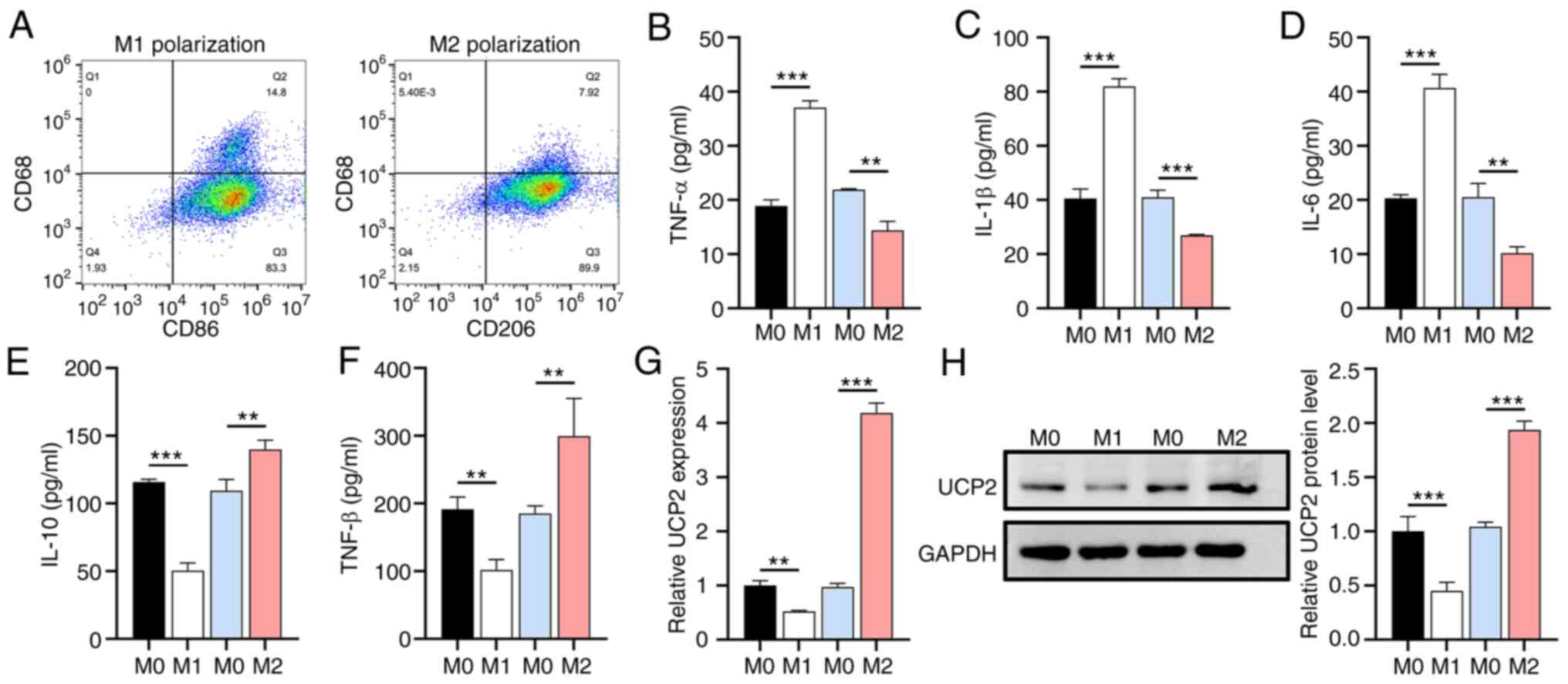
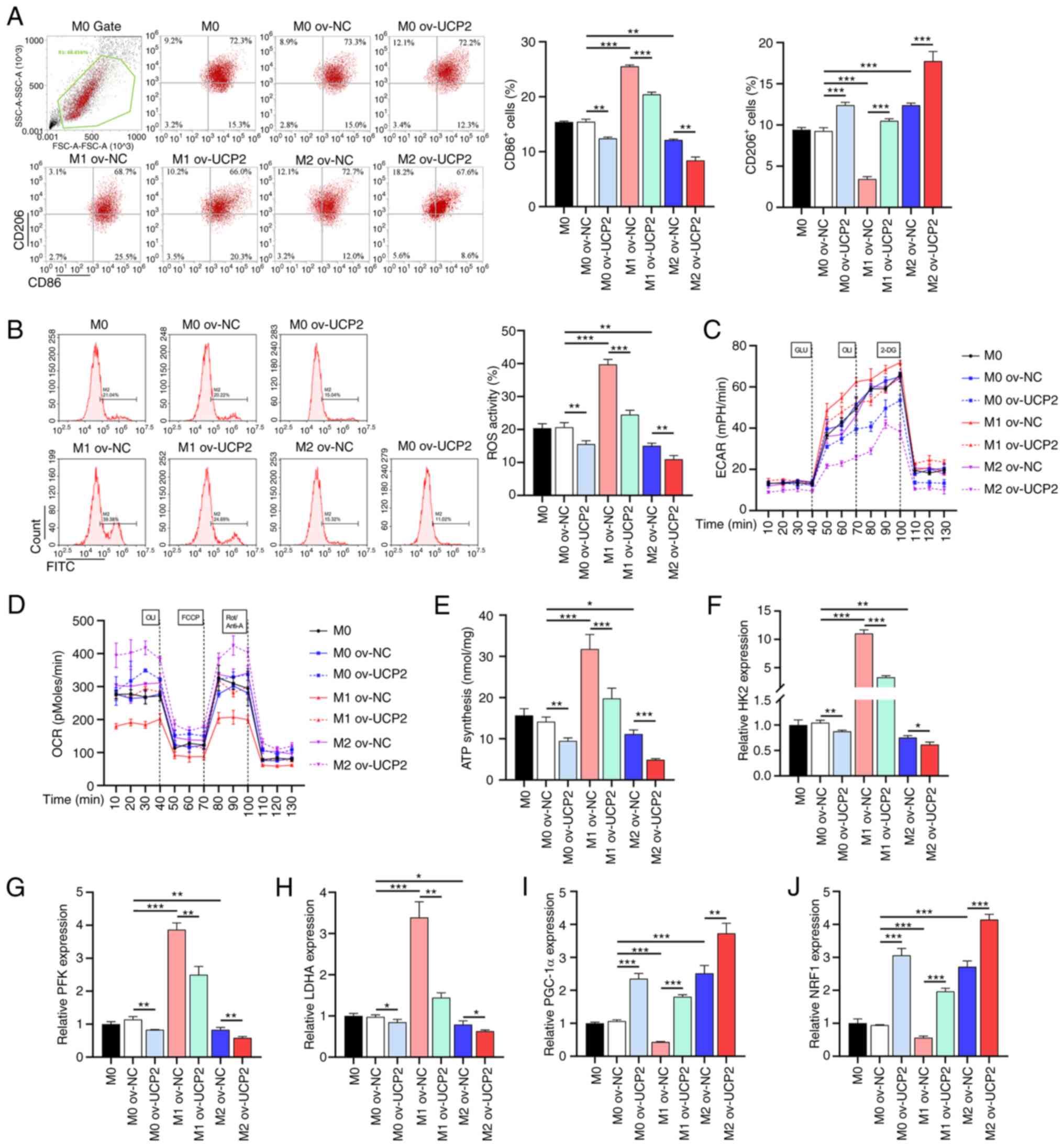
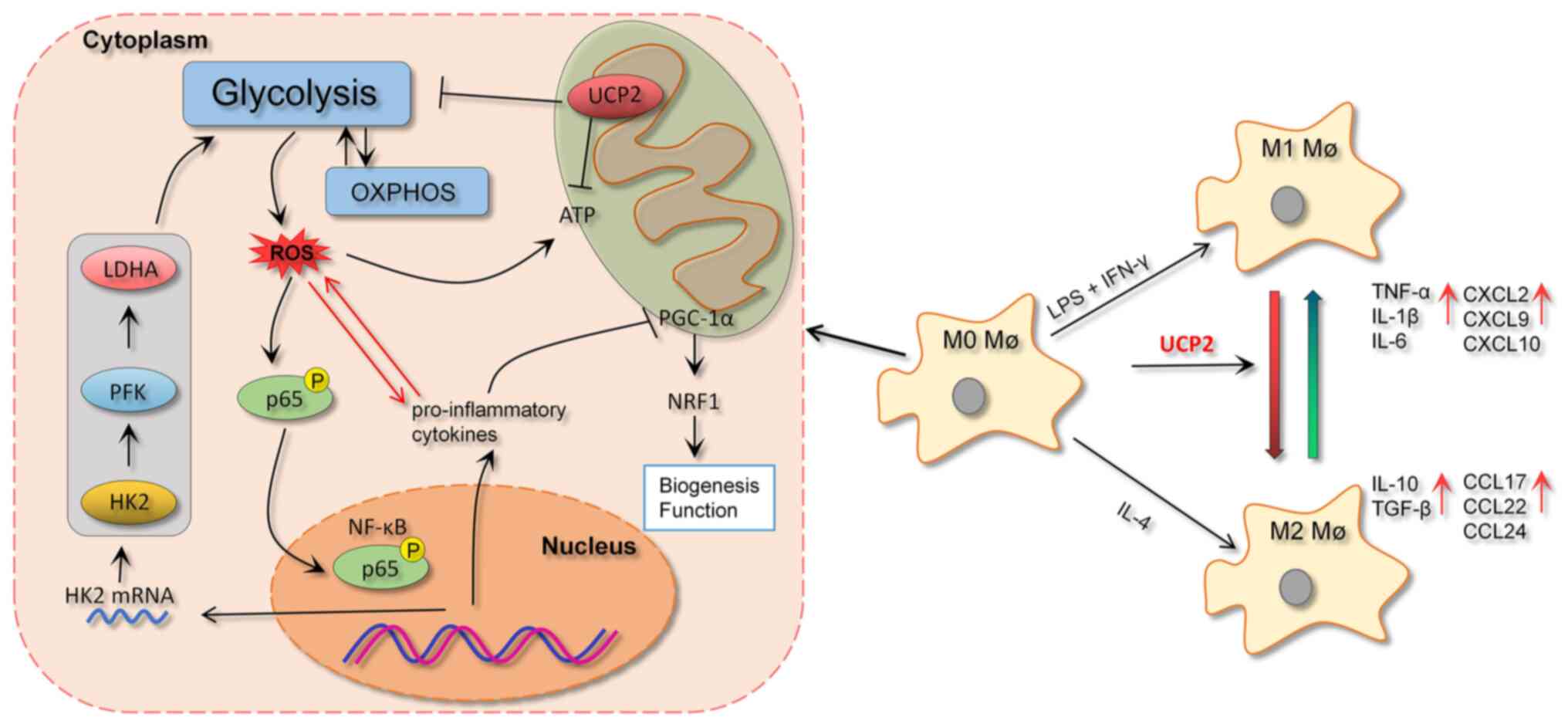
|
1
|
Mahroum N, Alghory A, Kiyak Z, Alwani A,
Seida R, Alrais M and Shoenfeld Y: Ferritin-from iron, through
inflammation and autoimmunity, to COVID-19. J Autoimmun.
126(102778)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li C, Xu MM, Wang K, Adler AJ, Vella AT
and Zhou B: Macrophage polarization and meta-inflammation. Transl
Res. 191:29–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ross EA, Devitt A and Johnson JR:
Macrophages: The good, the bad, and the gluttony. Front Immunol.
12(708186)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Viola A, Munari F, Sanchez-Rodriguez R,
Scolaro T and Castegna A: The metabolic signature of macrophage
responses. Front Immunol. 10(1462)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu L, Zhao Q, Yang T, Ding W and Zhao Y:
Cellular metabolism and macrophage functional polarization. Int Rev
Immunol. 34:82–100. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang S, Liu G, Li Y and Pan Y: Metabolic
reprogramming induces macrophage polarization in the tumor
microenvironment. Front Immunol. 13(840029)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marrocco A and Ortiz LA: Role of metabolic
reprogramming in pro-inflammatory cytokine secretion from LPS or
silica-activated macrophages. Front Immunol.
13(936167)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Peace CG and O'Neill LA: The role of
itaconate in host defense and inflammation. J Clin Invest.
132(e148548)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mouton AJ, Li X, Hall ME and Hall JE:
Obesity, hypertension, and cardiac dysfunction: novel roles of
immunometabolism in macrophage activation and inflammation. Circ
Res. 126:789–806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Dierendonck X, Sancerni T,
Alves-Guerra MC and Stienstra R: The role of uncoupling protein 2
in macrophages and its impact on obesity-induced adipose tissue
inflammation and insulin resistance. J Biol Chem. 295:17535–17548.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Steen KA, Xu H and Bernlohr DA: FABP4/aP2
regulates macrophage redox signaling and inflammasome activation
via control of UCP2. Mol Cell Biol. 37:e00282–e00216.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Esteves P, Pecqueur C, Ransy C, Esnous C,
Lenoir V, Bouillaud F, Bulteau AL, Lombes A, Prip-Buus C, Ricquier
D and Alves-Guerra MC: Mitochondrial retrograde signaling mediated
by UCP2 inhibits cancer cell proliferation and tumorigenesis.
Cancer Res. 74:3971–3982. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Emre Y, Hurtaud C, Karaca M, Nubel T,
Zavala F and Ricquier D: Role of uncoupling protein UCP2 in
cell-mediated immunity: How macrophage-mediated insulitis is
accelerated in a model of autoimmune diabetes. Proc Natl Acad Sci
USA. 104:19085–19090. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nishio K, Qiao S and Yamashita H:
Characterization of the differential expression of uncoupling
protein 2 and ROS production in differentiated mouse
macrophage-cells (Mm1) and the progenitor cells (M1). J Mol Histol.
36:35–44. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang S, Yu H, Gao J, Chen J, He P, Zhong
H, Tan X, Staines KA, Macrae VE, Fu X, et al: PALMD regulates
aortic valve calcification via altered glycolysis and
NF-κB-mediated inflammation. J Biol Chem.
298(101887)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kooshki L, Mahdavi P, Fakhri S, Akkol EK
and Khan H: Targeting lactate metabolism and glycolytic pathways in
the tumor microenvironment by natural products: A promising
strategy in combating cancer. Biofactors. 48:359–383.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu H, Zhu S, Han W, Cai Y and Liu C: DMEP
induces mitochondrial damage regulated by inhibiting Nrf2 and
SIRT1/PGC-1alpha signaling pathways in HepG2 cells. Ecotoxicol
Environ Saf. 221(112449)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Z, Zhang X, Meng L, Gong M, Li J,
Shi W, Qiu J, Yang Y, Zhao J, Suo Y, et al: Pioglitazone inhibits
diabetes-induced atrial mitochondrial oxidative stress and improves
mitochondrial biogenesis, dynamics, and function through the
PPAR-γ/PGC-1α signaling pathway. Front Pharmacol.
12(658362)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim MJ, Lee H, Chanda D, Thoudam T, Kang
HJ, Harris RA and Lee IK: The role of pyruvate metabolism in
mitochondrial quality control and inflammation. Mol Cells.
46:259–267. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY,
Yang LY, Chen JH and Yeh WL: Regulatory effects of quercetin on
M1/M2 macrophage polarization and oxidative/antioxidative balance.
Nutrients. 14(67)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Forte M, Bianchi F, Cotugno M, Marchitti
S, Stanzione R, Maglione V, Sciarretta S, Valenti V, Carnevale R,
Versaci F, et al: An interplay between UCP2 and ROS protects cells
from high-salt-induced injury through autophagy stimulation. Cell
Death Dis. 12(919)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu J, Shi L, Lin W, Lu B and Zhao Y: UCP2
promotes proliferation and chemoresistance through regulating the
NF-κB/β-catenin axis and mitochondrial ROS in gallbladder cancer.
Biochem Pharmacol. 172(113745)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meng L, Lu C, Wu B, Lan C, Mo L, Chen C,
Wang X, Zhang N, Lan L, Wang Q, et al: Taurine antagonizes
macrophages M1 polarization by mitophagy-glycolysis switch blockage
via dragging SAM-PP2Ac transmethylation. Front Immunol.
12(648913)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ji R, Chen W, Wang Y, Gong F, Huang S,
Zhong M, Liu Z, Chen Y, Ma L, Yang Z, et al: The warburg effect
promotes mitochondrial injury regulated by uncoupling protein-2 in
septic acute kidney injury. Shock. 55:640–648. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ahmed ST, Craven L, Russell OM, Turnbull
DM and Vincent AE: Diagnosis and treatment of mitochondrial
myopathies. Neurotherapeutics. 15:943–953. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jia JJ, Zhang X, Ge CR and Jois M: The
polymorphisms of UCP2 and UCP3 genes associated with fat
metabolism, obesity and diabetes. Obes Rev. 10:519–526.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rius-Perez S, Torres-Cuevas I, Millan I,
Ortega AL and Perez S: PGC-1 α, inflammation, and oxidative stress:
An integrative view in metabolism. Oxid Med Cell Longev.
2020(1452696)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Korbecki J, Kojder K, Barczak K, Siminska
D, Gutowska I, Chlubek D and Baranowska-Bosiacka I: Hypoxia alters
the expression of CC chemokines and CC chemokine receptors in a
tumor-a literature review. Int J Mol Sci. 21(5647)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Morrissey SM, Zhang F, Ding C,
Montoya-Durango DE, Hu X, Yang C, Wang Z, Yuan F, Fox M, Zhang HG,
et al: Tumor-derived exosomes drive immunosuppressive macrophages
in a pre-metastatic niche through glycolytic dominant metabolic
reprogramming. Cell Metab. 33:2040–2058 e2010. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hu S, Feng J, Wang M, Wufuer R, Liu K,
Zhang Z and Zhang Y: Nrf1 is an indispensable redox-determining
factor for mitochondrial homeostasis by integrating
multi-hierarchical regulatory networks. Redox Biol.
57(102470)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ohkouchi S, Block GJ, Katsha AM, Kanehira
M, Ebina M, Kikuchi T, Saijo Y, Nukiwa T and Prockop DJ:
Mesenchymal stromal cells protect cancer cells from ROS-induced
apoptosis and enhance the Warburg effect by secreting STC1. Mol
Ther. 20:417–423. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li D, Zhang Q, Li L, Chen K, Yang J, Dixit
D, Gimple RC, Ci S, Lu C, Hu L, et al: beta2-microglobulin
maintains glioblastoma stem cells and induces M2-like polarization
of tumor-associated macrophages. Cancer Res. 82:3321–3334.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luby A and Alves-Guerra MC: UCP2 as a
cancer target through energy metabolism and oxidative stress
control. Int J Mol Sci. 23(15077)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D,
Cheng Y, Huang S, Liu Y, Jiang S, et al: Spatiotemporal immune
landscape of colorectal cancer liver metastasis at single-cell
level. Cancer Discov. 12:134–153. 2022.PubMed/NCBI View Article : Google Scholar
|