Introduction
In ophthalmology, orbital fracture (OF) is a
frequent cause of orbital trauma, frequently leading to the
impaction of the extraocular muscles and the contents of the orbit.
Diplopia, eye movement abnormalities and enophthalmos are the
predominant clinical symptoms. This condition is usually found in
young adults, with male patients constituting a proportion of 72%,
which is mainly due to physical assault (1,2).
Facial trauma after a high-energy collision is a common finding in
patients with OF and the most common etiology is interpersonal
violence, followed by falls and motor vehicle collisions (3,4). The
medial wall of the orbital bone, which is the weakest part of the
bone, frequently sustains fractures. Following OF, neurological and
ocular sequelae are frequent (5).
The most frequent ophthalmologic problems that may affect vision
are retrobulbar hematoma and trauma to the optic nerve (6). Consequently, it is crucial to treat
OF appropriately and rapidly. Typically, the goal of treating an
orbital floor fracture is to eliminate any diplopia and
enophthalmos that may be present (7). Operations are prioritized during
treatment, with autologous bone implantation being the most
frequent procedure (8). The most
recent procedures are patient-specific computer-aided
design/computer-aided manufacturing ceramic implants (9) and three-dimensional (3D) printed
model implants (10). Studies have
demonstrated that patients have excellent outcomes, with shorter
surgical durations and successful functional and esthetic outcomes
(11).
Midway through the 1980s, computed facial tomography
(CT) offered the most accurate diagnostic evaluation and made it
possible to visualize the contents of the orbit in 3D (12). Aggressive surgical methods were
used as a result (13). The ocular
injuries caused by OF may be characterized using CT scans, but the
underlying neurological changes remain elusive. In this context,
functional magnetic resonance imaging (fMRI) is becoming a more
valuable clinical tool that accurately maps out functional brain
networks in individuals and offers a chance to comprehend the
neurobiological underpinnings of unique behavioral distinctions
(14). In studies on orbital
studies associated with brain function alterations, fMRI has been
widely used. For instance, patients with advanced monocular
blindness exhibited alterations in reduced gray matter volume in
particular regions of the brain (15). In addition, it was demonstrated
that neural activity in the inferior frontal cortex and superior
parietal lobule enable blind individuals to automatically assign
directional meaning to echoes (16). However, the mechanisms driving
internal brain activity modifications in patients with acute
unilateral vision loss following OF remain unclear despite research
showing altered visual and brain function in blind patients.
A technique to show the intrinsic connection
patterns of whole-brain functional networks is the voxel-wise
degree centrality (DC) method. Brain networks that exhibit
intrinsic coordinated activity may be identified by functional
connectivity (17). The DC method
is distinct from the voxel-based morphometry (18) approach, as the use of regions of
interest is not required. The DC approach has been used
extensively, since it may give inside looks at the functional
connectivity of the entire brain, particularly to research brain
pathological disorders such as autism (19) and Parkinson's disease (20), which are both neurological
conditions (21). On this basis,
the present study investigated the clinical correlations in terms
of functional connectivity in patients with OF.
Materials and methods
Study participants
The present study recruited 20 patients with OF
(sex, 12 males and 8 females; mean age, 51.21 years; age range,
35-61 years) from the Ophthalmology Department of the First
Affiliated Hospital of Nanchang University Hospital (Nanchang,
China). The present study was performed from July 2020 to June
2021. All patients were diagnosed with OF using CT.
The inclusion criteria were as follows: i) Patients
with OF who did not receive surgical treatment; and ii) patients
without other ocular diseases, such as cataracts, corneal ulcers,
glaucoma or macular degeneration.
The following exclusion criteria were used: i)
Presence of other ophthalmic diseases; ii) presence of central
nervous system diseases; iii) previous history of ophthalmic
surgery; and iv) patients unable to receive MRI examination.
In addition, an equal number of healthy controls
(HCs) who met the following criteria were recruited: i) Individuals
without ocular or central nervous system diseases; and ii)
individuals who had no contraindications for MRI scanning.
The 20 HCs (12 males and 8 females; mean age, 50.96
years; age range, 50.96±10.82 years) were pair-matched with
patients in the OF group according to sex, age, body weight and
education level (Table I). The
Medical Ethics Committee of the First Affiliated Hospital of
Nanchang University (Nanchang, China) authorized and approved the
methods used in the present study (approval no. cdyfy2021039),
which followed the tenets of The Declaration of Helsinki. All
participants were volunteers, to whom the purpose, methods,
procedures and underlying risks of the study had been explained.
All participants signed informed consent forms.
 | Table ICharacteristics of the study
participants. |
Table I
Characteristics of the study
participants.
|
Characteristics | OF | Healthy
controls | t |
P-valuea |
|---|
| Sex | | | N/A | >0.999 |
|
Male | 12 | 12 | | |
|
Female | 8 | 8 | | |
| Age, years | 51.21±11.42 | 50.96±10.82 | 0.242 | 0.871 |
| Body weight,
kg | 68.32±9.24 | 69.93±9.54 | 0.165 | 0.902 |
| Handedness, R | 20 | 20 | N/A | >0.999 |
| OF duration,
days | 11.61±4.14 | N/A | N/A | N/A |
| Best-corrected
VA | | | | |
|
Left
eye | 0.40±0.20 | 1.05±0.20 | -3.763 | 0.017 |
|
Right
eye | 0.45±0.15 | 1.00±0.15 | -3.064 | 0.011 |
| Latency right of
the VEP, msec | 118.16±8.29 | 100.98±6.17 | 3.554 | 0.017 |
| Amplitude right of
the VEP, µV | 6.87±2.42 | 14.16±1.93 | -6.643 | 0.009 |
| Latency left of the
VEP, msec | 116.12±7.11 | 101.21±1.32 | 4.532 | 0.022 |
| Amplitude left of
the VEP, µV | 7.42±2.73 | 16.74±2.52 | -5.732 | 0.012 |
Resting-state (Rs-)fMRI data
acquisition
Rs-fMRI data were acquired with a Siemens Trio 3.0 T
scanner associated with an 8-channel phased array probe coil
(Siemens AG). The Rs-fMRI scanning was performed using the
following parameters: Repetition time, 2,000 msec; echo time, 40
msec; flip angle, 90˚; slice thickness/gap, 4.0/1 mm; field of
view, 240x240 mm; in-plane resolution, 64x64,30 axial slices and
240 volumes.
Rs-fMRI data processing
MRIcro (www.MRIcro.com) was used to pre-filter and DPARSFA
(http://rfmri.org/DPARSF), SPM8 (http://www.fifil.ion.ucl.ac.uk/spm) and the
Resting-state Data Analysis Toolkit (http://www.restfmri.net) to preprocess the data as
performed in a previous study (22).
Degree centrality
The DC value was calculated using significant
suprathreshold correlations between the subjects, or the degree of
the binarized adjacency matrix, in the voxel function network based
on the individual voxel-wise functional network. The DC map for
each individual was transformed into a z-score map using the
following equation: Zi=DCi-mean/standard
deviation (SD), where Zi refers to the z score of the
ith voxel, DCi refers to DC value of the ith
voxel and mean refers to DC of all voxels in the brain mask
(22).
Clinical characteristics
Personal clinical data were collected after
admission. The OF duration (days) of all individuals was also
recorded. After examining the best-corrected visual acuity (VA),
the results were converted into the minimum logarithmic. In
addition, a visual evoked potential (VEP) examination was performed
with the patient's consent.
Statistical analysis
Demographic and clinical data were analyzed using
the independent samples t-test in SPSS 20.0 software (IBM Corp.) to
identify any significant differences in clinical features between
the patients with OF and HCs. P<0.05 was considered to indicate
a statistically significant difference. Data are presented as the
mean ± SD. An independent two-samples t-test in SPSS 20.0 was used
to compare the DC data between patients with OF and HCs. Receiver
operating characteristic (ROC) curve analyses, which were used to
classify the mean DC values in various brain areas of the patients
with OF, as well as the calculation of the T-value in Table II, were performed using SPSS 20.0.
The correlations between behavioral performance and mean DC values
were evaluated with Pearson's correlation analysis using GraphPad
Prism (version 7.0; GraphPad Software; Dotmatics).
 | Table IIBrain areas with significant
differences in voxel-wise degree centrality between the OF and HC
groups. |
Table II
Brain areas with significant
differences in voxel-wise degree centrality between the OF and HC
groups.
| | Montreal
Neurological Institute coordinates | |
|---|
| Brain areas | X | Y | Z | Brodmann area | Number of
voxels | T-value |
|---|
| Right cerebellum 9
region | -3 | -24 | -21 | - | 164 | 4.6322 |
| Left cerebellar
peduncles 2 area | -30 | -78 | -48 | 93 | 123 | 4.2018 |
Results
Demographics and visual
measurements
The present study found similar proportions in sex
(P>0.99), body weight (P=0.902) and age (P=0.871) among the
participants. However, statistically significant differences were
found in the best-corrected VA-right (P=0.011), best-corrected
VA-left (P=0.017), latency (msec)-right of the VEP (P=0.017),
latency (msec)-left of the VEP (P=0.022), amplitudes (µV)-right of
the VEP (P=0.009) and amplitudes (µV-left of the VEP (P=0.012)
between the two groups (Table
I).
Differences in DC
The DC values in patients with OF were significantly
higher in the right cerebellum 9 region (Cerebelum_9_R) and left
cerebellar peduncle 2 area (Cerebelum_Crus2_L) (Fig. 1A and B; Table
II) than in HCs. Fig. 2
presents the mean values of altered DC between patients with OF and
HCs.
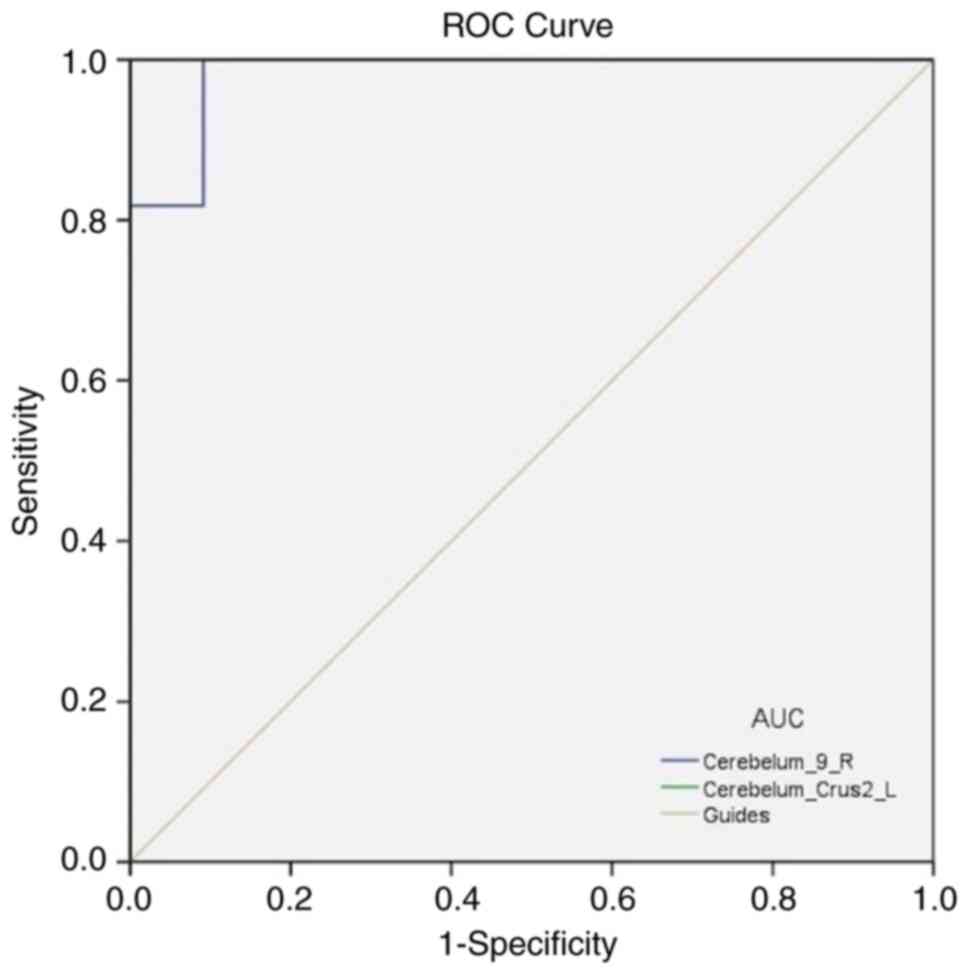
ROC curve
It was hypothesized that the OF and HC groups have
different DC values in distinct brain regions, and the ROC curves
were applied to distinguish between OF and HC. In the present
study, the areas under the ROC curve were 0.983 (P<0.0001; 95%
CI, 0.941-1.000) for the Cerebelum_9_R area and 1.000 (P<0.0001;
95% CI, 1.000-1.000) for the Cerebelum_Crus2_L area (Fig. 3).
Discussion
The orbit is a complex and important anatomical
structure. OF is frequently followed by acute injury to the brain,
retina, optic nerve, retrobulbar soft tissue and optic nerve, which
causes various issues, including eye movement dysfunction and
diplopia (23). Rs-fMRI is a type
of medical imaging that tracks brain activity using signals that
depend on blood oxygen levels. The changes in potential brain
functional network activity in patients with OF were examined in
the present study using Rs-fMRI and the association between
spontaneous brain activity and clinical features in patients with
OF was examined using DC technology (18,22).
To date, the DC method has been successfully applied to a variety
of neurogenic and ophthalmological diseases, with great potential
for development. Altered DC values may be a useful biomarker to
indicate the abnormal brain activity changes in diabetic
retinopathy (24), optic neuritis
(25) or Alzheimer's disease
(26) (Table III). High-energy trauma is a
common cause of OF, which may result in several complications and
harm to visual function (3,23).
The degree of the retinal, optic nerve and visual impairment may be
rapidly and objectively determined by visual electrophysiological
testing. The cerebral cortex generates bioelectricity when the
retina is activated and this is known as the VEP (27). The VEP has a decreased amplitude
and a delayed peak when the optic nerve is damaged by ocular trauma
(28). In the present study,
patients with OF had significantly worse VA, VEP amplitudes and VEP
latency. The present investigation discovered that patients with OF
had higher DC values in the Cerebelum_9_R and Cerebelum_Crus2_L
compared with those in HCs. Afferent visual pathways may become
aberrant as a result of cerebellum diseases (29). It may be hypothesized that the
decline in VA is connected to the aberrant cerebellar DC value in
patients with OF.
 | Table IIIVoxel-wise degree centrality method
applied in ophthalmologic and neurogenic diseases. |
Table III
Voxel-wise degree centrality method
applied in ophthalmologic and neurogenic diseases.
| A,
Ophthalmologic |
|---|
| First author,
year | Disease | (Refs.) |
|---|
| Chen et al,
2013 | Alternating
Horner's syndrome | (55) |
| Cai et al,
2015 | Glaucoma | (21) |
| Hu et al,
2018 | High myopia | (56) |
| Huang et al,
2019 | Visual loss | (57) |
| Huang et al,
2021 | Diabetic
retinopathy | (24) |
| Wei et al,
2022 | Optic neuritis | (25) |
| B, Neurogenic |
| First author,
year | Disease | (Refs.) |
| Guo et al,
2016 | Alzheimer's
disease | (26) |
| Deng et al,
2019 | Bipolar
disorder | (58) |
| Zhang et al,
2022 | Postpartum
depression | (59) |
The primary and posterolateral fissures divide the
cerebellum, which is found in the posterior cranial fossa and into
anterior, posterior and pompous lobes (30,31).
The control and coordination of muscle tension, voluntary movement
and body balance are all functions of the cerebellum. The
cerebellum not only immediately and effectively regulates eye
movement but may also contribute to long-term visual calibration.
More crucially, the cerebellum also ensures the correctness of eye
movements, ensuring visual sharpness and clarity (30,32-35).
The vestibular nucleus in the brain stem serves as the primary
afferent source for the vestibular cerebellum. The vestibular organ
integrates position data from the head and the entire body into the
vestibular cerebellum, which in turn controls the vestibular
nucleus and the pontine reticular structure. In addition to
regulating trunk activity, the vestibular cerebellum can also
control the activity of the extraocular muscles (36). A study has indicated that
cerebellar damage can impact spatial and temporal visual attention
(37). In addition, it has been
indicated that patients with cerebellar damage have less effective
eye tracking and fixation than HCs (30,32,34,38).
The current study suggested that patients with OF had higher
cerebellar DC (see spots 1 and 2 in Fig. 4). This indicates that patients with
OF may have aberrant cerebellar functional connections, which may
be hypothesized to be connected to issues such as eye movement
difficulties or a decline in VA in OF.
A total of three sets of cerebellar feet are present
in the cerebellum (superior, middle, inferior). Eye movements are
tightly associated with all cerebellar feet (33,35,39).
Starting from the bottom of the bridge, the middle cerebellar foot
is in the outermost of the three pairs and it is situated between
the cerebellum and the pons. The primary afferent fibers of the
cerebellum, which comprise white matter fibers of the opposing
pontine nucleus, are gathered in the middle peduncle of the
cerebellum. This nucleus is a gray matter structure responsible for
regulating the start, transmission and execution of movement
through a closed-loop connection between the cerebellum and the
precentral/prefrontal cortex (39-41).
Therefore, ipsilateral limb ataxia, nystagmus and vertigo are
frequently seen as clinical symptoms of cerebellar middle foot
lesions (39). The foot is a key
conduit for conveying information about eye movement; thus, a
middle cerebellar peduncle injury may result in aberrant eye
movement (33,39). A previous study described three
patients with cerebellar middle foot hemangiomas (42). During the vertical line of the
sight chase test, aberrant eye movement and significant twisting
nystagmus were present in all patients. When tracking with the
vertical line of sight, nystagmus was indicated to occur as a
result after unilateral cerebellar foot damage (42-44).
The cerebellum's middle foot includes the Cerebelum_Crus2_L area.
The present study demonstrated that the DC value of the
Cerebelum_Crus2_L area is larger in patients with OF compared with
that in HCs (Table IV),
indicating that OF may cause an overactive cerebellar middle
peduncle, also known as the pontine arm, and possibly a higher
level of functional connectivity.
 | Table IVBrain areas of altered voxel-wise
degree centrality values and potential effects. |
Table IV
Brain areas of altered voxel-wise
degree centrality values and potential effects.
| Brain areas | Experimental result
(DC values) | Brain function | Anticipated
results |
|---|
| Right cerebellum 9
region | OF > HCs | Physical balance,
muscular tension, motor coordination | Behavioral
disorders |
| Left cerebellar
peduncles 2 area | OF > HCs | Connecting
structure, associated with eye movement | Visual
impairment |
Ocular motor vermis and caudal fastigial nuclei,
ventral uvula and nodulus, and flocculus and para flocculus are the
three critical areas of cerebellar control of eye movement
(33). The neural network signal
encoding vertical line of sight tracking is transmitted to the
vestibular cerebellum through the middle foot of the cerebellum
(35,36). The posterior lobe of the
cerebellum, which contributes to the development of the vestibular
cerebellum, contains the right cerebellar region 9 (Cerebelum_9_R).
In a previous study, the vestibulo-ocular reflex suggested that eye
movement-counteracting head movement was able to maintain a stable
line of sight (45). It was
discovered that patients with OF had significantly higher DC values
in Cerebelum_9_R compared with those in HCs (Table IV), indicating that eye movement
impairment in patients with OF may be related to the compensatory
mechanism of vestibular cerebellum dysfunction. The paramedian
tract cell population and the inferior olivary nucleus are
responsible for transmitting retinal slip signals to the para
flocculus of the vestibular cerebellum. This is the precise process
by which the cerebellum regulates eye movement. In the
aforementioned process, the vestibular nucleus participates as the
afferent fibers of the flocculus and para flocculus. The vestibular
nucleus and vestibular cerebellum are linked via a two-way fiber.
To regulate the activity of the muscles in the trunk and
extremities, it receives projections from the vestibular nucleus
and its efferent fibers change through the vestibular nucleus
before reaching the motoneurons in the medial section of the
anterior horn of the spinal cord. The middle peduncle of the
cerebellum receives nerve fibers from the vestibular nucleus and
transmits them to the cerebellum (46-50).
Patients with OF had considerably higher DC values in the
cerebellar tonsillar and peduncle regions, which are crucial in the
development of the vestibular cerebellum. It may be hypothesized
that an OF may impair the cerebellum's ability to precisely
regulate eye movement, which would reduce VA, and this is
consistent with previously published results (51).
In the present study, the mechanism of cerebellar
dysfunction caused by OF remains elusive and the associated
inflammatory response cannot be ignored. Orbital cellulitis is one
of the severe complications of OF (52). Anti-inflammatory therapy is
critical in OF. A variety of active substances have been indicated
to have neuroprotective and powerful anti-inflammatory effects and
are expected to be applied in clinical therapy. Cerebrolysin, a
mixture of enzymatic processing peptides from pig brains, protects
neurons, primarily by reducing glutamate concentration in the
synaptic clefts. In addition, it may reduce oxidative stress and
harm to neuronal cells by boosting antioxidant activity and
lowering levels of inflammatory cytokines according to the data
from Avci et al (53). In
another study, a bioactive natural product called crocin was able
to not only inhibit the production of reactive oxygen species and
the secretion of pro-inflammatory cytokines but also reduce the
inflammation of various organs/systems (54). However, more relevant studies are
needed to further evaluate and improve the therapy for OF.
Although the DC technique is a useful tool for
observing whole-brain activity, it has certain drawbacks. First,
there are several variables to consider. For instance, differences
in disease time courses and physical circumstances among patients
may have led to measurement inaccuracies. In addition, the sample
size of the current study was small and this may have had an impact
on the present DC results. The present findings should be confirmed
by further investigations to address these issues and use reliable
brain function activity exams.
In conclusion, the present study demonstrated that
the cerebellum is involved in the regulation of eye movement and
that patients with OF exhibit abnormal spontaneous activity in the
middle cerebellar peduncle and posterior cerebellar lobe compared
with that in the HC group. In addition, focusing on the damage to
the extraocular muscle and oculomotor nerve, the approach to
treating eye movement disorders in patients with OF offers a fresh
perspective. The present findings may be helpful in the treatment
of individuals with OF. Attention should also be paid to the brain
tissue connected to eye movement and VA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and YG conceived, designed and supervised the
study. HS collected data and prepared the images. QL and QG
collected and interpreted the data and wrote the manuscript. XL,
YP, TS, JW, LZ and RL performed data analysis and critically
reviewed the manuscript. YS conceived and designed the present
study and critically revised the manuscript. All authors have read
and approved the final manuscript. HS and YS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The Medical Ethics Committee of the First Affiliated
Hospital of Nanchang University (Nanchang, China) authorized and
approved the methods used in the present study (approval no.
cdyfy2021039), which followed the tenets of The Declaration of
Helsinki. All participants were volunteers, to whom the purpose,
methods, procedures and underlying risks of the study had been
explained. All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seifert LB, Mainka T, Herrera-Vizcaino C,
Verboket R and Sader R: Orbital floor fractures: Epidemiology and
outcomes of 1594 reconstructions. Eur J Trauma Emerg Surg.
48:1427–1436. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gosau M, Schöneich M, Draenert FG, Ettl T,
Driemel O and Reichert TE: Retrospective analysis of orbital floor
fractures-complications, outcome, and review of literature. Clin
Oral Investig. 15:305–313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gebran SG, Lopez J, Wasicek PJ, Elegbede
A, Rasko YM, Liang F, Nam AJ, Manson PN and Grant MP: Surgical
treatment and visual outcomes of adult orbital roof fractures.
Plast Reconstr Surg. 147:82e–93e. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moffatt J, Hughes D, Bhatti N and Holmes
S: Orbital bone fractures in a Central London Trauma Center: A
retrospective study of 582 patients. J Craniofac Surg.
32:1334–1337. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koenen L and Waseem M: Orbital Floor
Fracture. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2022.
|
|
6
|
Austria QM, Tran AQ, Tooley AA, Kazim M
and Godfrey K: Orbital cavernous venous malformation with partial
bone encasement. Orbit: Mar 16, 2021 (Epub ahead of print).
|
|
7
|
Omura K, Nomura K, Okushi T, Tanaka Y and
Otori N: Endoscopic endonasal orbital floor fracture repair with
mucosal preservation to reinforce the fractured bone. J Craniofac
Surg. 32:541–545. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Strong EB: Orbital fractures:
Pathophysiology and implant materials for orbital reconstruction.
Facial Plast Surg. 30:509–517. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Falkhausen R, Mitsimponas K, Adler W,
Brand M and von Wilmowsky C: Clinical outcome of patients with
orbital fractures treated with patient specific CAD/CAM ceramic
implants-A retrospective study. J Craniomaxillofac Surg.
49:468–479. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weadock WJ, Heisel CJ, Kahana A and Kim J:
Use of 3D printed models to create molds for shaping implants for
surgical repair of orbital fractures. Acad Radiol. 27:536–542.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chattopadhyay C, Dev V, Pilania D and
Harsh A: Reconstruction of orbital floor fractures with titanium
micromesh: Our experience. J Maxillofac Oral Surg. 21:369–378.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dubois L, Steenen SA, Gooris PJ, Mourits
MP and Becking AG: Controversies in orbital reconstruction-I.
Defect-driven orbital reconstruction: A systematic review. Int J
Oral Maxillofac Surg. 44:308–315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gooris PJJ, Jansen J, Bergsma JE and
Dubois L: Evidence-Based decision making in orbital fractures:
Implementation of a clinical protocol. Atlas Oral Maxillofac Surg
Clin North Am. 29:109–127. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lynch CJ, Elbau I and Liston C: Improving
precision functional mapping routines with multi-echo fMRI. Curr
Opin Behav Sci. 40:113–119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jiang A, Tian J, Li R, Liu Y, Jiang T, Qin
W and Yu C: Alterations of regional spontaneous brain activity and
gray matter volume in the blind. Neural Plast.
2015(141950)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fiehler K, Schütz I, Meller T and Thaler
L: Neural correlates of human echolocation of path direction during
walking. Multisens Res. 28:195–226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu K, Liu M, He L and Tan Y: Abnormal
degree centrality in delayed encephalopathy after carbon monoxide
poisoning: A resting-state fMRI study. Neuroradiology. 62:609–616.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
De Groote S, Goudman L, Linderoth B, Buyck
F, Rigoard P, De Jaeger M, Van Schuerbeek P, Peeters R, Sunaert S
and Moens M: A regions of interest voxel-based morphometry study of
the human brain during high-frequency spinal cord stimulation in
patients with failed back surgery syndrome. Pain Pract. 20:878–888.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Di Martino A, Zuo XN, Kelly C, Grzadzinski
R, Mennes M, Schvarcz A, Rodman J, Lord C, Castellanos FX and
Milham MP: Shared and distinct intrinsic functional network
centrality in autism and attention-deficit/hyperactivity disorder.
Biol Psychiatry. 74:623–632. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li MG, Bian XB, Zhang J, Wang ZF and Ma L:
Aberrant voxel-based degree centrality in Parkinson's disease
patients with mild cognitive impairment. Neurosci Lett.
741(135507)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zuo XN, Ehmke R, Mennes M, Imperati D,
Castellanos FX, Sporns O and Milham MP: Network centrality in the
human functional connectome. Cereb Cortex. 22:1862–1875.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cai F, Gao L, Gong H, Jiang F, Pei C,
Zhang X, Zeng X and Huang R: Network centrality of resting-state
fMRI in primary angle-closure glaucoma before and after surgery.
PLoS One. 10(e0141389)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cossman JP, Morrison CS, Taylor HO, Salter
AB, Klinge PM and Sullivan SR: Traumatic orbital roof fractures:
Interdisciplinary evaluation and management. Plast Reconstr Surg.
133:335e–343e. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang X, Xie BJ, Qi CX, Tong Y and Shen Y:
Abnormal intrinsic functional network hubs in diabetic retinopathy
patients. Neuroreport. 32:498–506. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei R, Yan J, Wu H, Meng F, He F, Liu X
and Liang H: Irregular degree centrality in neuromyelitis optica
spectrum disorder patients with optic neuritis: A resting-state
functional magnetic resonance imaging study. Mult Scler Relat
Disord. 59(103542)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo Z, Liu X, Hou H, Wei F, Liu J and Chen
X: Abnormal degree centrality in Alzheimer's disease patients with
depression: A resting-state functional magnetic resonance imaging
study. Exp Gerontol. 79:61–66. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Firan AM, Istrate S, Iancu R, Tudosescu R,
Ciuluvică R and Voinea L: Visual evoked potential in the early
diagnosis of glaucoma. Literature review. Rom J Ophthalmol.
64:15–20. 2020.PubMed/NCBI
|
|
28
|
Zheng X, Xu G, Zhang K, Liang R, Yan W,
Tian P, Jia Y, Zhang S and Du C: Assessment of human visual acuity
using visual evoked potential: A review. Sensors (Basel).
20(5542)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Their P and Markanday A: Role of the
vermal cerebellum in visually guided eye movements and visual
motion perception. Annu Rev Vis Sci. 5:247–268. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Azizi SA: Role of the cerebellum in the
phenotype of neurodegenerative diseases: Mitigate or exacerbate?
Neurosci Lett. 760(136105)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Voogd J: The human cerebellum. J Chem
Neuroanat. 26:243–252. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Peterburs J, Liang Y, Cheng DT and Desmond
JE: Sensory acquisition functions of the cerebellum in verbal
working memory. Brain Struct Funct. 226:833–844. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee SU, Bae HJ and Kim JS: Ipsilesional
limb ataxia and truncal ipsipulsion in isolated infarction of the
superior cerebellar peduncle. J Neurol Sci. 349:251–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kheradmand A and Zee DS: Cerebellum and
ocular motor control. Front Neurol. 2(53)2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Beh SC, Frohman TC and Frohman EM:
Cerebellar control of eye movements. J Neuroophthalmol. 37:87–98.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hernandez E and Das JM: Neuroanatomy,
Nucleus Vestibular. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2022.
|
|
37
|
Craig BT, Morrill A, Anderson B, Danckert
J and Striemer CL: Cerebellar lesions disrupt spatial and temporal
visual attention. Cortex. 139:27–42. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hernandez E and Das JM: Neuroanatomy,
Nucleus Vestibular. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2021.
|
|
39
|
Kim SH and Kim JS: Eye movement
abnormalities in middle cerebellar peduncle strokes. Acta Neurol
Belg. 119:37–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Delgado-García JM: Structure and function
of the cerebellum. Rev Neurol. 33:635–642. 2001.PubMed/NCBI(In Spanish).
|
|
41
|
Cicirata F, Serapide MF, Parenti R, Pantò
MR, Zappalà A, Nicotra A and Cicero D: The basilar pontine nuclei
and the nucleus reticularis tegmenti pontis subserve distinct
cerebrocerebellar pathways. Prog Brain Res. 148:259–282.
2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
FitzGibbon EJ, Calvert PC, Dieterich M,
Brandt T and Zee DS: Torsional nystagmus during vertical pursuit. J
Neuroophthalmol. 16:79–90. 1996.PubMed/NCBI
|
|
43
|
Krauzlis RJ, Goffart L and Hafed ZM:
Neuronal control of fixation and fixational eye movements. Philos
Trans R Soc Lond B Biol Sci. 372(20160205)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kaski D, Bentley P, Lane R and Bronstein
A: Up-down asymmetry of saccadic contrapulsion in lateral medullary
syndrome. J Neuroophthalmol. 32:224–226. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dieterich M and Brandt T: Vestibulo-ocular
reflex. Curr Opin Neurol. 8:83–88. 1995.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Arnold DB and Robinson DA: The oculomotor
integrator: Testing of a neural network model. Exp Brain Res.
113:57–74. 1997.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nagao S, Kitamura T, Nakamura N, Hiramatsu
T and Yamada J: Location of efferent terminals of the primate
flocculus and ventral paraflocculus revealed by anterograde axonal
transport methods. Neurosci Res. 27:257–269. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Glickstein M, Gerrits N, Kralj-Hans I,
Mercier B, Stein J and Voogd J: Visual pontocerebellar projections
in the macaque. J Comp Neurol. 349:51–72. 1994.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Voogd J, Schraa-Tam CK, van der Geest JN
and De Zeeuw CI: Visuomotor cerebellum in human and nonhuman
primates. Cerebellum. 11:392–410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Leigh RJ and Zee DS: The Neurology of Eye
Movements. 5th edition. Oxford University Press, New York, NY,
2015.
|
|
51
|
Kim YS, Kim JH and Hwang K: The frequency
of decreased visual acuity in orbital fractures. J Craniofac Surg.
26:1581–1583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ben Simon GJ, Bush S, Selva D and McNab
AA: Orbital cellulitis: A rare complication after orbital blowout
fracture. Ophthalmology. 112:2030–2034. 2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Avci S, Gunaydin S, Ari NS, Karaca
Sulukoglu E, Polat OE, Gecili I, Yeni Y, Yilmaz A, Genc S,
Hacimuftuoglu A, et al: Cerebrolysin alleviating effect on
glutamate-mediated neuroinflammation via glutamate transporters and
oxidative stress. J Mol Neurosci. 72:2292–2302. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hashemzaei M, Mamoulakis C, Tsarouhas K,
Georgiadis G, Lazopoulos G, Tsatsakis A, Shojaei Asrami E and
Rezaee R: Crocin: A fighter against inflammation and pain. Food
Chem Toxicol. 143(111521)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen Z, Fan Y, Li J and Ma L: Alterations
of brain functional connectivity in a patient with alternating
Horner's syndrome: A functional magnetic resonance imaging study.
Nan Fang Yi Ke Da Xue Xue Bao. 33:1177–1180. 2013.PubMed/NCBI(In Chinese).
|
|
56
|
Hu YX, He JR, Yang B, Huang X, Li YP, Zhou
FQ, Xu XX, Zhong YL, Wang J and Wu XR: Abnormal resting-state
functional network centrality in patients with high myopia:
Evidence from a voxel-wise degree centrality analysis. Int J
Ophthalmol. 11:1814–1820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Huang X, Dan HD, Zhou FQ, Deng QQ and Shen
Y: Abnormal intrinsic functional network hubs and connectivity
following peripheral visual loss because of inherited retinal
degeneration. Neuroreport. 30:295–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Deng W, Zhang B, Zou W, Zhang X, Cheng X,
Guan L, Lin Y, Lao G, Ye B, Li X, et al: Abnormal degree centrality
associated with cognitive dysfunctions in early bipolar disorder.
Front Psychiatry. 10(140)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhang S, Li B, Liu K, Hou X and Zhang P:
Abnormal voxel-based degree centrality in patients with postpartum
depression: A resting-state functional magnetic resonance imaging
study. Front Neurosci. 16(914894)2022.PubMed/NCBI View Article : Google Scholar
|