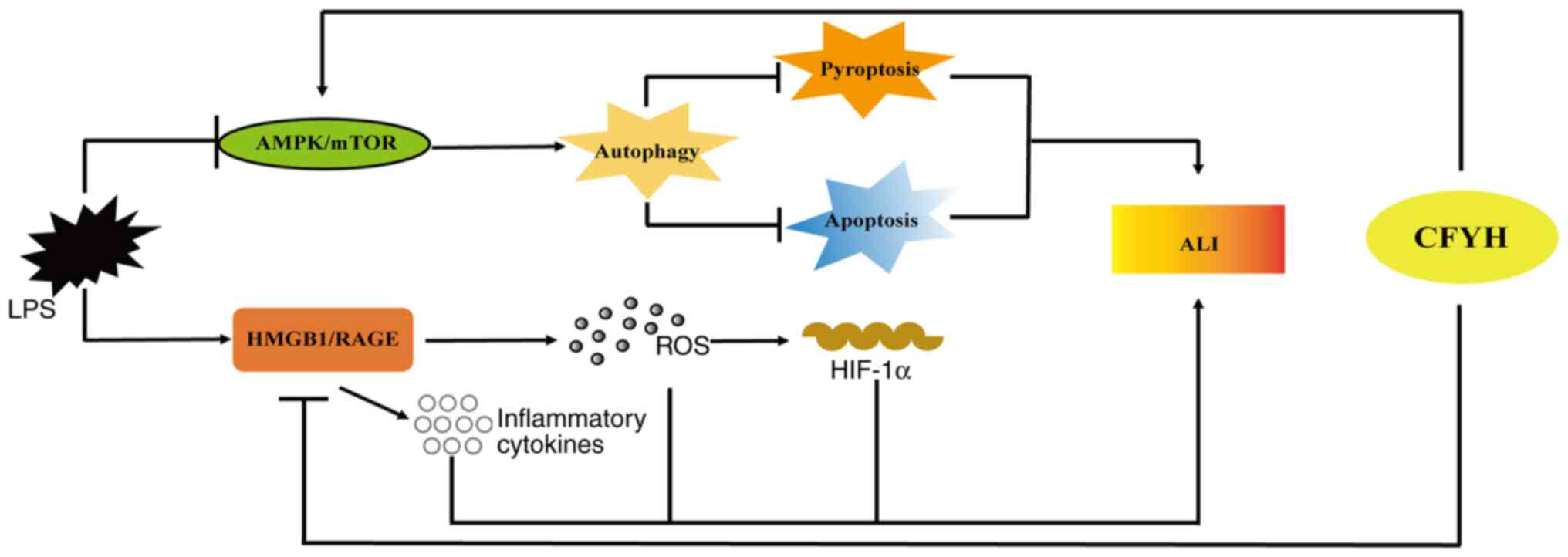
In conclusion, it was shown that CFYH exhibited a
favorable effect against LPS-induced ALI. This protective effect
may be mediated by downregulating the HMGB1/RAGE signaling pathway,
reducing the release of inflammatory factors, inhibiting oxidative
stress, activating the AMPK/mTOR signaling pathway, enhancing lung
cell autophagy, and inhibiting lung cell pyroptosis and apoptosis
(Fig. 8). These results suggest
that CFYH has anti-inflammatory, anti-stress, and anti-apoptosis
properties. The present study provides a theoretical basis for the
clinical application of CFYH in the treatment of ALI. However, an
in-depth study of these mechanisms requires further
investigation.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Sichuan Provincial
Administration of Traditional Chinese Medicine (grant no.
CKY2021155).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HF, XL, WT, and XH designed the study. HF, XL, and
WT performed the experiments. HF and XL were responsible for
primary data generation, analysis, and writing of the manuscript.
WT and XH revised the manuscript. All authors have read and
approved the final manuscript. HF, XL and XH confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals and were approved by the ethical committee for
the experimental use of animals at Sichuan Academy of Traditional
Chinese Medicine [approval no. SYLL(2022)-039].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Monsel A, Zhu YG, Gudapati V, Lim H and
Lee JW: Mesenchymal stem cell derived secretome and extracellular
vesicles for acute lung injury and other inflammatory lung
diseases. Expert Opin Biol Ther. 16:859–871. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang LP and Tang XQ: Study on early
warning signals of acute lung injury. Life Sci Res. 25:532–539.
2021.
|
|
3
|
Zambon M and Vincent JL: Mortality rates
for patients with acute lung injury/ARDS have decreased over time.
Chest. 133:1120–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee CS:
Acute respiratory distress syndrome. Nat Rev Dis Primers.
5(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al Masry A, Boules ML, Boules NS and Ebied
RS: Optimal method for selecting PEEP level in ALI/ARDS patients
under mechanical ventilation. J Egypt Soc Parasitol. 42:359–372.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Fan E, Del Sorbo L, Goligher EC, Hodgson
CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower
RG, et al: An official American thoracic society/European society
of intensive care medicine/society of critical care medicine
clinical practice guideline: Mechanical ventilation in adult
patients with acute respiratory distress syndrome. Am J Respir Crit
Care Med. 195:1253–1263. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Duggal A, Rezoagli E, Pham T, McNicholas
BA, Fan E, Bellani G, Rubenfeld G, Pesenti AM and Laffey JG: LUNG
SAFE Investigators and the ESICM Trials Group. Patterns of use of
adjunctive therapies in patients with early moderate to severe
ARDS: Insights from the LUNG SAFE study. Chest. 157:1497–1505.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rosenberg OA: Pulmonary surfactant
preparations and surfactant therapy for ARDS in surgical intensive
care (a literature review). Creat Surg Oncol. 9:50–65. 2019.
|
|
9
|
Badet M, Bayle F, Richard JC and Guérin C:
Comparison of optimal positive end-expiratory pressure and
recruitment maneuvers during lung-protective mechanical ventilation
in patients with acute lung injury/acute respiratory distress
syndrome. Respir Care. 54:847–854. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fukuda Y: Acute lung injury/acute
respiratory distress syndrome: progress in diagnosis and treatment.
Topics: I. Pathogenesis and pathophysiology: 4. Pathophysiology and
histopathology of ALI/ARDS. Nihon Naika Gakkai Zasshi.
100:1536–1540. 2011.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
11
|
Zhang X, Zheng J, Yan Y, Ruan Z, Su Y,
Wang J, Huang H, Zhang Y, Wang W, Gao J, et al:
Angiotensin-converting enzyme 2 regulates autophagy in acute lung
injury through AMPK/mTOR signaling. Arch Biochem Biophys.
672(108061)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Su JS, Liu ES and Zhao XM: Advances in the
treatment of acute lung injury/acute respiratory distress syndrome
with traditional chinese medicine. Jilin J Tradit Chin Med.
(37)2019.(In Chinese).
|
|
13
|
Wang SB: Clinical characteristics and risk
factors of severe pneumonia in 165 cases of novel coronavirus
pneumonia. Chengdu University of TCM, 2021 (In Chinese).
|
|
14
|
Liu M: Effect of chuanfang no: L exposure
on COVID-19 a retrospective cohort study based on propensity score
matching. Chengdu University of TCM, 2021 (In Chinese).
|
|
15
|
Huang JH, Huang XH, Chen ZY, Zheng QS and
Sun RY: Dose conversion among different animals and healthy
volunteers in pharmacological study. Chin J Clin Pharmacol Ther.
9:1069–1072. 2004.
|
|
16
|
Arck PC: When 3 Rs meet a forth R:
Replacement, reduction and refinement of animals in research on
reproduction. J Reprod Immunol. 132:54–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: NC3Rs Reporting Guidelines Working Group. Animal
research: Reporting in vivo experiments: the ARRIVE guidelines. Br
J Pharmacol. 160:1577–1579. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang GQ: The effect and mechanism of
Lianggesan on fluid transportion in endotoxin acute lung injury
tissue. So Med Univ, 2014 (In Chinese).
|
|
19
|
Wang XH, Yan SG, Hui Y, Shi J and Li JT:
Mechanism of ephedrae Herba-rhei Radix et Rhizoma drug pair on
treating acute lung injury based on alveolar macrophage M2
polarization. Chin Tradit Herbal Drugs. 53:2715–2722. 2022.(In
Chinese).
|
|
20
|
Pei CX, Wang XM, Wu YC, Wang ZX, Huang DM,
Yang Q, et al: Studies on mechanism of platycodin D inhibiting
apoptosis and protecting acute lung injury via Bax/bcl-2/caspase-3
signaling pathway. Modernization Tradit Chin Med Materia
Medica-World Sci and Tech. 23:3551–3558. 2021.(In Chinese).
|
|
21
|
Mikawa K, Nishina K, Takao Y and Obara H:
ONO-1714, a nitric oxide synthase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
97:1751–1755. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cumming G, Fidler F and Vaux DL: Error
bars in experimental biology. J Cell Biol. 177:7–11.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang X, Xiu H, Zhang S and Zhang G: The
role of macrophages in the pathogenesis of ALI/ARDS. Mediators
Inflamm. 2018(1264913)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Janz DR and Ware LB: Biomarkers of
ALI/ARDS: Pathogenesis, discovery, and relevance to clinical
trials. Semin Respir Crit Care Med. 34:537–548. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bos LD, Martin-Loeches I and Schultz MJ:
ARDS: Challenges in patient care and frontiers in research. Eur
Respir Rev. 27(170107)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Spieth PM and Zhang H: Pharmacological
therapies for acute respiratory distress syndrome. Curr Opin Crit
Care. 20:113–121. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao R, Wang B, Wang D, Wu B, Ji P and Tan
D: Oxyberberine prevented lipopolysaccharide-induced acute lung
injury through inhibition of mitophagy. Oxid Med Cell Longev.
2021(6675264)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu WY, Gao CX, Zhang HH, Wu YG and Yu CH:
Herbal active ingredients: potential for the prevention and
treatment of acute lung injury. Biomed Res Int.
2021(5543185)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hao X, Liu Y, Zhou P, Jiang Q, Yang Z, Xu
M, Liu S, Zhang S and Wang Y: Integrating network pharmacology and
experimental validation to investigate the mechanisms of
huazhuojiedu decoction to treat chronic atrophic gastritis. Evid
Based Complement Alternat Med. 2020(2638362)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li C: Multi-compound pharmacokinetic
research on Chinese herbal medicines: Approach and methodology.
Zhongguo Zhong Yao Za Zhi. 42:607–617. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Li Z, Li WJ, Shang XY and Yu KY: Effect of
Zhenwu Decoction on myocardial mitochondrial damage and
cardiomyocyte apoptosis in heart failure rats by SIRT1 signaling
pathway. Chin Arch Tradit Chin Med. 36:1062–1067. 2018.(In
Chinese).
|
|
33
|
Wang YH, Li WJ and Li Z: Effect of serum
containing Zhenwu Decoction on cardiomyocyte apoptosis and Bcl-2
and Bax protein expression induced by isoproterenol in Rats.
Liaoning J Trad Chin Med. 45:1305–1308+1346. 2018.(In Chinese).
|
|
34
|
Yang PQ, Li QY and Fang S: Effect of
Zhenwu Decoction combined with Benazepril on the curative effect
and cardiopulmonary function of chronic pulmonary heart disease in
acute stage. J Emerg Trad Chin Med. 31:854–856. 2022.(In
Chinese).
|
|
35
|
Zhang Z, Zhao S, Han YP, Jin F, Shang JR,
Wang JP and Fang CY: Effect of Buyang Huanwu Decoction on
Keap1/Nrf2/HO-1 antioxidant signaling pathway in rats with
idiopathic pulmonary fibrosis. Chin J Exp Trad Med formula. 1–10,
(In Chinese).
|
|
36
|
National Pharmacopoeia Commission,
Pharmacopoeia of the People's Republic of China (2020 Edition),
China Medical Sciences Press, 2020.
|
|
37
|
Gao XM: Traditional Chinese pharmacy.
China Traditional Chinese Medicine Press, 2002-1.
|
|
38
|
Li H, Li Y, Song C, Hu Y, Dai M, Liu B and
Pan P: Neutrophil extracellular traps augmented alveolar macrophage
pyroptosis via AIM2 inflammasome activation in LPS-induced
ALI/ARDS. J Inflamm Res. 14:4839–4858. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Song C, Li H, Li Y, Dai M, Zhang L, Liu S,
Tan H, Deng P, Liu J, Mao Z, et al: NETs promote ALI/ARDS
inflammation by regulating alveolar macrophage polarization. Exp
Cell Res. 382(111486)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fujita M, Kuwano K, Kunitake R, Hagimoto
N, Miyazaki H, Kaneko Y, Kawasaki M, Maeyama T and Hara N:
Endothelial cell apoptosis in lipopolysaccharide-induced lung
injury in mice. Int Arch Allergy Immunol. 117:202–208.
1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fukatsu M, Ohkawara H, Wang X, Alkebsi L,
Furukawa M, Mori H, Fukami M, Fukami SI, Sano T, Takahashi H, et
al: The suppressive effects of Mer inhibition on inflammatory
responses in the pathogenesis of LPS-induced ALI/ARDS. Sci Signal.
15(eabd2533)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li N, Geng C, Hou S, Fan H and Gong Y:
Damage-associated molecular patterns and their signaling pathways
in primary blast lung injury: New research progress and future
directions. Int J Mol Sci. 21(6303)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang G, Han D, Zhang Y, Xie X, Wu Y, Li S
and Li M: A novel hypothesis: Up-regulation of HO-1 by activation
of PPARγ inhibits HMGB1-RAGE signaling pathway and ameliorates the
development of ALI/ARDS. J Thorac Dis. 5:706–710. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu Y, Li J, Lin Z, Liang W, Qin L, Ding J,
Chen S and Zhou L: Isorhamnetin alleviates airway inflammation by
regulating the Nrf2/Keap1 pathway in a mouse model of COPD. Front
Pharmacol. 13(860362)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Meyer A, Zoll J, Charles AL, Charloux A,
de Blay F, Diemunsch P, Sibilia J, Piquard F and Geny B: Skeletal
muscle mitochondrial dysfunction during chronic obstructive
pulmonary disease: Central actor and therapeutic target. Exp
Physiol. 98:1063–1078. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bouitbir J, Charles AL, Echaniz-Laguna A,
Kindo M, Daussin F, Auwerx J, Piquard F, Geny B and Zoll J:
Opposite effects of statins on mitochondria of cardiac and skeletal
muscles: A ‘mitohormesis’ mechanism involving reactive oxygen
species and PGC-1. Eur Heart J. 33:1397–1407. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yu J, Wang Y, Li Z, Dong S, Wang D, Gong
L, Shi J, Zhang Y, Liu D and Mu R: Effect of heme oxygenase-1 on
mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Sci Rep.
6(36530)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee SY, Li MH, Shi LS, Chu H, Ho CW and
Chang TC: Rhodiola crenulata extract alleviates hypoxic pulmonary
edema in rats. Evid Based Complement Alternat Med.
2013(718739)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Eltzschig HK and Carmeliet P: Hypoxia and
inflammation. N Engl J Med. 364:656–665. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Devraj G, Beerlage C, Brüne B and Kempf
VA: Hypoxia and HIF-1 activation in bacterial infections. Microbes
Infect. 19:144–156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cummins EP, Keogh CE, Crean D and Taylor
CT: The role of HIF in immunity and inflammation. Mol Aspects Med.
47-48:24–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao J, Jiang P, Guo S, Schrodi SJ and He
D: Apoptosis, autophagy, NETosis, necroptosis, and pyroptosis
mediated programmed cell death as targets for innovative therapy in
rheumatoid arthritis. Front Immunol. 12(809806)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kesavardhana S, Malireddi RKS and
Kanneganti TD: Caspases in cell death, inflammation, and
pyroptosis. Annu Rev Immunol. 38:567–595. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu X, Gao C, Wang Y, Niu L, Jiang S and
Pan S: BMSC-derived exosomes ameliorate LPS-induced acute lung
injury by miR-384-5p-controlled alveolar macrophage autophagy. Oxid
Med Cell Longev. 2021(9973457)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wu D, Zhang H, Wu Q, Li F, Wang Y, Liu S
and Wang J: Sestrin 2 protects against LPS-induced acute lung
injury by inducing mitophagy in alveolar macrophages. Life Sci.
267(118941)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhao X, Yu Z, Lv Z, Meng L, Xu J, Yuan S
and Fu Z: Activation of alpha-7 nicotinic acetylcholine receptors
(α7nAchR) promotes the protective autophagy in LPS-induced acute
lung injury (ALI) in vitro and in vivo. Inflammation. 42:2236–2245.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ning L, Wei W, Wenyang J, Rui X and Qing
G: Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung
injury induced by lipopolysaccharide. Clin Transl Med.
10(e228)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Klionsky DJ, Codogno P, Cuervo AM, Deretic
V, Elazar Z, Fueyo-Margareto J, Gewirtz DA, Kroemer G, Levine B,
Mizushima N, et al: A comprehensive glossary of autophagy-related
molecules and processes. Autophagy. 6:438–448. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mizushima N and Levine B: Autophagy in
human diseases. N Engl J Med. 383:1564–1576. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Qu L, Chen C, He W, Chen Y, Li Y, Wen Y,
Zhou S, Jiang Y, Yang X, Zhang R and Shen L: Glycyrrhizic acid
ameliorates LPS-induced acute lung injury by regulating autophagy
through the PI3K/AKT/mTOR pathway. Am J Transl Res. 11:2042–2055.
2019.PubMed/NCBI
|
|
67
|
Wei F, Jiang X, Gao HY and Gao SH:
Liquiritin induces apoptosis and autophagy in cisplatin
(DDP)-resistant gastric cancer cells in vitro and xenograft
nude mice in vivo. Int J Oncol. 51:1383–1394.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Masuda GO, Yashiro M, Kitayama K, Miki Y,
Kasashima H, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T,
Sakurai K, et al: Clinicopathological correlations of
autophagy-related proteins LC3, beclin 1 and p62 in gastric cancer.
Anticancer Res. 36:129–136. 2016.PubMed/NCBI
|
|
70
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Choudhary GS, Al-Harbi S and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 1219:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Zhang ZQ and Sun LL: Clinical significance
of IL-1β and IL-18 in acute lung injury. Hainan Med J.
28:1954–1956. 2017.(In Chinese).
|
|
75
|
McVey MJ, Steinberg BE and Goldenberg NM:
Inflammasome activation in acute lung injury. Am J Physiol Lung
Cell Mol Physiol. 320:L165–L178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Karmakar M, Minns M, Greenberg EN,
Diaz-Aponte J, Pestonjamasp K, Johnson JL, Rathkey JK, Abbott DW,
Wang K, Shao F, et al: N-GSDMD trafficking to neutrophil organelles
facilitates IL-1β release independently of plasma membrane pores
and pyroptosis. Nat Commun. 11(2212)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu Y, Zhou J, Luo Y, Li J, Shang L, Zhou
F and Yang S: Honokiol alleviates LPS-induced acute lung injury by
inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2
activation in vitro and in vivo. Chin Med. 16(127)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tan S and Chen S: The mechanism and effect
of autophagy, apoptosis, and pyroptosis on the progression of
silicosis. Int J Mol Sci. 22(8110)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ge Y, Xu X, Liang Q, Xu Y and Huang M:
α-Mangostin suppresses NLRP3 inflammasome activation via promoting
autophagy in LPS-stimulated murine macrophages and protects against
CLP-induced sepsis in mice. Inflamm Res. 68:471–479.
2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Wong WT, Li LH, Rao YK, Yang SP, Cheng SM,
Lin WY, Cheng CC, Chen A and Hua KF: Repositioning of the β-blocker
carvedilol as a novel autophagy inducer that inhibits the NLRP3
inflammasome. Front Immunol. 9(1920)2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Guo R, Wang H and Cui N: Autophagy
regulation on pyroptosis: Mechanism and medical implication in
sepsis. Mediators Inflamm. 2021(9925059)2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ma C, Zhang D, Ma Q, Liu Y and Yang Y:
Arbutin inhibits inflammation and apoptosis by enhancing autophagy
via SIRT1. Adv Clin Exp Med. 30:535–544. 2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Nakahira K, Cloonan SM, Mizumura K, Choi
AMK and Ryter SW: Autophagy: A crucial moderator of redox balance,
inflammation, and apoptosis in lung disease. Antioxid Redox Signal.
20:474–494. 2014.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Changle Z, Cuiling F, Feng F, Xiaoqin Y,
Guishu W, Liangtian S and Jiakun Z: Baicalin inhibits inflammation
of lipopolysaccharide-induced acute lung injury toll like
receptor-4/myeloid differentiation primary response 88/nuclear
factor-kappa B signaling pathway. J Tradit Chin Med. 42:200–212.
2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Liu Z, Wang P, Lu S, Guo R, Gao W, Tong H,
Yin Y, Han X, Liu T, Chen X, et al: Liquiritin, a novel inhibitor
of TRPV1 and TRPA1, protects against LPS-induced acute lung injury.
Cell Calcium. 88(102198)2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|